Abstract
Nucleic acids show immense potential to treat cancer, acquired immune deficiency syndrome, neurological diseases and other incurable human diseases. Upon systemic administration, they encounter a series of barriers and hence barely reach the site of action, the cell. Intracellular delivery of nucleic acids is facilitated by nanovectors, both viral and non-viral. A major advantage of non-viral vectors over viral vectors is safety. Nanovectors evaluated specifically for nucleic acid delivery include polyplexes, lipoplexes and other cationic carrier-based vectors. However, more recently there is an increased interest in inorganic nanovectors for nucleic acid delivery. Nevertheless, there is no comprehensive review on the subject. The present review would cover in detail specific properties and types of inorganic nanovectors, their preparation techniques and various biomedical applications as therapeutics, diagnostics and theranostics. Future prospects are also suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nucleic acids, a special class of therapeutic agents, treat a disease either by modifying the expression of a gene or correcting an abnormal gene. Their applications in the treatment of incurable human diseases like cancer [1], acquired immune deficiency syndrome (AIDS) [2], neurological diseases [3], ocular diseases [4] and many others have generated immense interest amongst researchers globally. Intracellular delivery of nucleic acids is a prime requisite for their exploitation as therapeutic agents. This, however, poses a serious challenge and is limited by their physicochemical properties. Naked nucleic acids cannot directly enter the cell. Studies on naked DNA have shown that it is cleared off substantially on first pass through the liver. The uptake of naked DNA occurs by non-parenchymal cells, mainly Kupffer cells, following interaction of DNA with scavenger receptors for polyanions. Neutralization of DNA is a proven strategy to minimize uptake by non-parenchymal cells [5]. Direct plasmid injection is well tolerated and requires minimal manipulations. Nevertheless, levels of gene transfer observed are low. Although high expression levels in murine muscles enough to induce desired immune responses by ‘DNA vaccination’ are seen, transfection levels in tumours are moderate and variable. Moreover, in vivo data have shown regression of tumours following intratumoural delivery of a cytosine deaminase suicide gene [6].
Nanovectors, both viral and non-viral, as vehicles for intracellular delivery are well reported in literature both for xenobiotics and biopharmaceuticals. Commonly evaluated non-viral vectors including liposomes [7, 8], lipoplexes [9, 10], polymeric nanoparticles (NPs) [11, 12], polyplexes [13, 14], cationic polysaccharides (chitosan derivatives) [15, 16], dextran–spermine [17, 18]), acrylates [19, 20], dendrimers [21–23] and polymeric micelles [24] are well reviewed. Inorganic nanovectors present immense opportunity for the delivery of nucleic acids because of their many specific advantages like resistance to microbial attack, low toxicity and good storage stability. Moreover, they are amenable to easy surface functionalization for targeted delivery because of their advanced chemistry. Multiple functionalizations are also feasible because of their unique optical, electrical and physical properties, and they may provide a solution for many physical barriers of the cell that limit biomedical applications [25]. Although extremely promising, reviews on inorganic nanovectors are limited. M. Epple et al. [26] have reviewed the application of inorganic nanovectors for intracellular delivery of nucleic acids while another review discusses specifically the use of various inorganic nanovectors in cancer imaging and therapy [27]. In the present review, we intend to present the state-of-the-art inorganic nanovectors for nucleic acid delivery. The review would cover the need for intracellular delivery of nucleic acids, barriers to intracellular delivery, strategies to achieve the same, specific properties and types of inorganic nanovectors, their methods of preparation and applications as therapeutic, diagnostic and theranostic agents.
Need for intracellular delivery
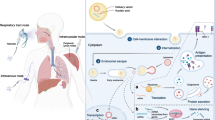
Nucleic acids include biopharmaceuticals that may act intracellularly in the cytoplasm (siRNA, mRNA, oligonucleotides) or cell nucleus (plasmid DNA (pDNA), ribozymes, DNAzymes) [28]. The various sites of action and mechanism of action are schematically depicted in Fig. 1. Nucleic acids can therefore elicit their action only when delivered intracellularly. The mechanism of action of nucleic acids is described in detail in [2, 30–42].
Mechanism of gene silencing by various nucleic acids used in therapy [29]
Nanovectors—overcoming barriers to intracellular delivery of nucleic acids
Nucleic acids are hydrophilic, negatively charged, high molecular weight compounds. Following intravenous (i.v.) administration, nucleic acids have to traverse through the plasma, across the capillary endothelium to the tissues to finally enter the cells. During the course, they are prone to rapid degradation by nucleases in vivo [43]. Nanovectors have been extensively researched for their potential to deliver nucleic acids intracellularly. Physicochemical properties of these nanovectors profoundly influence their capability to deliver nucleic acids into the cell and their subsequent transfection. Properties like size, charge and stealth need to be considered during developing these nanovectors [44, 45]. The various barriers to their delivery at the site of action are succinctly depicted in Fig. 2 and discussed below.
Barriers for nucleic acid delivery [29]
Serum as a barrier
Serum presents the first barrier following nucleic acid administration (i.v.). The nucleic acid may undergo degradation due to extreme pH, proteases and nucleases, the immune defence and scavenger systems [46]. The surface charge of the vector can greatly influence its stability in serum. In vitro, a positive surface charge can facilitate its binding to the negatively charged cell membranes and thereby induce cell uptake. In case of in vivo applications, a positive surface charge can attract the negatively charged serum proteins such as serum albumin, lipoproteins or IgG proteins, which may inhibit cell uptake. Zelphati et al. [47] showed that non-specific interactions between cationic lipids and serum proteins may cause neutralisation of the positive charges resulting in reduced interaction with the cell membrane and/or an increase in size leading to reduced internalization efficiency. Neutralized complexes may undergo aggregation resulting in lung embolism [48]. Moreover, nanoparticles that are smaller than the renal filtration cutoff of 50 kDa or 5–6 nm are rapidly cleared from the bloodstream by way of renal excretion [49]. Studies reveal that opsonisation of nanovectors can be limited by including cholesterol, or ‘stealth molecules’ such as gangliosides or polyethylene glycol (PEG). PEG-modified phospholipids increased liposome stability in blood and reduced rapid clearance, thereby improving the biodistribution profile. This can also inhibit scavenging by macrophages and greatly improve in vitro colloidal stability [50, 51]. Polyplexes based on polyethyleneimine (PEI) and poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA), respectively, were covalently coated with PEG, resulting in evasion of aggregation with blood components due to surface charge shielding. Upon i.v. administration, these complexes displayed enhanced circulation times and increased tumour transfection [52, 53].
Vascular endothelial barrier
Large molecules like nucleic acids cannot readily pass across the capillary endothelium to enter the extracellular fluid that bathes tissue cells [54]. Transcytosis facilitated by plasmalemmal vesicles or transport through pores or clefts interspersed in the capillary endothelium represent two transport pathways across the endothelium [55].
The cell membrane barrier
The cell membrane is a phospholipid bilayer membrane that is hydrophobic and is negatively charged primarily due to the carboxyl group of sialic acids of mucopolysaccharides (N-acetylneuraminic acid, N-glycolylneuraminic acid and others) and also due to presence of glycoprotein-based structures like heparin, heparin sulphate and others. The hydrophilicity and negative charge of the nucleic acid molecules prevents interaction with the hydrophobic and negatively charged cell membrane. This hinders their subsequent internalization into the cell [56–58]. Although physical approaches, such as the hydrodynamics method, gene gun, electroporation, sonoporation and laser irradiation, have been attempted for naked nucleic acid delivery, clinical applicability of these methods is severely restricted by the vulnerability of naked DNA to nuclease degradation in systemic circulation [59]. Cellular attachment of the nanovector can be mediated by non-specific hydrophobic or electrostatic interactions, or specific recognition by membrane anchored receptor proteins eventually resulting in transmembrane signalling, activation of the endocytic machinery and finally endocytosis of the nanovectors [28]. To promote cellular entry, polyplexes and lipoplexes are formulated by combining cationic polymers or lipids, respectively, with anionic nucleic acids that have an overall positive charge. These nanovectors interact with anionic cell membrane, via electrostatic interactions, which subsequently trigger internalization via endocytosis [60].
Barriers within the cell
Internalization into the cell can occur through receptor-mediated endocytosis, and nanovectors can modulate the entry of nucleic acids either to the endolysosomal pathway or into the cytoplasm [61]. Clathrin-mediated endocytosis tethers to the lysosomal compartment leading to degradation while caveolae lead to cytoplasmic delivery thereby enabling efficient transfection in case of mRNA, siRNA, oligonucleotides etc. [62]. Following internalization, two crucial events encompass membrane destabilization for release of nucleic acid into the cytoplasm and dissociation of the nucleic acid from the nanovector [63]. Positively charged polymers (PEI or PAMAM (poly(amidoamine)) dendrimers) can enable endosomal release of nucleic acids by the ‘proton sponge effect mechanism’ [64]. Polymers like PEI, PDMAEMA, PAMAM dendrimers, poly(amidoamine)s and histidylated polylysine that have a buffering capacity at pH 5 possess the intrinsic ability to destabilize endosomes. This activity can be augmented by the addition of endosome destabilizing compounds like chloroquine, poly-l-lysine (PLL), lipids (dioleoylphosphatidylethanolamine) or fusogenic peptides derived from viruses and artificial amphipathic peptides [51, 53, 65]. Despite escape from endolysosomal pathway, cytoplasmic nucleases, cytosolic viscosity and dense organelles can also impede their movement towards target sites [66]. Diffusion of DNA fragments >250 bp is markedly diminished in the cytosol giving rise to enhanced residence time of DNA in the cytoplasm which can be deleterious as the DNA would be subjected to degradation by cytoplasmic nucleases. Diffusion into the nucleus would also be hindered. The decreased mobility of DNA is ascribed to molecular crowding effects, as small solutes diffuse relatively freely in the cytosol [44]. Polyethyleneimine (PEI)/DNA nanocomplexes have been shown to be actively transported by motor proteins along microtubules within the cytoplasm to enter the nucleus [67]. To elicit effect following entry into the nucleus, by passing nuclear membranes, the nucleic acid must be released for further transcription and translation [63, 68]. Nucleocytoplasmic transport occurs via the nuclear pore complexes (NPCs) which form channels in the nuclear envelope with a diameter of ∼40 nm [69, 70]. The trafficking of pDNA into the nucleus can be limited by the nuclear envelope due to the slowly or non-dividing nature of most target cells. Nuclear localization signals (NLS sequences) primarily contain basic amino acids and can be utilized for import of DNA into the nucleus. Proteins like yeast GAL4 amino terminal domain that contain the NLS are known to form stable complexes with cytosolic factors called karyopherins a and b, which are docked at the NPC and then translocated to the nucleus [71]. A number of proteins of viral origin especially those that contain highly basic sequences, rich in arginines, show good DNA binding as well as act as a good guide to the nucleus [72]. pH-sensitive liposomes containing bovine serum albumin were successfully delivered to the cytosol and subsequently to the nucleus after chemically conjugating the NLS of SV40 large T antigen to the liposomes [73].
Targeted delivery of nucleic acids to specific sites can be achieved through use of appropriate ligands in the nanovectors. Upon i.v. injection of a PEGylated form of branched PEI with an RGD (arginylglycylaspartic acid) peptide of siRNA into tumour-bearing mice, increased siRNA uptake in the tumour was observed while there was decreased uptake in the lung and the liver compared to the ‘naked’ polyplex. siRNA induced sequence-specific inhibition of vascular endothelial growth factor (VEGF) in the tumour, resulting in the inhibition of both tumour angiogenesis and growth [74].
Nanovectors for intracellular delivery of nucleic acids
Currently, the emphasis is on nanotechnology (size range 1–100 nm) because it offers advantages like improvements in target-to-non-target concentration ratios, increased drug residence at the target site and improved cellular uptake and intracellular stability [75]. Nanovectors for nucleic acid delivery must efficiently protect the genetic materials from premature degradation in the systemic blood stream and transfer the therapeutic genes to target cells. Broadly, these nanovectors can be classified as viral (use of recombinant viruses) and non-viral vectors.
Viral vectors
Viral vectors like retrovirus [76], adenovirus [77], adeno-associated virus [78] and herpes simplex virus [79] are very efficient transfection vectors. The presence of proteins on the outer surface of viruses enables docking on specific proteins in the cell membrane thereby facilitating cellular internalization and hence higher transfection levels as compared to the present generation of non-viral system. This way viral vector enters directly in the cytosol or is present in endosomal vesicles. In case of cellular entry by receptor-mediated endocytosis, viruses have the capability to destabilize the endosomal membrane upon acidification of this vesicle [53]. Although these vectors are efficient transfection agents, their utilization needs to be carefully considered. Direct injection of an adenovirus vector into the hepatic artery resulted in death of a patient in 1999. Recombinant viral vectors have shown significant problems, such as short-term transgene expression, an inability to persist in host cells, immunogenicity and toxicity [80]. Three clinical trial participants developed leukaemia-like complications post-retroviral-based gene therapy [19]. Other drawbacks of viral vectors include limitations in the size of inserted DNA and difficult large-scale pharmaceutical grade production. For details on viral vectors for gene delivery, readers are directed to [81–87].
Non-viral vectors
The earliest non-viral vectors to be investigated were the cationic lipids followed by many cationic polymers of amino acids, carbohydrates, dendrimers and, more recently, nanovectors [88]. Cationic polymer and lipid molecules can neutralize the negative charges of nucleic acids to form condensed electrostatic complex, called polyplexes and lipoplexes, respectively. Cationic lipids consist of three structural domains: a cationic head group, a hydrophobic part and a linker between the two domains. Some examples of commercially available lipid reagents include N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethyl-ammonium chloride [89–93], 2,3-dioleyloxy-N-[2(sperminecarboxamido) ethyl]-N,N-dimethyl-1-propanaminium trifluoroacetate [94–97], 1,2-dioleoyl-3-trimethylammoniumpropane [9, 47, 98–102] and dioctadecylamidoglycylspermine [103–106]. Cationic polymers explored for gene delivery include PEI (high transfection efficiency, high cytotoxicity) [107–111], PLL (PLL polyplexes rapidly bind to plasma proteins and cleared from circulation) [13, 14, 112, 113], cationic polysaccharides (chitosan derivatives [15, 16, 21], dextran–spermine [17, 18]) and acrylates [19, 20]. The cationic siRNA–poly(dl-lactide-co-glycolide/LPEI micelles have been shown to have superior intracellular uptake and enhanced gene silencing effect, compared to naked siRNA/LPEI complexes [24]. Dendrimers for gene delivery include the PAMAM [21–23] and PPI dendrimers [114, 115] or the dendritic polylysine [116]. The integration of viruses or viral peptides (to mimic viruses) into traditional cationic-amphiphile-based carriers gives rise to hybrid vectors. For example, the adenovirus hexon protein enhances the nuclear delivery and increases the transgene expression of polyethyleneimine–pDNA vectors. Such hybrid vectors can also be found in [117–119]. Amphiphilic alpha-helical peptides, containing cationic amino acids and polymeric nanoparticles, are also being utilized for nucleic acid delivery [120]. Table 1 compares viral and non-viral vectors.
Amongst the non-viral vectors, more recently, there is a tremendous focus on inorganic nanovectors on which there are limited reviews available. Inorganic nanovectors like carbon nanotubes (CNT), noble metals, calcium phosphate (CaP), quantum dots (QDs), magnetic, manganese phosphate, layered double hydroxides (LDHs) (anionic clay) etc. show good promise for nucleic acid delivery and imaging (theranostics) because of their characteristics like resistance to microbial attack, low toxicity and good storage stability. These inorganic nanovectors and their application in nucleic acid are discussed in detail in the sections below.
Inorganic nanovectors—how are they different?
Inorganic nanomaterials for intracellular delivery of nucleic acid comprise of carbon nanotubes, noble metals, calcium phosphate, quantum dots, magnetic, manganese phosphate, layered double hydroxide (anionic clay) etc. Unique optical, electrical, magnetic and/or electrochemical properties, microbial resistance and good storage stability and favourable physicochemical properties like compatible sizes and shape represent special features of inorganic nanomaterials. Capability of conjugating with molecular targeting ligands and ease of integrating multiple functions have evoked their widespread applications in biomedical fields including therapeutic and diagnostic applications. Figure 3 depicts the various inorganic nanovectors used for intracellular delivery of nucleic acid. Cellular uptake of inorganic nanovectors follows the same mechanism like other nanovectors. Figure 4 portrays the cellular uptake pathways in healthy and tumour tissues.
Preparation techniques
Methods of synthesis of nanovectors must enable manipulation of composition, shape, size, drug loading and stability. Furthermore, functionalization should be readily facilitated using simplistic approaches.
Carbon nanotubes
CNT consist of a rolled sheet of graphite terminated by two end caps similar to a half C60. Such CNT could be single-walled (SWNT) or multi-walled (MWNT). SW generally comprise monolayer graphene sheets while MW possess several concentric layers [123]. They can easily pass through plasma membrane and enter the cytoplasm to deliver the nucleic acids. This direct translocation process is termed as ‘nano-needle’ mechanism. Depending on nature of the functional groups covalently linked or non-covalently complexed to the CNTs, CNTs follow either the nano-needle mechanism or an energy dependent endocytic pathway [124]. They possess high aspect ratio, ultralight weight, high mechanical strength, high electrical conductivity and high thermal conductivity which make them very viable for biomedical applications [125]. CNTs can be primarily produced by three major techniques: electric arc discharge (EAD), laser ablation and thermal or plasma-enhanced chemical vapour deposition (CVD) [126]. EAD system generates a plasmon across carbon electrodes. This brings about deposition of CNTs on a substrate. The anode constructed from pure graphite results in MWNTs as the main product, whereas the incorporation of nanometer-sized metal catalysts in the anode material gives rise to SWNTs [127]. Laser ablation involves generation of atomic carbon at T > 3,000 °C through laser irradiation of graphite, with appropriate catalyst particles (Fe, Co or Ni) to form nanotubes [128]. CVD process employs a mixture of hydrocarbon gas, acetylene, methane or ethylene and nitrogen which is introduced into the reaction chamber. Decomposition of the hydrocarbon at temperatures 700–900 °C and atmospheric pressure occurs leading to the formation of nanotubes on the substrate on which catalyst particles are deposited [129]. CNT display large surface area that can be easily functionalized and loaded with nucleic acids and scaled up for industrial production [130].
Silica nanoparticles
Silica nanoparticles have well-defined silanol groups at the surface which offer many possibilities of modification for site specific gene delivery [131]. The traditional approach for design of silica nanoparticles is the sol–gel route. This technique involves one synthetic step wherein a solution is prepared containing the material whose nanoparticles are to be prepared, dispersants which control particle/crystal growth rates, surfactants, mixed metal oxide and metallic compositions and the source for addition of functional group. Generally crystal growth which influences the particle size is restricted by controlling the temperature and pH during preparation. Hence, this is a suitable route for laboratory-scale preparations. Yet another class of theranostics includes the mesoporous silica nanoparticles (MSNs) that possess unique features, such as tunable porosity, high surface area, large pore volume, facile functionalization, good biocompatibility, high physicochemical and biochemical stability etc. [132]. In case of mesoporous silica nanoparticles, an organic template like amphiphilic surfactant within a sol–gel synthesis of silica or other metal oxide particles is often used for synthesis. A mild interaction between the template and the hydrolysing and condensing silica species gives rise to an ordered organic micellar template supported by an amorphous silica wall. The mesoscale pores may be revealed thereafter by thermal calcination or solvent extraction of the template [133].
Quantum dots
QDs are semiconductor NPs comprising elements from the periodic groups II–VI or III–V (e.g. CdS, CdSe, ZnS, ZnSe, ZnO, GaAs, InAs; sometimes in a core–shell structure), with a size range of 2–10 nm in diameter. This limits the mobility of charge carriers (electrons and holes) within the nanoscale dimensions, and this quantum confinement confers QDs with unique optical and electronic properties. QDs portray an array of favourable features like broad absorption spectra that can lead to Stokes’ shifts in excess of 100 nm; lifetimes longer than 10 ns; very large molar extinction coefficients in the order of 0.5–5.0 × 106 M−1 cm−1 and, often, increased stability to photobleaching than many fluorophores [134, 135]. QDs are usually synthesized in different media, including aqueous solution, high-temperature organic solvents and solid substrates. Monodisperse QDs are usually made from cadmium sulphide, cadmium selenide (CdSe) or cadmium telluride (CdTe), of which CdSe/ZnS is most commonly used for biological applications [136]. QDs are made hydrophilic by silanization or surface exchange with bifunctional molecule. The bifunctional molecule has a hydrophobic side (e.g. a thiol group) and a hydrophilic side (e.g. a carboxyl group –COOH). The hydrophobic side binds to the ZnS layer of the QD. Another strategy involves encapsulation of QDs within phospholipid micelles, polymer beads or shells, amphiphilic polysaccharides or block–copolymer micelles [137]. Their aggregation can be prevented by use of suitable capping agents which can also be functionalized.
Calcium phosphate nanoparticles
CaP is a naturally occurring inorganic material present in teeth and bones. Hence, nanoparticles of CaP are non-toxic. CaP precipitates can bind and encapsulate polyanions/nucleic acids probably due to interaction between the calcium ions and the negatively charged phosphate groups in the backbone of the nucleic acids by an easy and inexpensive way. Such interactions provide enhanced protection to nucleic acids from enzymes to enable intracellular delivery. However, there may be uncontrollable rapid growth of calcium phosphate crystal after preparation, resulting in the formation of large agglomerates (>mm) that can drastically decrease transfection efficiency [138, 139]. Various methods used for the synthesis of these nanoparticles include wet chemical routes, solid-state reactions and hydrothermal reactions at elevated temperature, biosynthetic routes and microemulsions [140]. The CaP nanoparticles can be prepared by rapid mixing of an aqueous solution of calcium nitrate (pH = 9) with an aqueous solution of diammonium hydrogen phosphate (pH = 9). This is followed by mixing the CaP dispersion with an aqueous solution of oligonucleotides (CpG or polyinosinic–polycytidylic acid) and the addition of 100 μL of the model-antigen hemagglutinin (HA) (1 mg mL−1) [141]. Another approach is microemulsion-based. Microemulsions are produced spontaneously without the need for significant mechanical agitation, thereby making it a simple technique useful for large-scale production of nanoparticles. Microemulsions have been used for synthesis of variety of monodispersed nanomaterials such as metallic and bimetallic nanoparticles, single metal oxide as well as mixed and doped metal oxides, quantum dots and even complex ceramic materials [142–146]. Nanocatalyst prepared by w/o microemulsions has displayed better performance (activity, selectivity) than those prepared by other methods [147]. One such method utilizes sodium bis(ethylhexyl)sulphosuccinate (AOT) in hexane solution containing CaCl2 aqueous solution as microemulsion A. Microemulsion B consists of AOT dispersed in hexane with aqueous solution of Na2HPO4 containing pDNA. Mixing of the two microemulsions by continuous stirring results in generation of nanoparticles that are subsequently washed with hexane, redispersed in double-distilled water by sonication, dialyzed and stored at −4 °C for further use [148]. In order to address the issue of aggregation, many schemes have been worked upon. CaP nanoparticles with a pre-coating agent, an anionic phospholipid, dioleoylphosphatidic acid (DOPA) have been utilized for siRNA delivery that resulted in prevention of aggregation during the centrifugal separation with very small (25–30 nm) and hollow structured nanoparticles [149]. CaP-NPs are relatively insoluble at physiological pH (7.4), and its solubility increases below pH 6. Dissolution of CaP-NPs is facilitated by pH changes following cellular uptake to release the encapsulated agents [148].
Gold nanoparticles
Gold nanoparticles have controllable optical properties termed surface plasmon resonance. They have received much attention in nucleic acid delivery because of their attractive features like straightforward synthesis, easy modification of surfaces with thiolated molecules and biocompatibility with cells or tissues [150]. The ‘top down’ and ‘bottom up’ are two widely used procedures for preparation of gold nanoparticles (AuNPs). In case of the ‘top down’ preparation method, Au bulk is broken down by a strong attack force, for example, ion irradiation in air or arc discharge in water to generate AuNPs. These are usually used in suspensions and stabilized by addition of suitable surfactants. The ‘bottom up’ method involves wet chemical process like chemical reduction of Au salts (most common being aqueous reduction of gold salt by sodium citrate at reflux), electrochemical pathways and decomposition of organometallic compounds. Nanoparticles of various sizes and shapes can be prepared by the chemical reduction method which is simple and controllable [151–153]. Spherical AuNPs of narrow size distribution in a broad size range from 6 up to 200 nm can be obtained by reduction methods. Alternative reducing agents like sodium borohydride or carrying the synthesis out in organic solvents also can be employed in order to obtain smaller NPs [154]. Other reducing agents used include formaldehyde, ethanol, white phosphorus, sodium citrate (for an average particle diameter of 20 nm), ascorbic acid (12 nm), ethylenediaminetetraacetic acid (EDTA) (20 nm), sodium citrate in the presence of tannin (5 nm), borohydride in a mixture with sodium citrate or EDTA and cyanoborohydride [155]. In the one-pot protocol synthesis developed by Brust et al. [156], AuCl4 − salts are reduced with NaBH4 in the presence of the desired thiol capping ligand or ligands, wherein varying the thiol–gold stoichiometry can give rise to range of core size of the particles from 1.5 to ∼6 nm. Alternative methods used to synthesize AuNPs comprise of physical reduction (hollow Au nanostructures in large-scale), photochemical reduction (cubic AuNPs), biological reduction (molecular hydrogels of peptide amphiphiles for producing various shapes of AuNPs) and solvent evaporation techniques (2D Au super lattices) [157]. [158, 159] have proposed that non-spherical AuNPs of varying shapes can be obtained from the anisotropic growth of the nuclei or seed particles, primarily facilitated by addition of surfactants like cetyltrimethylammonium bromide, sodium dodecyl sulphonate and poly(vinylpyrrolidone). Song et al. [160] synthesized stable gold nanoparticles by treating an aqueous HAuCl4 solution using the plant leaf extracts of Magnolia kobus and Diopyros kaki as reducing agents. Synthesis of gold nanoparticles, with a particle size of 5 to 15 nm, using Zingiber officinale extract which acts both as reducing and stabilizing agent and which portrayed good blood biocompatibility and physiological stability compared to the citrate capped nanoparticles is reported. Biosynthesis of gold nanoparticles by plants such as lemongrass, Aloe vera, alfalfa, neem, tamarind, Cinnamomum camphora, Emblica officianalis, Mangifera indica leaf, Hibiscus rosa-sinensis, Murraya koenigii leaf and Ocimum sanctum is also reported in literature [161, 162]. A green procedure to synthesize gold nanoparticles in aqueous solution using sodium citrate dihydrate as reducing agent and stabilizer is reported [163]. Biological systems employing microorganisms like fungus Penicillium rugulosum [164], Sclerotium rolfsii [165], Verticillium sp. and Fusarium oxysporum; extremophilic actinomycete Thermomonospora sp. [166–168]; metal-reducing bacteria Shewanella oneidensis [169] and Shewanella algae [170]; marine alga Sargassum wightii [171]; alga Tetraselmis kochinensis [172]; thermophilic bacterium Geobacillus stearothermophilus [173]; yeast Yarrowia lipolytica [174] among others for synthesis of gold nanoparticles are also reported.
Magnetic nanoparticles
This class of NPs includes metallic (Fe, Pt, Co, Ni), bimetallic (FePt, FeCo, FeNi) and superparamagnetic iron oxide nanoparticles (SPIONs). Colloidal iron oxide nanoparticles, such as SPION and ultra-SPION, are the most studied magnetic nanoparticles (MNPs) for biomedical applications due to their biocompatibility. They normally comprise of magnetite (Fe3O4) or maghemite (γFe2O3) and are ease to synthesize. These SPIONs can be fabricated by either top-down (mechanical attrition) or bottom-up (chemical synthesis) approaches. The solution chemical methods comprise of standard iron chloride co-precipitation, co-precipitation in constrained environments, thermal decomposition and/or reduction, hydrothermal synthesis and polyol synthesis [175]. The co-precipitation method involves the simultaneous precipitation of Fe2+ and Fe3+ ions in basic aqueous media. The commercially available T2 contrast agents are prepared by this method. During or after preparation, nanoparticles are coated with hydrophilic polymers like dextran, PEG, chitosan, poly(vinyl alcohol) and their derivatives amongst others to ensure physiological stability [176, 177]. Recently employed is the high-temperature decomposition method. Controlled synthesis (size, shape, composition) is achieved by decomposition of organometallic precursors hosting the desired element, in the presence of surfactants (oleic acid, lauric acid, alkane sulphonic acids and alkane phosphonic acids), in organic phase (toluene, dichlorobenzene) and under inert atmosphere conditions. Size can be controlled by varying reaction temperature or by changing metal precursor. The use of oleic acid may give rise to a hydrophobic surface which may be rendered water soluble by synthesizing nanoparticles in organic media through surface surfactant exchange or use of amphiphilic polymer [178]. Magnetite nanoparticles can be surface coated, dispersed into suitable solvents to form homogenous suspensions called ferrofluids. With application of external magnetic field, these ferrofluids can be positioned to specific areas thereby enabling magnetic resonance imaging (MRI) for medical diagnosis and AC magnetic field-assisted cancer therapy [179, 180]. The use of Fe3O4 MNPs is sometimes questionable due to their potential cytotoxicity arising from the release of Fe3+ into the cellular environment. Coating iron oxide nanoparticles with surface modifiers, such as 2,3-dimercaptosuccinic acid (DMSA), can substantially overcome this cytotoxicity [181]. Table 2 lists the methods for synthesis of SPIONS.
Layered double hydroxide nanoparticles
LDHs are a family of anionic clay materials, exemplified by the natural mineral hydrotalcite [Mg6Al2(OH)16CO3·4H2O]. Most LDH minerals can be described using the general formula [MII n MIII(OH)2 + 2n ]+(Am−)1/m × H2O (n = 2–4), where MII represents a divalent metal cation, MIII a trivalent metal cation and Am− an anion. Structurally, cationic brucite-like layers ([MII n MIII(OH)2 + 2n ]+) are bound together by the interlayer counter-anions as well as water molecules [(An−) x/n mH2O] [201]. The most widely adopted method of synthesis is direct synthesis that involves nucleating and growing the metal hydroxide layer by mixing an aqueous solution containing a mixture of two metal salts (MII+/MIII+) and a base mostly sodium hydroxide. Variations of the method include titration at constant or varied pH and buffered precipitation [202]. Other methods of synthesis reported are urea method; induced hydrolysis; reconstruction; sol–gel technique; hydrothermal, microwave and ultrasound treatments and anion-exchange reactions [203]. LDH materials, especially MgAl/LDH, are biocompatible; show high loading of anionic/polar drugs, pH-controlled release of cargo, protection of drugs in the interlayer and finely tunable particle size; display low-cost preparation, less toxic to mammalian cells and can be quickly absorbed by various cell lines based on the conjugation of specific antibody and biodegraded in the cytoplasm [204, 205]. LDHs with their ability to exchange their interlayer anions can intercalate bioactive molecules such as nucleic acids with high loading capacity [206, 207]. They primarily enter cells via the most common energy-dependent endocytosis, clathrin-mediated endocytic pathway [208].
Applications
The myriad applications of inorganic nanovectors in nucleic acid therapeutics are detailed below. Table 3 summarizes the therapeutic applications of inorganic nanovectors in nucleic acid delivery.
Carbon nanotubes
Various functionalized CNTs have been fabricated for the purpose of nucleic acid delivery [240]. CNTs display strong optical absorbance in the NIR region and hence used for photothermal therapy [241]. CNTs functionalised with either a fluorescent group (fluorescein isothiocyanate) or a fluorescent peptide could move across a cell membrane and showed nuclear localization. Plasmid DNA gene delivery into mammalian HeLa cells revealed 10-fold higher uptake via the similarly-functionalised CNT vector than that without using CNTs [242, 243]. Varkouhi et al. [130] have shown two cationically functionalized CNTs (CNT-PEI and CNT-pyridinium) for siRNA delivery, and they achieved 10–30 % silencing activity. Podesta et al. [244] demonstrated greater tumour inhibition from intratumoural administration of functionalized MWNTs (MWNT-NH3+) of a proprietary toxic siRNA sequence (siTOX) as compared to that of liposome of siTOX. This may be attributed to better translocation of CNTs across plasma membrane as compared to that of liposomes. The ‘CNT spearing’ involves the transportation of nickel (Ni)-embedded MWCNTs containing DNA plasmids, including a green fluorescent protein (GFP) sequence into the cell membranes which is driven by an external magnetic field. This technique resulted in higher transduction efficiency and higher viability after transduction in Bal17 B lymphoma, ex vivo B cells and primary neurons [245]. In hypoxic stress conditions, tumour cells including pancreatic cancer cells overexpress the hypoxia inducible factor 1 alpha (HIF-1α) transcription factor, which regulates the transcription of many anti-apoptotic genes giving rise to therapy resistance. SWCNT-HIF-1α siRNA complexes could significantly inhibit the activity of tumour HIF-1α in mice-bearing pancreatic tumour cells [246].
Silica nanoparticles
Silica nanoparticles display favourable properties for gene delivery like biocompatibility, low toxicity, easy preparation and low polydispersity. Ultrafine silica NPs, functionalized with amino groups, can effectively bind and protect plasmid DNA from enzymatic digestion and enable cell transfection in vitro [247]. MSN coupled with mannosylated polyethyleneimine enabled efficient transfection of plasmid DNA in vitro to macrophages via mannose receptor-mediated endocytosis [248]. Amino-functionalized MSNs containing large cage-like pores, with an inner diameter of 20 nm and a particle size of 70–300 nm, allowed successful adsorption of about 150 μg g−1 MSN of firefly luciferase plasmid DNA (5,256 bp) from phosphate-buffered saline due to attractive interaction of the surface amino groups with the negatively charged DNA and thereby protected it against enzymatic degradation [249]. Amino silica nanoparticles (NH2SiNPs) could greatly improve the inhibition efficiency of anti-ODNs for the proliferation and survivin expression in HeLa cells and A549 cells. The NH2SiNPs showed greater biocompatibility and were virtually non-cytotoxic at the concentrations required for efficient transfection as compared with liposomes [250]. Bharali et al. [251] reported that amino-surface-functionalized SiNPs could be efficiently used for gene delivery. When SiNPs, plasmid DNA-binding SiNPs and free plasmid were injected into mouse brains, only plasmid DNA-binding SiNPs produced robust gene expression, implying that the gene was very well protected and transferred into the cell nuclei.
Quantum dots
Their favourable optical properties (highly efficient fluorescence owing to quantum confinement effects and a good resistance towards photobleaching) make them useful for biomedical imaging. Apart from imaging, they have also been utilized for transfection. A PEGylated QD core as a carrier, conjugated with siRNA and tumour-homing peptides (F3) as functional groups on the QDs surface, enabled efficient delivery of these F3/siRNA-QDs to HeLa cells and QDs fluorescence facilitated monitoring of siRNA release from endosomes. Moreover, siRNA attached to the particle by disulphide cross-linkers showed greater silencing efficiency than when attached by a nonreducible thioether linkage [252]. Positively charged PEI was covalently conjugated to the surface of QDs and complexed with cyanine dye labelled vascular endothelial growth factor siRNA (cy5-VEGF siRNA), and Flourescence resonance energy transfer (FRET) was achieved between cy5-VEGF siRNA and PEI-conjugated QDs (QD625) which established that PEI-conjugated QDs are efficient siRNA carrier and can be used to analyze intracellular trafficking and unpacking pathway as well as to effectively silence a target gene [253]. The colloidal and chemical properties of QDs can be refined for siRNA delivery, ensuing highly effective and safe RNA interference and fluorescence contrast. This is accomplished by converting the carboxylic acid groups on a QD into tertiary amines thereby effecting endosomal release of the siRNA through the proton sponge effect. Upon linking these two functional groups to the surface of nanoparticles, steric and electrostatic interactions arise that respond to the acidic organelles, necessary for siRNA binding and cellular entry. This not only enhances gene silencing activity 10–20-fold but also reduces cellular toxicity 5–6-fold. Moreover, QDs are innate dual-modality optical and electron microscopy probes thereby making possible real-time tracking and ultrastructural localization of QDs during transfection [254]. QD complexed with MMP-9-siRNA (nanoplex) could downregulate the expression of MMP-9 gene in brain microvascular endothelial cells that constitute the BBB reflected by the upregulation of extracellular matrix proteins like collagens I, IV and V and a decrease in endothelial permeability thereby maintaining the integrity of BBB in neurological diseases such as HIV-1, AIDS dementia and cerebral ischemia [255]. Phospholipid-coated, CdSe/ZnS core/shell QDs conjugated to plasmid DNA via a peptide nucleic acid–N-succinimidyl-3-(2-pyridylthio) propionate linker displayed enhanced expression of green fluorescent protein in the presence of Lipofectamine 2000 that could be effectively monitored by fluorescence microscopy, and the cellular uptake of QD—DNA conjugates was monitored in real-time at the same time. There was 62 % enhancement in transfection efficiency than unconjugated plasmid DNA [256].
Calcium phosphate nanoparticles
Nanoparticles of calcium phosphate encapsulating pDNA of size 100–120 nm portrayed higher transfection efficiency than that of the commercial transfecting reagent Polyfect in HeLa cell lines using pSVβgal as a marker plasmid [257]. Biodegradable calcium phosphate nanoparticles as carriers for the immunoactive toll-like receptor ligands CpG and polyinosinic–polycytidylic acid for the activation of dendritic cells combined with the viral antigen HA was able to induce an antigen specific T cell response in vitro [141]. Sokolova et al. [258] developed stable CaP NPs of 100–200 nm in size by rapid precipitation followed by immediate adsorption of DNA or siRNA, which could transfect cells with a GFP plasmid or silenced the expression of GFP in the treated cells. Poly(ethylene glycol)-b-poly(2-methacryloyloxyethyl phosphate)/CaP/pDNA nanoparticles are known to have great potential as gene delivery carriers due to their high transfection efficiency, low toxicity and good stability under physiological conditions [259]. Nouri et al. [260] have shown that CaP-SBF/DNA complexes that were prepared in simulated body fluid displayed higher transfection efficiency and showed reduced aggregation tendency of CaP particles over time as compared to the nanoparticles prepared in water. The CaP/DNA complex precipitates enter cells through endocytosis by forming an intracellular vesicle. The vesicles merge with lysosomes with subsequent release of the CaP/DNA particles into the cytoplasm. The DNA molecules ultimately enter the nuclei and facilitate gene transfer and expression [261].
Gold nanoparticles
Gold nanoparticles are known to be noncytotoxic, nonimmunogenic and biocompatible. Intramuscular gold therapy in human trials revealed marked clinical improvements in rheumatoid arthritis patients [262, 263]. Thiol-modified antisense oligonucleotides when directly conjugated onto gold nanoparticles were able to regulate protein expression in cells [264]. Nanovector was developed based on dendrimer-entrapped gold nanoparticles (Au DENPs) that showed much greater gene transfection efficiency than that of dendrimers without AuNPs entrapped [265]. Polyvalent AuNPs of siRNAs and oligoethylene glycol, markedly enhanced the stability of bound siRNAs, could release the siRNAs in a controlled manner and exhibited extended gene knockdown compared with lipofectamine transfected siRNAs [266]. Thiolated double-stranded (ds) DNA fragments (enhanced green fluorescence protein, EGFP) directly conjugated to AuNPs using ligase-dependent strategy could be digested by restriction enzymes and expressed as functional proteins inside mammalian cells, demonstrating retained activity of the dsDNA conjugated to AuNPs [267]. Kim et al. [268] have revealed functionalized AuNP as a universal carrier for the delivery of DNA oligonucleotides (oligos) into the nucleus which when injected into a xenograft tumour in a mouse model system resulted in inhibition of tumour development by redirecting the alternative splicing of the pre-mRNA. Plasmid DNA encoding for murine interleukin-2 (pVAXmIL-2) complexed with positively charged colloidal gold nanoparticles portrayed enhanced cellular delivery and transfection efficiency of pVAXmIL-2 into C2C12 cells as evident by higher murine IL-2 protein expression compared to the PEI vectors [269]. It is well established that gold nanorods exhibit strong and tunable surface plasmon absorption in the near infrared (NIR) range. Hence, Chen et al. [270] attached EGFP genes to the surface of gold nanorods by linking of thiolated EGFP DNA through Au–S bonds. When femtosecond NIR irradiation was applied to the gold nanorod–EGFP DNA conjugates, a change of shape from rod to sphere induced DNA release in HeLa cells (Fig. 5). Cationic gold nanoparticles prepared by NaBH4 reduction in the presence of 2-aminoethanethiol complexed with plasmid DNA containing a luciferase gene enabled delivery of the gene into the target HeLa cells in about 3 h [271]. Morais et al. [272] studied the biodistribution of AuNPs (∼20 nm) with different surface coatings: citrate, 11-mercaptoundecanoic acid and three pentapeptides, Cys-Ala-Leu-Asn-Asn, Cys-Ala-Leu-Asn-Asp and Cys-Ala-Leu-Asn-Ser, after i.v. administration to rats and concluded that peptide capping lead to considerable increase in hepatic uptake, thereby demonstrating the significance of AuNPs functionalization in biodistribution. Functionalized AuNP system for the delivery of AMOs (anti-miRNA oligonucleotides)-miR29 was developed that successfully blocked miRNAs, resulting in the upregulation of its target protein, myeloid cell leukemia-1, a factor responsible for promoting cell survival [273]. Functionalized AuNP has been used to deliver small, highly structured RNA aptamers specific to the β-catenin protein into the nucleus of human cells more efficiently than liposome-based delivery that resulted in nearly complete inhibition of β-catenin binding to the p50 subunit of NF-κB in the nucleus [274].
Confocal microscope images showing the expression of EGFP in HeLa cells after transfection with gold nanorod–EGFP DNA conjugates in combination with NIR irradiation. The left image is the bright-field image of cells (in white circles). The right-hand image shows EGFP expression of the same cells under the fluorescent mode of confocal microscopy [reprinted (adapted) with permission from [270] Copyright (2006) American Chemical Society]
Magnetic nanoparticles
Iron oxide nanoparticles (IONPs) for delivery of therapeutic DNAzyme for the treatment of hepatitis C was investigated, and it could efficiently induce knockdown of hepatitis C virus gene, NS3, that encodes helicase and protease which are essential for the virus replication [275]. In vitro studies based on COS-7 cells were carried out by Ang et al. [276] using pEGFP-N1 and pMIR-REPORT-complexed, PEI-coated iron oxide MNPs. pEGFP-N1 codes for GFP under the control of a cytomegalovirus (CMV) promoter and pMIR-REPORT contains a firefly luciferase reporter gene under the control of a CMV promoter. Their experiments reveal that the highest transfection efficiency was achieved in normal magnetic transfection mode due to clustering of the PEI AMNPs (aggregated MNPs) on the cells wherein mechanism of magnetic transfection is endocytosis rather than cell wounding. Intra-carotid administration of polyethyleneimine-modified iron oxide nanoparticles (GPEI) in conjunction with magnetic targeting resulted in 30-fold increase in brain tumour entrapment of GPEI compared to that seen with intravenous administration and holds great potential for gene delivery [277]. Chen et al. [278] conjugated a T cell-specific ligand, the CD3 single chain antibody (scAbCD3), to PEG-g-PEI stabilized IONPs for gene delivery to T cells for immunosuppression that led to 16-fold gene transfection enhancement in rat T lymphocyte HB8521 cells with low cytotoxicity, thereby effecting T-lymphocyte-targeted immunotherapy which was successfully imaged by MRI. Antisense RNA was covalently linked to cross-linked iron oxide NPs, and a cell penetrating peptide was used to facilitate transfection of therapeutic siRNAs to human colorectal carcinoma tumours in vivo and signifies the first targeted/siRNA MNP used for therapeutic application [279].
Layered double hydroxides
The encapsulation of mononucleotides and DNA into Mg–Al LDH by intercalation reaction ensured protection of mononucleotides and DNA and enabled their complete release confirming it to be a potential carrier for gene delivery [280]. Magnesium–aluminium LDH with a full gene and promoter encoding GFP intercalated between layers of LDH upon delivery to 9 L glioma cells, JEG3 choriocarcinoma placental cells and cardiac myocytes were able to internalize the LDH nanoparticles and express the gene with some cell lines having up to 90 % transfection efficiency [281]. Chung et al. [282] have studied the intracellular trafficking pathway of LDH nanoparticles in human cells and shown that it is size-dependent. They propose that most 100-nm nanoparticles could escape a typical endolysosomal degradation. However, nanoparticles of about 50 nm followed uptake by an endosome–lysosome pathway but were prone to exocytosis. LDH(R1)/DNA plasmid complex has been shown to be a promising approach for vaccination against tumour. Intradermal immunization in C57BL/6 mice induced an enhanced serum antibody response much greater than naked DNA vaccine. This was further demonstrated in B16-OVA melanoma tumour model, as evident by enhanced immune priming and protection from tumour challenge in vivo [149]. Wong et al. [283] have efficiently delivered siRNA to cortical neurons using LDH nanoparticles. siRNA–LDH complexes were shown to be internalized by clathrin-dependent endocytosis at the cell body and in neurites, and subsequent release into the cytoplasm enabled effective silencing of neuronal gene expression thereby displaying its potential for neurodegenerative disease.
Theranostic applications
The integration of inorganic nanovectors in diagnosis and therapeutics has resulted in their application as theranostics. While the nanovector enables accumulation at the target site, the special features of the inorganic nanovectors enables visualization and real-time monitoring. Inorganic nanovectors consisting of semiconductor QDs, magnetic nanoparticles and gold colloids are readily visualized by different imaging techniques that include fluorescence imaging, MR imaging, photoacoustic therapy, surface enhanced Raman spectroscopy, multiphoton luminescence imaging, X-ray computed tomography (CT) [284, 285]. The high photostability and brightness of QDs are their distinguishing features that allow long–term acquisition of photoluminescence emissions with a good signal-to–noise ratio. Moreover, they have broader range of emission spectra from UV to near infrared with larger absorption coefficients making them suitable contrast agents for fluorescence imaging modalities including both single- and multiphoton microscopy and for photoacoustic imaging [286, 287]. Iron oxide NPs are biocompatible, inexpensive and possess superior magnetic properties due to which they are suitable contrast probes for MRI [288]. Strong surface plasmon absorption, stability, biosafety and ease of modification have rendered AuNPs amenable to functionalization for both imaging and therapy applications.
Silica particles can be organically modified in order to self-assemble micelles wherein various biomolecules, detection agents, hydrophilic or hydrophobic dyes can be loaded into the core. For instance, when FRET was applied between ethidium monoazide and another dye, ethidium homodimer-1, inserted between the DNA and the surface of the silica NPs, fluorescence microscopy enabled not only visualization of the silica NPs entering into the cell but also uptake of DNA into nucleus as it detached from the silica micelle. Electrostatic attachment of DNA to the triethoxyvinylsilane on the surface of the silica NPs ensured protection of DNA from enzymes during intracellular trafficking [289, 290]. Multifunctional hollow manganese oxide nanoparticles functionalized with therapeutic monoclonal antibody, herceptin by a bio-inspired method, using 3,4-dihydroxy-l-phenylalanine as an adhesive moiety, has shown good promise for cancer targeted delivery of therapeutic siRNA and simultaneous diagnosis via MRI [291]. Wang et al. [292] have proposed dual-purpose chitosan and PEI-coated magnetic micelles (CP-mag-micelles) for delivery of nucleic acid-based therapeutic agents and also to provide MRI. These ‘theranostic’ CP-mag-micelles are composed of monodisperse hydrophobic SPIONs loaded into the cores of micelles that are self-assembled from a block copolymer of poly(d,l-lactide) and monomethoxy polyethylene glycol. Superparamagnetic iron oxide nanoparticle-loaded with water-soluble chitosan–linoleic acid nanoparticles (SCLNs) could form gene complexes. Upon i.v. injection to mice, they accumulated mainly in the liver as evident by nuclear and magnetic resonance imaging (Fig. 6). SCLN/pEGFP complexes were transfected into primary hepatocytes, where GFP expression was observed in the cytoplasm and injection of the gene complexes into mice resulted in significantly increased expression of GFP in hepatocytes in vivo. Additionally, administration of gene complexes loaded with specific siRNAs enabled efficient gene silencing [293]. A superparamagnetic γFe2O3 nanocarrier was developed for the delivery of a decoy oligonucleotide in human colon carcinoma SW 480 cells to target the signal transducer and activator of transcription 3 a key regulator of cell survival and proliferation. Moreover, due to the nonlinear magnetic behaviour of superparamagnetic nanoparticles, a new method to quantify their internalization by cells is also shown [294]. Lee et al. [295] developed a multifunctional ‘all-in-one’ magnetic NP that consisted of cell-specific targeting moiety, a fluorescent dye and a therapeutic siRNA payload enabling simultaneous targeting of images and diseased cells. Kam et al. [296] prepared phospholipid–CNT conjugates for both imaging and therapy wherein they coupled siRNA to CNTs via a disulfide bond, which was susceptible to enzymatic breakage in the endolysosome and showed high transfection efficiency, as compared to lipofectamine in inducing RNAi.
Nuclear images (a) of mice at 30 min and 1 h after injection of 99mTc-labeled SCLNs. MR images (b) of the middle part of the mouse liver before and after injection of SCLNs [293]
Toxicity
The manifold applications of inorganic nanovectors for nucleic acid delivery and imaging are also a cause of concern. CNTs are non-biodegradable. Studies have demonstrated that intraperitoneal injection of CNTs resulted in carcinogenesis as high as asbestos [297]. Unpurified CNTs generally can cause oxidative stress, depletion of glutathione, an increase of dermal cell number, localized alopecia and skin thickening in mice [298]. CNT toxicity may arise due to several factors like oxidative stress induced by the formation of reactive oxygen species (ROS) generated at the surface of CNTs, residual catalyst during the CNT manufacturing process, physical contact between a CNT and a cell, dispersant used for the stabilization of CNTs in suspension or blend of these factors [299]. Toxicity can occur due to CNT/DNA interaction as shown in a 3-h incubation study with 96 μg SWCNT/cm2, which induced DNA damage (through micronucleus generation) in lung fibroblasts [300]. CNTs are known to cause pulmonary inflammation and fibrosis. Accumulation of eosinophils and neutrophils, increase in inflammatory and cytotoxicity markers and mechanical blockage in the lungs are other limitations. There could be a significant increase in total bronchoalveolar lavage cells, polymorphonuclear leukocytes and mucin. Other components like IL-1β, protein, tumour necrosis factor-α, lactate dehydrogenase levels may also increase [301]. CNTs show genotoxicity and mutagenic effects like micronucleus induction, chromosome aberration and DNA damage [302].
SiO2 nanoparticles, 46 nm, showed similar cytotoxicity as 15 nm SiO2 nanoparticles in the 10–100-mg mL−1 dosage range, and both sizes of SiO2 nanoparticles were found to be more cytotoxic than crystalline silica in the 50–100-mg mL–1 range. This toxic effect was a result of ROS generation leading to oxidative stress as evident by reduced glutathione levels and the elevated production of malondialdehyde and lactate dehydrogenase, indicative of lipid peroxidation and membrane damage [303]. Silica nanoparticles are known to cause endothelial cells ROS generation, which induces apoptosis via JNK/p53-dependent mitochondrial pathways. At high concentrations, it can activate NF-kB due to oxidative stress in endothelial cells, resulting in the upregulation of CD54, CD62E, TF, IL-6, IL-8 and MCP-1 suggesting possible development of cardiovascular diseases such as atherosclerosis and thrombus [304]. Other authors have demonstrated ROS-mediated induction of apoptosis in human liver cells, which is regulated through p53, bax/bcl-2 and caspase pathways [305]. Size-dependent genotoxic effect of silica nanoparticles has been demonstrated wherein 16 nm nano-SiO2 induced a greater formation of micronucleus than 60 and 104 nm nano-SiO2 [306].
Toxicity of QDs may arise from oxidative degradation of its heavy metal core releasing metal ions, which bind to sulfhydryl groups on intracellular proteins and disrupt the function of subcellular organelles [307]. Although in vitro cytotoxicity assays may provide vital information on the toxicity of inorganic nanovectors, it cannot mimic cellular signalling that occurs in vivo. This is exemplified by a biodistribution study of quantum dots in isolated perfused porcine skin, wherein a phenomenon of skin–quantum dot interaction was observed which went undetected in vitro [308]. QDs can activate the intrinsic mitochondrial apoptosis pathway, thereby increasing the risk for cardiovascular diseases [309]. Cellular studies have shown 2.2 nm CdTe-QD to be more toxic than larger, 5.2 nm particles [310]. Acute genotoxicity of CeSe/ZnS-QD in mammalian cells is reported by using the Comet assay. They demonstrated that genotoxicity of CdSe/ZnS-QD varied with the QD coating material. 11-Mercaptoundecanoic acid coated QD was more damaging than other particle coatings tested [311].
AuNPs (below 4–5 nm) can penetrate the nuclear compartment and bind to DNA resulting in potential toxicity. Gold by virtue of being the most electronegative metal exhibits high affinity to the negatively charged DNA grooves. This is more prominent with NPs of about 1.4 nm diameter that perfectly matches the size of the major DNA groove, resulting in strong potential toxicity of AuNPs [316]. AuNPs caused oxidative stress in human lung fibroblasts after internalization, thereby inducing formation of ROS which resulted in lipid peroxidation and malondialdehyde protein adducts [312].
Highly cationic MNPs, particularly those with PEI coating, exhibit poor stability in biological solutions. Moreover, they reveal potential for in vivo toxicity [317]. Existence of gas vesicles in SPION-treated cells (by staining with the crystal violet dye) with increased granularity of the cells suggested autophagy to be the possible cause of cytotoxicity [313]. SPION–protein interaction can result in fibrillation, denaturation of proteins thereby exposing new antigenic epitopes and loss of function which may eventually induce a new immune response [314]. Intratracheal administration of iron oxide nanoparticles to mice resulted in elevated levels of many inflammatory cytokines, including interleukin-1 and tumour necrosis factor-α, as well as increased expression of matrix metalloproteinases and heat shock protein [315].
It is critical to elucidate the correlation between particle characteristics (size, surface charge, surface chemistry, shape), dose, route of administration and response of the host immune system. Toxicity can arise due to cell-nanovector interactions by probable mechanisms like inflammatory cell infiltration, cellular necrosis, ROS induced apoptosis [318] etc. A vast discrepancy exists between in vitro cytotoxicity assays and its extrapolation in vivo. The need of the hour is to establish suitable toxicity study protocols that can accurately predict in vivo behaviour.
Future prospects
The immense potential of inorganic nanovectors in nucleic acid delivery and theranostics is evident from the aforementioned illustrations. Despite the plethora of applications reported, the question of safety of these nanovectors and their impact on the ecology still prevails. Appropriate design and fabrication of these nanovectors with suitable coating agents, tailoring their physicochemical properties like size, surface charge and surface chemistry, optimizing dose, choosing the right kind of route of administration and targeted delivery, can avert the toxic effects and improve their safety profile. For instance, the use of various PEI-coated IONPs reported for in vitro magnetic NP-mediated nonviral gene delivery was not used for in vivo applications due to probable cellular toxicity. PEGylated PEI was synthesized and was found to overcome this limitation [279]. First generation QDs utilized toxic Cd and Pb which are now replaced with InAs/ZnSe and InAs/InP/ZnSe [319, 320]. QDs can be directed to a specific target by surface modification with antibodies, peptides and small molecules, and this holds good promise for gene delivery and imaging. High-affinity peptide neurotoxin quantum dot nanoconjugates were used to image endogenous proteins in living cells and ex vivo tissue. Moreover, intracellular delivery can be mediated by attaching protein transduction domains such as HIV TAT, Pep-1, polyarginine and SV40 T antigen to quantum dots [321]. Huang et al. [322] have investigated multifunctional nanoprobes for imaging and therapy. They developed folic-acid-conjugated silica-modified gold nanorods X-ray/CT imaging-guided dual-mode radiation and photothermal therapy. This system can be considered for gene delivery and imaging.
The advent of inorganic nanovectors has unearthed new gateways for development of multifunctional nanovectors that integrate imaging (single or dual), therapy (one or more actives) and targeting (one or more ligands) into one platform. Future research should be directed towards development of biocompatible, biodegradable nanovectors and biosafe coatings to improvise the safety profile. Influence of labelling on inherent properties of nanovectors must be considered. Nanotoxicology studies in vitro must extrapolate to in vivo events as efforts to abate toxicity are a prime requirement. It is also important to keep in mind that attempts to achieve targeting or minimize toxicity should not compromise the functionality of these nanovectors. Based on the existing scenario, addressing these numerous factors can eventually dictate the transfer of these nanovectors from research laboratories to clinical settings.
References
Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63:170–83.
Radhakrishnan SK, Layden TJ, Gartel AL. RNA interference as a new strategy against viral hepatitis. Virol. 2004;323:173–81.
Silva GA. Nanotechnology approaches for drug and small molecule delivery across the blood brain barrier. Surg Neurol. 2007;67:113–6.
Bloquel C, Bourges JL, Touchard E, Berdugo M, BenEzraa D, Behar-Cohen F. Non-viral ocular gene therapy: potential ocular therapeutic avenues. Adv Drug Deliv Rev. 2006;58:1224–42.
Pouton CW, Seymour LW. Key issues in non-viral gene delivery. Adv Drug Deliv Rev. 1998;34:3–19.
Wagner E, Kircheis R, Walker GF. Targeted nucleic acid delivery into tumors: new avenues for cancer therapy. Biomed Pharmacother. 2004;58:152–61.
Felgner PL, Ringold GM. Cationic liposome-mediated transfection. Nat. 1989;337:387–8.
de Lima MCP, Neves S, Filipe A, Duzgunes N, Simoes S. Cationic liposomes for gene delivery: from biophysics to biological applications. Curr Med Chem. 2003;10:1221–31.
Cardoso AL, Simoes S, de Almeida LP, Plesnila N, Pedroso de Lima MC, Wagner E, Culmsee C. Tf-lipoplexes for neuronal siRNA delivery: a promising system to mediate gene silencing in the CNS. J Control Rel. 2008;132:113–23.
Mignet N, Richard C, Seguin J, Largeau C, Bessodes M, Scherman D. Anionic pH-sensitive pegylated lipoplexes to deliver DNA to tumors. Int J Pharm. 2008;361:194–201.
Saxena A, Mozumdar S, Johri AK. Ultra-low sized cross-linked polyvinylpyrrolidone nanoparticles as non-viral vectors for in vivo gene delivery. Biomater. 2006;27:5596–602.
Anderson MO, Lichawska A, Arpanaei A, Rask Jensen SM, Kaur H, Oupicky D, Besenbacher F, Kingshott P, Kjems J, Howard KA. Surface functionalisation of PLGA nanoparticles for gene silencing. Biomater. 2010;31:5671–7.
Wang S, Cheng L, Yu F, Pan W, Zhang J. Delivery of different length poly(L-lysine)-conjugated ODN to HepG2 cells using N-stearyllactobionamide-modified liposomes and their enhanced cellular biological effects. Int J Pharm. 2006;311:82–8.
Galetich I, Kosevich M, Shelkovsky V, Stepanian SG, Blagoi YP, Adamowicz L. Structure and energy of nucleic acid base-amino acid complexes: 1. 1-Methyl-uracil-acrylamide. J Mol Struct. 1999;478:155–62.
Tahara K, Sakai T, Yamamoto H, Takeuchi H, Kawashima Y. Establishing chitosan coated PLGA nanosphere platform loaded with wide variety of nucleic acid by complexation with cationic compound for gene delivery. Int J Pharm. 2008;354:210–6.
Wang J, Tao X, Zhang Y, Wei D, Ren Y. Reversion of multidrug resistance by tumor targeted delivery of antisense oligodeoxynucleotides in hydroxypropyl-chitosan nanoparticles. Biomater. 2010;31:4426–33.
Eliyahu H, Joseph A, Azzam T, Barenholz Y, Domb AJ. Dextran–spermine-based polyplexes—evaluation of transgene expression and of local and systemic toxicity in mice. Biomater. 2006;27:1636–45.
Eliyahu H, Joseph A, Schillemans JP, Azzam T, Domb AJ, Barenholz Y. Characterization and in vivo performance of dextran–spermine polyplexes and DOTAP/cholesterol lipoplexes administered locally and systemically. Biomater. 2007;28:2339–49.
Wong SY, Pelet JM, Putnam D. Polymer systems for gene delivery—past, present, and future. Prog Polym Sci. 2007;32:799–837.
Hongtao LV, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Rel. 2006;114:100–9.
Wang P, Zhao XH, Wang ZY, Meng M, Li X, Ning Q. Generation 4 polyamidoamine dendrimers is a novel candidate of nano-carrier for gene delivery agents in breast cancer treatment. Cancer Lett. 2010;298:34–49.
Navarro G, de ILarduya CT. Activated and non-activated PAMAM dendrimers for gene delivery in vitro and in vivo. Nanomed: Nanotechnol Biol Med. 2009;5:287–97.
Perez AP, Romero EL, Morilla MJ. Ethylenediamine core PAMAM dendrimers/siRNA complexes as in vitro silencing agents. Int J Pharm. 2009;380:189–200.
Lee SH, Mok H, Yuhan L, Park TG. Self-assembled siRNA–PLGA conjugate micelles for gene silencing. J Control Rel. 2011;152:152–8.
Son SJ, Bai X, Lee SB. Inorganic hollow nanoparticles and nanotubes in nanomedicine Part 1. Drug/gene delivery applications. Drug Discov Today. 2007;12:650–6.
Epple M, Sokolova V. Nucleic acid carriers; inorganic nanoparticles as carriers of nucleic acids into cells. Angew Chem Int Ed. 2008;47:1382–95.
Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Rel. 2011;155:344–57.
Vercauteren D, Rejman J, Martens TF, Demeester J, De Smedt SC, Braeckmans K. On the cellular processing of non-viral nanomedicines for nucleic acid delivery: mechanisms and methods. J Control Rel. 2012;161:566–81.
D’Souza A, Pranatharthiharan S, Devarajan PV. Nanomedicine in nucleic acid therapy. In: Souto E editor. Patenting nanomedicines. Berlin: Springer, 2012. Part 2, pp. 205–49
Han Y, Liu S, Ho J, Danquah MK, Forde GM. Using DNA as a drug—bioprocessing and delivery strategies. Chem Eng Res des. 2009;87:343–8.
Bertoni C, Jarrahian S, Wheeler TM, Li Y, Olivares EC, Calos MP, Rando TA. Enhancement of plasmid-mediated gene therapy for muscular dystrophy by directed plasmid integration. PNAS. 2006;103:419–24.
Schmidts T, Dobler D, von den Hoff S, Schlupp P, Garn H, Runkel F. Protective effect of drug delivery systems against the enzymatic degradation of dermally applied DNAzyme. Int J Pharm. 2011;410:75–82.
Sioud M, Leirdal M. Therapeutic RNA and DNA enzymes. Biochem Pharmacol. 2000;60:1023–6.
Sen D, Geyer CR. DNA enzymes. Curr Opin Chem Biol. 1998;2:680–7.
James HA, Gibson I. The therapeutic potential of ribozymes. Blood. 1998;91:371–82.
Scott WG. Ribozymes. Curr Opin Struct Biol. 2007;17:280–6.
Fattal E, Bochot A. State of the art and perspectives for the delivery of antisense oligonucleotides and siRNA by polymeric nanocarriers. Int J Pharm. 2008;364:237–48.
Uprichard SL. The therapeutic potential of RNA interference. FEBS Lett. 2005;579:5996–6007.
Lieberman J, Song E, Lee SK, Shankar P. Interfering with disease: opportunities and roadblocks to harnessing RNA interference. Trends Mol Med. 2003;9:397–403.
Akhtar S. Oral delivery of siRNA and antisense oligonucleotides. J Drug Targeting. 2009;17:491–5.
Tebes SJ, Kruk PA. The genesis of RNA interference, its potential clinical applications, and implications in gynecologic cancer. Gynecol Oncol. 2005;99:736–41.
Pfeifer A, Lehmann H. Pharmacological potential of RNAi—focus on miRNA. Pharmacol Ther. 2010;126:217–27.
O’Neil MJ, Bourre L, Melgar S, O’Driscoll CM. Intestinal delivery of non-viral gene therapeutics: physiological barriers and preclinical models. Drug Discov Today. 2011;16:203–18.
Belting M, Sandgren S, Wittrup A. Nuclear delivery of macromolecules: barriers and carriers. Adv Drug Deliv Rev. 2005;57:505–27.
Wiethoff CM, Middaugh CR. Barriers to nonviral gene delivery. J Pharm Sci. 2003;92:203–17.
Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–52.
Zelphati O, Uyechi LS, Barron LG, Szoka Jr FC. Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim Biophys Acta. 1998;1390:119–33.
David S, Pitard B, Benoît JP, Passirani C. Non-viral nanosystems for systemic siRNA delivery. Pharmacol Res. 2010;62:100–14.
Guo J, Bourre L, Soden DM, O’Sullivan GC, O’Driscoll C. Can non-viral technologies knockdown the barriers to siRNA delivery and achieve the next generation of cancer therapeutics? Biotechnol Adv. 2011;29:402–17.
Woodle MC, Matthay KK, Newman MS, Hidayat JE, Collins LR, Redemann C, Martin FJ, Papahadjopoulos D. Versatility in lipid compositions showing prolonged circulation with sterically stabilized liposomes. Biochim Biophys Acta—Biomembranes. 1992;1105:193–200.
Zuhorn IS, Engberts JBFN, Hoekstra D. Gene delivery by cationic lipid vectors: overcoming cellular barriers. Eur Biophys J. 2007;36:349–62.
Funhoff AM, Monge S, Teeuwen R, Koning GA, Schuurmans-Nieuwenbroek NME, Crommelin DJA, Haddleton DM, Hennink WE, van Nostrum CF. PEG shielded polymeric double-layered micelles for gene delivery. J Control Rel. 2005;102:711–24.
Funhoff AM, van Nostrum CF, Lok MC, Kruijtzer JAW, Crommelin DJA, Hennink WE. Cationic polymethacrylates with covalently linked membrane destabilizing peptides as gene delivery vectors. J Control Rel. 2005;101:233–46.
Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36:4158–417.
Rippe B, Rosengren BI, Carlsson O, Venturoli D. Transendothelial transport: the vesicle controversy. J Vasc Res. 2002;39:375–90.
Bolhassani A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim Biophys Acta. 2011;1816:232–46.
Ingle NP, Malone B, Reineke TM. Poly(glycoamidoamine)s: a broad class of carbohydrate-containing polycations for nucleic acid delivery. Trends Biotechnol. 2011;29:443–53.
Hughes MD, Hussain M, Nawaz Q, Sayyed P, Akhtar S. The cellular delivery of antisense oligonucleotides and ribozymes. Drug Discov Today. 2001;6:303–15.
Kawakami S, Higuchi Y, Hashida M. Nonviral approaches for targeted delivery of plasmid DNA and oligonucleotide. J Pharm Sci. 2008;97:726–45.
de Ilarduyaa CT, Sun Y, Düzgünes N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40:159–70.
Woodle MC, Scaria P. Cationic liposomes and nucleic acids. Curr Opin Colloid Interface Sci. 2001;6:78–84.
Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12:468–74.
Bally MB, Harvie P, Wong FMP, Kong S, Wasan EK, Reimer DL. Biological barriers to cellular delivery of lipid-based DNA carriers. Adv Drug Deliv Rev. 1999;38:291–315.
Edinger D, Wagner E. Bioresponsive polymers for the delivery of therapeutic nucleic acids. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:33–46.
Kakizawa Y, Kataoka K. Block copolymer micelles for delivery of gene and related compounds. Adv Drug Deliv Rev. 2002;54:203–22.
Wang T, Upponi JR, Torchilin VP. Design of multifunctional non-viral gene vectors to overcome physiological barriers: dilemmas and strategies. Int J Pharm. 2012;427:3–20.
Suh J, Dawson M, Hanes J. Real-time multiple-particle tracking: applications to drug and gene delivery. Adv Drug Deliv Rev. 2005;57:63–78.
Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MCA, Engberts JBFN, Hoekstra D. Nonbilayer phase of lipoplex–membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol Ther. 2005;11:801–10.
Lechardeur D, Verkman AS, Lukacs GL. Intracellular routing of plasmid DNA during non-viral gene transfer. Adv Drug Deliv Rev. 2005;57:755–67.
Ledley FD, Ledley TS. Pharmacokinetic considerations in somatic gene therapy. Adv Drug Deliv Rev. 1998;30:133–50.
Tachibana R, Harashima H, Shinohara Y, Kiwada H. Quantitative studies on the nuclear transport of plasmid DNA and gene expression employing nonviral vectors. Adv Drug Deliv Rev. 2001;52:219–26.
Vazquez E, Ferrer-Miralles N, Villaverde A. Peptide-assisted traffic engineering for nonviral gene therapy. Drug Discov Today. 2008;13:23–4.
Tachibana R, Harashima H, Shono M, Azumano M, Niwa, Futaki S, Kiwada H. Intracellular regulation of macromolecules using pH-sensitive liposomes and nuclear localization signal: qualitative and quantitative evaluation of intracellular trafficking. Biochem Biophys Res Commun. 1998;251:538–44.
Li SD, Huang L. Gene therapy progress and prospects: non-viral gene therapy by systemic delivery. Gene Ther. 2006;13:1313–9.
Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Rel. 2008;126:187–204.
Guo ZS, Li Q, Bartlett DL, Yang JY, Fang B. Gene transfer: the challenge of regulated gene expression. Trends Mol Med. 2008;14:410–8.
Reynolds PN, Feng M, Curiel DT. Chimeric viral vectors—the best of both worlds? Mol. Med. Today. 1999; 1357–4310
Raper SE. Gene therapy: the good, the bad, and the ugly. Surg. 2005;137:487–92.
Lundstrom K. Latest development in viral vectors for gene therapy. Trends Biotechnol. 2003;21:117–22.
Itaka K, Kataoka K. Recent development of nonviral gene delivery systems with virus-like structures and mechanisms. Eur J Pharm Biopharm. 2009;71:475–83.
Monahan PE, Samulski RJ. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today. 2000;6:433–40.
El-Aneed A. An overview of current delivery systems in cancer gene therapy. J Control Rel. 2004;94:1–14.
Galanis E, Vile R, Russell SJ. Delivery systems intended for in vivo gene therapy of cancer: targeting and replication competent viral vectors. Crit Rev Oncol/Hematol. 2001;38:177–92.
Hida K, Hanes J, Ostermeier M. Directed evolution for drug and nucleic acid delivery. Adv Drug Deliv Rev. 2007;59:1562–78.
Lim ST, Airavaara M, Harvey BK. Viral vectors for neurotrophic factor delivery: a gene therapy approach for neurodegenerative diseases of the CNS. Pharmacol Res. 2010;61:14–26.
Zhang X, Godbey WT. Viral vectors for gene delivery in tissue engineering. Adv Drug Deliv Rev. 2006;58:515–34.
Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80:35–47.
Rao NM. Cationic lipid-mediated nucleic acid delivery: beyond being cationic. Chem Phys Lipids. 2010;63:245–52.
Tagalakis AD, He L, Saraiva L, Gustafsson KT, Hart SL. Receptor-targeted liposome peptide nanocomplexes for siRNA delivery. Biomater. 2011;32:6302–15.
Kurosaki T, Kishikawa R, Matsumoto M, Kodama Y, Hamamoto T, To H, Niidome T, Takayama K, Kitahara TY, Sasaki H. Pulmonary gene delivery of hybrid vector, lipopolyplex containing N-auroylsarcosine, via the systemic route. J Control Rel. 2009;136:213–9.
Kawakami S, Harada A, Sakanaka K, Nishida K, Nakamura J, Sakaeda T, Ichikawa N, Nakashima M, Sasaki H. In vivo gene transfection via intravitreal injection of cationic liposome/plasmid DNA complexes in rabbits. Int J Pharm. 2004;278:255–62.
Kariko K, Kuo A, Barnathan ES, Langer DJ. Phosphate-enhanced transfection of cationic lipid-complexed mRNA and plasmid DNA. Biochim Biophys Acta Biomembr. 1998;1369:320–34.
Hagigit T, Nassar T, Behar-Cohen F, Lambert G, Benita S. The influence of cationic lipid type on in-vitro release kinetic profiles of antisense oligonucleotide from cationic nanoemulsions. Eur J Pharm Biopharm. 2008;70:248–59.
Hirsch-Lerner D, Zhang M, Eliyahu H, Ferrari ME, Wheeler CJ, Barenholz Y. Effect of “helper lipid” on lipoplex electrostatics. Biochim Biophys Acta Biomembr. 2005;1714:71–84.
Garcia-Chaumont C, Seksek O, Grzybowska J, Borowski E, Bolard J. Delivery systems for antisense oligonucleotides. Pharmacol Ther. 2000;87:255–77.
Blanc I, Da Costa MHB, Bolard J, Chazalet MS. Oligonucleotide delivery by a cationic derivative of the polyene antibiotic amphotericin B. I: Interaction oligonucleotide/vector as studied by optical spectroscopy and electron microscopy. Biochim Biophys Acta. 2000;1464:299–308.
Zhao XB, Lee RJ. Tumor-selective targeted delivery of genes and antisense oligodeoxyribonucleotides via the folate receptor. Adv Drug Deliv Rev. 2004;56:1193–204.
Kim HK, Davaa E, Myung CS, Park JS. Enhanced siRNA delivery using cationic liposomes with new polyarginine-conjugated PEG-lipid. Int J Pharm. 2010;392:141–7.
Ma Z, Li J, He F, Wilson A, Pitt B, Li S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem Biophys Res Commun. 2005;330:755–9.
Geusens B, Lambert J, Smedt SC, Buyens K, Sanders NN, Gele MV. Ultradeformable cationic liposomes for delivery of small interfering RNA (siRNA) into human primary melanocytes. J Control Rel. 2009;133:214–20.
Zohra FT, Chowdhury EH, Toshihiro AT. High performance mRNA transfection through carbonate apatite cationic liposome conjugates. Biomater. 2009;30:4006–13.
Bui HT, Umakoshi H, Suga K, Tanabe T, Ngo KX, Shimanouchi T, Kuboi R. Cationic liposome can interfere mRNA translation in an E. coli cell-free translation system. Biochem Eng J. 2010;52:38–43.
Zhang XX, McIntosh TJ, Grinstaff MW. Functional lipids and lipoplexes for improved gene delivery. Biochimie. 2012;94:42–58.
Resina S, Abes S, Turner JJ, Prevot P, Travo A, Clair P, Gait MJ, Thierry AR, Bernard Lebleu B. Lipoplex and peptide-based strategies for the delivery of steric-block oligonucleotides. Int J Pharm. 2007;344:96–102.
Boulanger C, Giorgio CD, Vierling P. Synthesis of acridine-nuclear localization signal (NLS) conjugates and evaluation of their impact on lipoplex and polyplex-based transfection. Eur J Med Chem. 2005;40:1295–306.
Trabulo S, Cardoso AL, Cardoso AMS, Düzgünes N, Jurado AS, Pedroso de Lima MC. Cell-penetrating peptide-based systems for nucleic acid delivery: a biological and biophysical approach. Methods Enzymol. 2012;509:277–300.
Takashima Y, Saito R, Nakajima A, Oda M, Kimura A, Kanazawa T, Okada H. Spray drying preparation of microparticles containing cationic PLGA nanospheres as gene carriers for avoiding aggregation of nanospheres. Int J Pharm. 2007;343:262–9.
Rejman J, Tavernier G, Bavarsad N, Demeester J, De Smedt SC. mRNA transfection of cervical carcinoma and mesenchymal stem cells mediated by cationic carriers. J Control Rel. 2010;147:385–91.
Ko YT, Bhattacharya R, Bickel U. Liposome encapsulated polyethylenimine/ODN polyplexes for brain targeting. J Control Rel. 2009;133:230–7.
Jeong JH, Kim SW, Park TG. A new antisense oligonucleotide delivery system based on self-assembled ODN–PEG hybrid conjugate micelles. J Control Rel. 2003;93:183–91.
Lee CH, Ni YH, Chen CC, Chou CK, Chang FH. Synergistic effect of polyethylenimine and cationic liposomes in nucleic acid delivery to human cancer cells. Biochim Biophys Acta Biomembr. 2003;1611:55–62.
Read ML, Dash PR, Clark A, Howard KA, Oupicky D, Toncheva V, Alpar HO, Schacht EH, Ulbrich K, Seymour LW. Physicochemical and biological characterisation of an antisense oligonucleotide targeted against the bcl-2 mRNA complexed with cationic–hydrophilic copolymers. Eur J Pharm Sci. 2000;10:169–77.
Midoux P, Monsigny M. Efficient gene transfer by histidylated polylysine pDNA complexes. Bioconjug Chem. 1999;10:406–11.
Taratula O, Garbuzenko OB, Kirkpatrick P, Pandya I, Savla R, Pozharov VP, He H, Minko T. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J Control Rel. 2009;140:284–93.
Pedziwiatr-Werbicka E, Ferenc M, Zaborski M, Gabara B, Klajnert B, Bryszewska M. Characterization of complexes formed by polypropylene imine dendrimers and anti-HIV oligonucleotides. Colloids Surf B: Biointerfaces. 2011;83:360–6.
Dufes C, Uchegbu IF, Schatzlein AG. Dendrimers in gene delivery. Adv Drug Deliv Rev. 2005;57:177–202.
Waelti ER, Gluck R. Delivery to cancer cells of antisense L-myc oligonucleotides incorporated in fusogenic, cationic-lipid reconstituted influenza-virus envelopes (cationic virosomes). Int J Cancer. 1996;77:728–33.
Scardino A, Correale P, Firat H, Pellegrini M, Kosmatopoulos K, Opolon P, Alves P, Zurbriggen R, Glück R, Lemonnier FA, Francini G, Cusi MG. In vivo study of the GC90/IRIV vaccine for immune response and autoimmunity into a novel humanised transgenic mouse. Br J Cancer. 2003;89:199–205.
Cusi MG, Fischer S, Sedlmeier R, Valassina M, Valensin PE, Donati M, Neubert WJ. Localization of a new neutralizing epitope on the mumps virus hemagglutinin–neuraminidase protein. Virus Res. 2001;74:133–7.
Schmidt-Wolf GD, Schmidt-Wolf IGH. Non-viral and hybrid vectors in human gene therapy: an update. Trends Mol Med. 2003;9:67–72.
Khlebtsov NG, Dykman LA. Optical properties and biomedical applications of plasmonic nanoparticles. J Quant Spectrosc Radiat Transf. 2010;111(1):1–35.
David G. Evans and Xue Duan. Preparation of layered double hydroxides and their applications as additives in polymers, as precursors to magnetic materials and in biology and medicine. Chem. Commun. 2006; 485
Klumpp C, Kostarelos K, Prato M, Bianco A. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics. Biochim Biophys Acta. 2006;1758:404–12.
Lacerda L, Russier J, Pastorin G, Herrero MA, Venturelli E, Dumortier H, Al-Jamal KT, Prato M, Kostarelos K, Bianco A. Translocation mechanisms of chemically functionalised carbon nanotubes across plasma membranes. Biomater. 2012;33:3334–43.
Ji SR, Liu C, Zhang B, Yang F, Xu J, Long J, Jin C, Fu D, Ni Q, Yu X. Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta. 2010;1806:29–35.
Sinha N, Yeow JTW. Carbon nanotubes for biomedical applications. IEEE Trans Nanobiosci. 2005;4:180–95.
Foldvari M, Bagonluri M. Carbon nanotubes as functional excipients for nanomedicines: I. Pharmaceutical properties. Nanomed: Nanotechnol Biol Med. 2008;4:173–82.
Thess A, Lee R, Nikolaev P, Dai H, Petit P, Robert J, Xu C, Lee YH, Kim SG, Rinzler AG, Colbert DT, Scuseria GE, Tománek D, Fisher JE, Smalley RE. Crystalline nanotubes of metallic carbon nanotubes. Sci. 1996;273:483–7.
Capek I. Dispersions, novel nanomaterial sensors and nanoconjugates based on carbon nanotubes. Adv Colloid Interface Sci. 2009;150:63–89.
Varkouhi AK, Foillard S, Lammers T, Schiffelers RM, Doris E, Hennink WE, Storm G. siRNA delivery with functionalized carbon nanotubes. Int J Pharm. 2011;416:419–25.
Sameti M, Bohr G, RaviKumar MNV, Kneuer C, Bakowsky U, Nacken M, Schmidt H, Lehr CM. Stabilisation by freeze-drying of cationically modified silica nanoparticles for gene delivery. Int J Pharm. 2003;266:51–60.
He Q, Ma M, Wei C, Shi J. Mesoporous carbon@silicon-silica nanotheranostics for synchronous delivery of insoluble drugs and luminescence imaging. Biomater. 2012;33:4392–402.
Fadeel B, Garcia-Bennett AE. Better safe than sorry: understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv Drug Deliv Rev. 2010;62:362–74.
Wang Y, Chen L. Quantum dots, lighting up the research and development of nanomedicine. Nanomed: Nanotechnol Biol Med. 2011;7:385–402.
Algar WR, Massey M, Krull UJ. The application of quantum dots, gold nanoparticles and molecular switches to optical nucleic-acid diagnostics. Trend Anal Chem. 2009;28:292–306.
Smith AM, Duan H, Mohs AM, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60:1226–40.
Azzazy HME, Mansour MMH, Kazmierczak SC. From diagnostics to therapy: prospects of quantum dots. Clin Biochem. 2007;40:917–27.
Pittella F, Zhang M, Lee Y, Kim HJ, Tockary T, Osada K, Ishii T, Miyata K, Nishiyama N, Kataoka K. Enhanced endosomal escape of siRNA-incorporating hybrid nanoparticles from calcium phosphate and PEG-block charge-conversional polymer for efficient gene knockdown with negligible cytotoxicity. Biomater. 2011;32:3106–14.
Giger EV, Puigmartí-Luis J, Schlatter R, Castagner B, Dittrich PS, Leroux JC. Gene delivery with bisphosphonate-stabilized calcium phosphate nanoparticles. J Control Rel. 2011;150:87–93.
Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorgan Med Chem. 2009;17:2950–62.
Sokolova V, Knuschke T, Buer J, Westendorf AM, Epple M. Quantitative determination of the composition of multi-shell calcium phosphate–oligonucleotide nanoparticles and their application for the activation of dendritic cells. Acta Biomaterialia. 2011;7:4029–36.
Boutonnet M, Kizling J, Stenius P, Maire G. The preparation of monodisperse colloidal metal particles from microemulsions. Colloids Surf. 1982;5:209–25.
Sanchez-Dominguez M, Pemartin K, Boutonnet M. Preparation of inorganic nanoparticles in oil-in-water microemulsions: a soft and versatile approach. Curr Opin Colloid Interface Sci. 2012;17:297–305.
Malik MA, Wani MY, Hashim MA. Microemulsion method: a novel route to synthesize organic and inorganic nanomaterials: 1st nano update. Arab J Chem. 2012;5:397–417.
Destrée C, Debuigne F, Jeunieau L, Nagy JB. Mechanism of formation of inorganic and organic nanoparticles from microemulsions. Adv Colloid Interface Sci. 2006;123–126:353–67
Salas G, Costo R, del Puerto Morales M. Chapter 2—synthesis of inorganic nanoparticles. Front Nanosci. 2012;4.
Boutonnet M, Lögdberg S, Svensson EE. Recent developments in the application of nanoparticles prepared from w/o microemulsions in heterogeneous catalysis. Curr Opin Colloid Interface Sci. 2008;13:270–86.
Roy I, Mitra S, Maitra A, Mozumdar S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. Int J Pharm. 2003;250:25–33.
Li A, Qin L, Wang W, Zhu R, Yu Y, Liu H, Wang S. The use of layered double hydroxides as DNA vaccine delivery vector for enhancement of anti-melanoma immune response. Biomater. 2011;32:469–77.
Lee SH, Bae KH, Kim SH, Lee KR, Park TG. Amine-functionalized gold nanoparticles as non-cytotoxic and efficient intracellular siRNA delivery carriers. Int J Pharm. 2008;364:94–101.
Nguyen DT, Kim DJ, Kim KS. Controlled synthesis and biomolecular probe application of gold nanoparticles. Micron. 2011;42:207–27.
Zhou J, Ralston J, Sedev R, Beattie DA. Functionalized gold nanoparticles: synthesis, structure and colloid stability. J Colloid Interface Sci. 2009;331:251–62.
Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110:15700–7.
Durazo SA, Kompella UB. Functionalized nanosystems for targeted mitochondrial delivery. Mitochondrion. 2011;12:190–201.
Dykman LA, Bogatyrev VA. Gold nanoparticles: preparation, functionalisation and applications in biochemistry and immunochemistry. Russ Chem Rev. 2007;76:181–94.
Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol derivatized gold nanoparticles in a 2-phase liquid–liquid system. J. Chem. Soc., Chem. Commun. 1994;801–802
Chen PC, Mwakwari SC, Oyelere AK. Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnol Sci Appl. 2008;1:45–66.
Hu J, Zhang Y, Liu B, Liu J, Zhou H, Xu Y, Jiang Y, Yang J, Tian ZQ. Synthesis and properties of tadpole-shaped gold nanoparticles. J Am Chem Soc. 2004;126:9470–1.
Kuo CH, Chiang TF, Chen LJ, Huang MH. Synthesis of highly faceted pentagonal- and hexagonal-shaped gold nanoparticles with controlled sizes by sodium dodecyl sulfate. Langmuir. 2004;20:7820–4.
Song JY, Jang HK, Kim BS. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009;44:1133–8.
Kumar KP, Paul W, Sharma CP. Green synthesis of gold nanoparticles with Zingiber officinale extract: characterization and blood compatibility. Process Biochem. 2011;46:2007–13.
Noruzi M, Zare D, Khoshnevisan K, Davoodi D. Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim Acta Part A. 2011;79:1461–5.
Cubillana-Aguilera LM, Franco-Romano M, Gil MLA, Naranjo-Rodríguez I, de Cisneros JLH, Palacios-Santander JM. New, fast and green procedure for the synthesis of gold nanoparticles based on sonocatalysis. Ultrason Sonochem. 2011;18:789–94.
Mishra A, Tripathy SK, Yun SI. Fungus mediated synthesis of gold nanoparticles and their conjugation with genomic DNA isolated from Escherichia coli and Staphylococcus aureus. Process Biochem. 2012;47:701–11.
Narayanan KB, Sakthivel N. Facile green synthesis of gold nanostructures by NADPH-dependent enzyme from the extract of Sclerotium rolfsii. Colloids Surf, A Physicochem Eng Asp. 2011;380:156–61.
Sastry M, Ahmad A, Khan MI, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 2003;85:162.
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Ramani R, Parischa R, Ajayakumar PV, Alam M, Sastry M, Kumar R. Bioreduction of AuCl4-ions by the fungus Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew Chem Int Ed. 2001;40:19.
Shankar SS, Ahmad A, Pasricha R, Khan MI, Kumar R, Sastry M. Immobilization of biogenic gold nanoparticles in thermally evaporated fatty acid and amine thin films. J Colloid Interface Sci. 2004;274:69–75.
Suresh AK, Pelletier DA, Wang W, Broich ML, Moon JW, Gu B, Allison DP, Joy DC, Phelps TJ, Doktycz MJ. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomaterialia. 2011;7:2148–52.
Konishi Y, Tsukiyama T, Tachimi T, Saitoh N, Nomura T, Nagamine S. Microbial deposition of gold nanoparticles by the metal-reducing bacterium Shewanella algae. Electrochim Acta. 2007;53:186–92.
Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B: Biointerfaces. 2007;57:97–101.
Senapati S, Syed A, Moeez S, Kumar A, Ahmad A. Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater Lett. 2012;79:116–8.
Fayaz AM, Girilal M, Rahman M, Venkatesan R, Kalaichelvan PT. Biosynthesis of silver and gold nanoparticles using thermophilic bacterium Geobacillus stearothermophilus. Process Biochem. 2011;46:1958–62.
Agnihotri M, Joshi S, Kumar AR, Zinjarde S, Kulkarni S. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater Lett. 2009;63:1231–4.
Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010;62:284–304.
Figuerola A, Di Corato R, Manna L, Pellegrino T. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol Res. 2010;62:126–43.
Masoudi A, Hosseini HRM, Shokrgozar MA, Ahmadi R, Oghabian MA. The effect of poly(ethylene glycol) coating on colloidal stability of superparamagnetic iron oxide nanoparticles as potential MRI contrast agent. Int J Pharm. 2012;433:129–41.
Xu CJ, Sun SH. Monodisperse magnetic nanoparticles for biomedical applications. Polym Int. 2007;56:821–6.
Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomater. 2005;26:3995–4021.
Sun C, Lee JSH, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60:1252–65.
Liu Y, Chen Z, Gu N, Wang J. Effects of DMSA-coated Fe3O4 magnetic nanoparticles on global gene expression of mouse macrophage RAW264.7 cells. Toxicol Letts. 2011;205:130–9.
Kumfer BM, Shinoda K, Jeyadevan B, Kennedy IM. Gas-phase flame synthesis and properties of magnetic iron oxide nanoparticles with reduced oxidation state. J Aerosol Sci. 2010;41:257–65.
Tartaj P, Serna CJ. Synthesis of monodisperse superparamagnetic Fe/silica nanospherical composites. J Am Chem Soc. 2003;125:15754–5.
Wang Z, Li X, Gao M, Zeng X. One-step preparation of amorphous iron nanoparticles by laser ablation. Powder Technol. 2012;215–216:147–50.
Bomatí-Miguel O, Tartaj P, Morales MP, Bonville P, Golla-Schindler U, Zhao XQ, Veintemillas-Verdaguer S. Core–shell iron–iron oxide nanoparticles synthesized by laser-induced pyrolysis. Small. 2006;2:1476–83.
Morjan I, Alexandrescu R, Dumitrache F, Birjega R, Fleaca C, Soare I, Luculescu CR, Filoti G, Kuncer V, Vekas L, Popa NC, Prodan G, Ciupina V. Iron oxide-based nanoparticles with different mean sizes obtained by the laser pyrolysis: structural and magnetic properties. J Nanosci Nanotechnol. 2010;10:1223–34.
Janot R, Guerard D. One-step synthesis of maghemite nanometric powders by ball-milling. J Alloy Compd. 2002;333:302–7.
Hasenpusch G, Geiger J, Wagner K, Mykhaylyk O, Wiekhorst F, Trahms L, Heidsieck A, Gleich B, Bergemann C, Aneja MK, Rudolph C. Magnetized aerosols comprising superparamagnetic iron oxide nanoparticles improve targeted drug and gene delivery to the lung. Pharm Res. 2012;29:1308–18.
Sugimoto T, Matijevic E. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J Colloid Interface Sci. 1980;74:227–43.
Itoh H, Sugimoto T. Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemite particles. J Colloid Interface Sci. 2003;265:283–95.
Zhang DE, Tong ZW, Li SZ, Zhang XB, Ying AL. Fabrication and characterization of hollow Fe3O4 nanospheres in a microemulsion. Mater Lett. 2008;62:4053–5.
Sun SH, Zeng H. Size-controlled synthesis of magnetite nanoparticies. J Am Chem Soc. 2002;124:8204–5.
Khollam YB, Dhage SR, Potdar HS, Deshpande SB, Bakare PP, Kulkarni SD, Date SK. Microwave hydrothermal preparation of submicron-sized spherical magnetite (Fe3O4)powders. Mater Lett. 2002;56:571–7.
Pascal C, Pascal JL, Favier F, Moubtassim MLE, Payen C. Electrochemical synthesis for the control of gamma-Fe2O3 nanoparticle size. Microstruct Magn Behav Chem Mater. 1999;11:141–7.
Vijayakumar R, Koltypin Y, Felner I, Gedanken A. Sonochemical synthesis and characterization of pure nanometer-sized Fe3O4 particles. Mater Sci Eng A-Struct Mater Prop Microstruct Process. 2000;286:101–5.
Grzeta B, Ristic M, Nowik I, Music S. Formation of nanocrystalline magnetite by thermal decomposition of iron choline citrate. J Alloy Compd. 2002;334:304–12.
Philipse AP, Maas D. Magnetic colloids from magnetotactic bacteria: chain formation and colloidal stability. Langmuir. 2002;18:9977–84.
Amemiya Y, Arakaki A, Staniland SS, Tanaka T, Matsunaga T. Controlled formation of magnetite crystal by partial oxidation of ferrous hydroxide in the presence of recombinant magnetotactic bacterial protein Mms6. Biomater. 2007;28:5381–9.
Prozorov T, Mallapragada SK, Narasimhan B, Wang LJ, Palo P, Hamilton MN, Williams TJ, Bazylinski DA, Prozorov R, Canfield PC. Protein mediated synthesis of uniform superparamagnetic magnetite nanocrystals. Adv Funct Mater. 2007;17:951–7.
Bhargava A, Jain N, Barathi ML, Panwar J. Fungal mediated synthesis of iron oxide nanoparticles and their characterization. In: 52nd Annual Conference of Association of Microbiologists of India (AMI-2011), 2011: 359. Punjab University, Chandigarh
Xu ZP, Niebert M, Porazik K, Walker TL, Cooper HM, Middelberg AP, Gray PP, Bartlett PF, Lu GQ. Subcellular compartment targeting of layered double hydroxide nanoparticles. J Control Rel. 2008;130:86–94.
Ladewig K, Niebert M, Xu ZP, Gray PP, Lu GQ. Controlled preparation of layered double hydroxide nanoparticles and their application as gene delivery vehicles. Appl Clay Sci. 2010;48:280–9.
Forano C, Hibino T, Leroux F, Ho CTG. Layered double hydroxides. In: Bergaya F, Theng BKG, Lagaly G, editors. Handbook of clay science, Dev. Clay Sci, vol. 1. Amsterdam: Elsevier; 2006. p. 743–52.
Kong X, Jin L, Wei M, Duan X. Antioxidant drugs intercalated into layered double hydroxide: structure and in vitro release. Appl Clay Sci. 2010;49:324–9.
Ladewig K, Niebert M, Xu ZP, Gray PP, Lu GQ. Efficient siRNA delivery to mammalian cells using layered double hydroxide nanoparticles. Biomater. 2010;31:1821–9.
Xu ZP, Zeng QH, Lu GQ, BingYu A. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Eng Sci. 2006;61:1027–40.
Ladewig K, Xu ZP, Lu GQ. Layered double hydroxide nanoparticles in gene and drug delivery. Expert Opin Drug Deliv. 2009;6:907–22.
Rajesh S, James WL. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–23.
Sharma A, Tandon A, Tovey JC, Gupta R, Robertson JD, Fortune JA, Klibanov AM, Cowden JW, Rieger FG, Mohan RR. Polyethylenimine-conjugated gold nanoparticles: gene transfer potential and low toxicity in the cornea. Nanomed. 2011;7:505–13.
Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimine’s transfer of plasmid DNA into mammalian cells. PNAS Proc Natl Acad Sci USA. 2003;100:9138–43.
Slowing II, Trewyn BG, Lin VSY. Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. J Am Chem Soc. 2007;129:8845–9.
Radu DR, Lai CY, Jeftinija K, Rowe EW, Jeftinija S, Lin VSY. Polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J Am Chem Soc. 2004;126:13216–7.
Torney F, Trewyn BG, Lin VSY, Wang K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotech. 2007;2:295–300.
Tolou H. Administration of oligonucleotides to cultured cells by calcium phosphate precipitation method. Anal Biochem. 1993;215:156–8.
Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–52.
Nakamura E, Isobe H, Nakanishi W, Tomita N, Jinno S, Okayama H. Nonviral gene delivery by tetraamino fullerene. Mol Pharm. 2006;3:124–34.
Nakamura E, Isobe H, Nakanishi W, Tomita N, Jinno S, Okayama H. Gene delivery by aminofullerenes: structural requirements for efficient transfection. Chem-Asian J. 2006;1:167–75.
Liu YL, Chen WH, Chang YH. Preparation and properties of chitosan/carbon nanotube nanocomposites using poly(styrene sulfonic acid)-modified CNTs. Carbohydr Polym. 2009;76:232–8.
ILSI Risk Science Institute Workshop Participants. The relevance of the rat lung response to particle overload for human risk assessment: a workshop consensus report. ILSI Risk Science Institute Workshop Participants. Inhal Toxicol. 2000;12:1–17.
Aisawa S. Nucleotide transport efficiency to leukemia cells by layered double hydroxide, vol. 7. 55th ed. Tokyo: Kagaku to Kogyo; 2002. p. 809–17.
Xu ZP, Walker TL, Liu KL, Cooper HM, Lu GQM, Bartlett PF. Layered double hydroxide nanoparticles as cellular delivery vectors of supercoiled plasmid DNA. Int J Nanomedicine. 2007;2:163–74.
Choy JH, Kwak SY, Jeong YJ, Park JS. Inorganic layered double hydroxides as nonviral vectors. Angew Chem Int Ed. 2000;39:4042–5.
Choy JH, Park JS, Kwak SY, Jeong YJ, Han YS. Layered double hydroxide as gene reservoir. Mol Crystal Liq Cryst. 2000;341:425–9.
Nakayama H, Wada N, Tsuhako M. Intercalation of amino acids and peptides into Mg-Al layered double hydroxide by reconstruction method. Int J Pharm. 2004;269:469–78.
Fudala A, Palinko I, Kiricsi I. Preparation and characterization of hybrid organic–inorganic composite materials using the amphoteric property of amino acids: amino acid intercalated layered double hydroxide and montmorillonite. Inorg Chem. 1999;38:4653–8.
Bonnet S, Forano C, de Roy A, Besse JP. Synthesis of hybrid organo-mineral materials: anionic tetraphenylporphyrins in layered double hydroxides. Chem Mater. 1996;8:1962–8.
Rahman MBA, Basri M, Hussein MZ, Idris MNH, Rahman RNZRA, Salleh AB. Immobilization of lipase from Candida rugosa on layered double hydroxides of Mg/Al and its nanocomposite as biocatalyst for the synthesis of ester. Catal Today. 2004;93–95:405–10.
Rahman MBA, Basri M, Hussein MZ, Idris MNH, Rahman RNZRA, Salleh AB. Immobilization of lipase from Candida rugosa on layered double hydroxides for esterification reaction. Appl Biochem Biotechnol. 2004;118(1–3):313–20.
Tamura H, Chiba J, Ito M, Takeda T, Kikkawa S. Synthesis and characterization of hydrotalcite-ATP intercalates. Solid State Ionics. 2004;172(1–4):607–9.
Lee SJ, Lee HJ, Moon MJ, Vu-Quang H, Lee HJ, Muthiah M, Che HL, Heo SU, Jeong HJ, Jeong YY, Park IK. Superparamagnetic iron oxide nanoparticles-loaded polymersome-mediated gene delivery guided by enhanced magnetic resonance signal. J Nanosci Nanotechnol. 2011;11(8):7057–60.
Hsu SH, Ho TT, Tseng TC. Nanoparticle uptake and gene transfer efficiency for MSCs on chitosan and chitosan–hyaluronan substrates. Biomater. 2012;33:3639–50.
McBain SC, Yiu HHP, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 2008;3:169–80.
Chorny M, Fishbein I, Alferiev I, Levy RJ. Magnetically responsive biodegradable nanoparticles enhance adenoviral gene transfer in cultured smooth muscle and endothelial cells. Mol Pharm. 2009;6:1380–7.
Mykhaylyk O, Antequera YS, Vlaskou D, Plank C. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat Protoc. 2007;2:2391–411.
Chen AA, Derfus AM, Khetani SR, Bhatia SN. Quantum dots to monitor RNAi delivery and improve gene silencing. Nucleic Acids Res. 2005;33:190.
Tan WB, Jiang S, Zhang Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomater. 2007;28(8):1565–71.
Jia N, Lian Q, Shen H, Wang C, Li X, Yang Z. Intracellular delivery of quantum dots tagged antisense oligodeoxynucleotides by functionalized multiwalled carbon nanotubes. Nano Lett. 2007;7(10):2976–80.
McNamara JO, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24(8):1005–15.
Baqalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, Farokhzad OC. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007;7(10):3065–70.
Cheung W, Pontoriero F, Taratula O, Chen AM, He H. DNA and carbon nanotubes as medicine. Adv Drug Deliv Rev. 2010;62:633–49.
Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62:1064–79.
Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem. Commun. 2004;16–17
Pantarotto D, Singh R, McCarthy D, Erhardt M, Briand JP, Prato M, Kostarelos K, Bianco A. Gene technology: functionalised carbon nanotubes for plasmid DNA gene delivery. Angew Chem Int Ed. 2004;43:5242–6.
Podesta JE, A-Jamal KT, Herrero MA, Tian B, Ali-Boucetta H, Hegde V, Bianco A, Prato M, Kostarelos K. Antitumor activity and prolonged survival by carbon-nanotube-mediated therapeutic siRNA silencing in a human lung xenograft model. Small. 2009;5:1176–85.
Vashist SK, Zheng D, Pastorin G, Al-Rubeaan K, Luong JHT, Sheu FS. Delivery of drugs and biomolecules using carbon nanotubes. Carbon. 2011;4(9):4077–97.
Bartholomeusz G, Cherukuri P, Kingston J, Cognet L, Lemos R, Leeuw TK, Gumbiner-Russo L, Weisman RB, Powis G. In vivo therapeutic silencing of hypoxia-inducible factor 1 alpha (HIF-1 alpha) using single-walled carbon nanotubes noncovalently coated with siRNA. Nano Res. 2009;2:279–91.
Bhakta G, Sharma RK, Gupta N, Cool S, Nurcombe V, Maitra A. Multifunctional silica nanoparticles with potentials of imaging and gene delivery. Nanomed: Nanotechnol Biol Med. 2011;7:472–9.
Park IY, Kim IY, Yoo MK, Choi YJ, Cho MH, Cho CS. Mannosylated polyethylenimine coupled mesoporous silica nanoparticles for receptor-mediated gene delivery. Int J Pharm. 2008;359:280–7.
Gao F, Botella P, Corma A, Blesa J, Dong L. Monodispersed mesoporous silica nanoparticles with very large pores for enhanced adsorption and release of DNA. J Phys Chem B. 2009;113:1796–804.
Peng J, He X, Wang K, Tan W, Li H, Xing X, Wang Y. An antisense oligonucleotide carrier based on amino silica nanoparticles for antisense inhibition of cancer cells. Nanomed: Nanotechnol Biol Med. 2006;2:113–20.
Bharali DJ, Klejbor I, Ewa K, Stachowiak EK, Dutta P, Indrajit Roy I, Kaur N, Earl J, Bergey EJ, Prasad PN, Stachowiak MK. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. PNAS Proc Natl Acad Sci USA. 2005;102:11539–44.
Derfus AM, Chen AA, Min DH, Ruoslahti, Bhatia SN. Targeted quantum dot conjugates for siRNA delivery. Bioconjug Chem. 2007;18:1391–6.
Zhang S, Zhao Y, Zhi D, Zhang S. Non-viral vectors for the mediation of RNAi. Bioorgan Chem. 2012;40:10–8.
Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–76.
Bonoiu A, Mahajan SD, Ye L, Kumar R, Ding H, Yong KT, Roy I, Aalinkeel R, Nair B, Reynolds JL, Sykes DE, Imperiale MA, Bergey EJ, Schwartz SA, Prasad PN. MMP-9 gene silencing by a quantum dot–siRNA nanoplex delivery to maintain the integrity of the blood brain barrier. Brain Res. 2009;1282:142–55.
Liu G, Swierczewskaa M, Leea S, Chen X. Functional nanoparticles for molecular imaging guided gene delivery. Nano Today. 2010;5:524–39.
Bisht S, Bhakta G, Mitra S, Maitra A. pDNA loaded calcium phosphate nanoparticles: highly efficient non-viral vector for gene delivery. Int J Pharm. 2005;288:157–68.
Sokolova VV, Radtke I, Heumann R, Epple M. Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomater. 2006;27:3147–53.
Jang S, Lee S, Kim H, Ham J, Seo J-H, Mok Y, Noh M, Lee Y. Preparation of pH-sensitive CaP nanoparticles coated with a phosphate-based block copolymer for efficient gene delivery. Polym. 2012. doi:10.1016/j.polymer.2012.08.043
Nouri A, Castro R, Santos JL, Fernandes C, Rodrigues J, Tomás H. Calcium phosphate-mediated gene delivery using simulated body fluid (SBF). Int J Pharm. 2012;434:199–208.
Orrantia E, Chang PL. Intracellular distribution of DNA internalized through calcium phosphate precipitation. Exp Cell Res. 1990;190:170–4.
Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir. 2005;21:10644–54.
Lehman AJ, Esdaile JM, Klinkhoff AV, Grant E, Fitzgerald A, Canvin J, METGO Study Group. A 48-week, randomized, double-blind, double-observer, placebo-controlled multicenter trial of combination methotrexate and intramuscular gold therapy in rheumatoid arthritis: results of the METGO study. Arthritis Rheum. 2005;52:1360–70.
Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Sci. 2006;312:1027–30.
Shan Y, Luo T, Peng C, Sheng R, Cao A, Cao X, Shen M, Guo R, Tomás H, Shi X. Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors. Biomater. 2012;33:3025–35.
Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. Gene regulation with polyvalent siRNA-nanoparticle conjugates. J Am Chem Soc. 2009;131:2072–3.
Tsai CY, Shiau AL, Cheng PC, Shieh DB, Chen DH, Chou CH, Yeh CS, Wu CL. A biological strategy for fabrication of Au/EGFP nanoparticle conjugates retaining bioactivity. Nano Lett. 2004;4:1209–12.
Kim DW, Kim JH, Park M, Yeom JH, Go H, Kim S, Han MS, Lee K, Bae J. Modulation of biological processes in the nucleus by delivery of DNA oligonucleotides conjugated with gold nanoparticles. Biomater. 2011;32:2593–604.
Noh SM, Kim WK, Kim SJ, Kim JM, Baek KH, Oh YK. Enhanced cellular delivery and transfection efficiency of plasmid DNA using positively charged biocompatible colloidal gold nanoparticles. Biochim Biophys Acta. 2007;1770:747–52.
Chen CC, Lin YP, Wang CW, Tzeng HC, Wu CH, Chen YC, Chen CP, Chen LC, Wu YC. DNA-gold nanorod conjugates for remote control of localized gene expression by near infrared irradiation. J Am Chem Soc. 2006;128:3709–15.
Niidome T, Nakashima K, Takahashi H, Niidome Y. Preparation of primary amine-modified gold nanoparticles and their transfection ability into cultivated cells. Chem. Commun. 2004;1978–1979.
Morais T, Soares ME, Duarte JA, Soares L, Maia S, Gomes P, Pereira E, Fraga S, Carmo H, De Lourdes Bastos M. Effect of surface coating on the biodistribution profile of gold nanoparticles in the rat. Eur J Pharm Biopharm. 2012;80:185–93.
Kim JH, Yeom JH, Ko JJ, Han MS, Lee K, Na SY, Bae J. Effective delivery of anti-miRNA DNA oligonucleotides by functionalized gold nanoparticles. J Biotechnol. 2011;155:287–92.
Ryou SM, Kim JM, Yeom JH, Hyun S, Kim S, Han MS, Kim SW, Bae J, Rhee S, Lee K. Gold nanoparticle-assisted delivery of small, highly structured RNA into the nuclei of human cells. Biochem Biophys Res Commun. 2011;416:178–83.
Ryoo SR, Jang H, Kim KS, Lee B, Kim KB, Kim YK, Yeo WS, Lee Y, Kim DE, Min DH. Functional delivery of DNAzyme with iron oxide nanoparticles for hepatitis C virus gene knockdown. Biomater. 2012;33:2754–61.
Ang D, Nguyen QV, Kayal S, Preiser PR, Rawat RS, Ramanujan RV. Insights into the mechanism of magnetic particle assisted gene delivery. Acta Biomater. 2011;7:1319–26.
Chertok B, David AE, Yang VC. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomater. 2010;31:6317–24.
Chen G, Chen W, Wu Z, Yuan R, Li H, Gao J, Shuai X. MRI-visible polymeric vector bearing CD3 single chain antibody for gene delivery to T cells for immunosuppression. Biomater. 2009;30:1962.
Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13:372–7.
Nakayama H, Hatakeyama A, Tsuhako M. Encapsulation of nucleotides and DNA into Mg–Al layered double hydroxide. Int J Pharm. 2010;393:104–11.
Tyner KM, Roberson MS, Berghorn KA, Li L, Gilmour RF, Batt CA, Giannelis EP. Intercalation, delivery, and expression of the gene encoding green fluorescence protein utilizing nanobiohybrids. J Control Rel. 2004;100:399–409.
Chung HE, Park DH, Choy JH, Choi SJ. Intracellular trafficking pathway of layered double hydroxide nanoparticles in human cells: size-dependent cellular delivery. Appl Clay Sci. 2012;65–66:24–30.
Wong Y, Markham K, Xu ZP, Chen M, Lu GQ, Bartlett PF, Cooper HM. Efficient delivery of siRNA to cortical neurons using layered double hydroxide nanoparticles. Biomater. 2010;31:8770–9.
Kim CS, Tonga GY, Solfiell D, Rotello VM. Inorganic nanosystems for therapeutic delivery: status and prospects. Adv. Drug Deliv. Rev. 2012. doi:10.1016/j.addr.2012.08.011
Cho EC, Glaus C, Chen J, Welch MJ, Xia Y. Inorganic nanoparticle-based contrast agents for molecular imaging. Trends Mol Med. 2010;16:561–73.
Nam J, Won N, Bang J, Jin H, Park J, Jung S, Jung S, Park Y, Kim S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv. Drug Deliv. Rev. 2012. doi:10.1016/j.addr.2012.08.015.
Shashkov EV, Everts M, Galanzha EI, Zharov VP. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett. 2008;8:3953–8.
Chouly C, Pouliquen D, Lucet I, Jeune JJ, Jallet P. Development of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface nature on biodistribution. J Microencapsul. 1996;13:245–55.
Jain TK, Roy I, De TK, Maitra A. Nanometer silica particles encapsulating active compounds: a novel ceramic drug carrier. J Am Chem Soc. 1998;120:11092–5.
Roy I, Ohulchanskyy TY, Bharali DJ, Pudavar HE, Mistretta RA, Kaur N, Prasad PN. Optical tracking of organically modified silica nanoparticles as DNA carriers: a nonviral, nanomedicine approach for gene deliver. PNAS Proc Natl Acad Sci USA. 2005;102:279
Bae KH, Lee K, Kim C, Park TG. Surface functionalized hollow manganese oxide nanoparticles for cancer targeted siRNA delivery and magnetic resonance imaging. Biomater. 2011;32:176–84.
Wang C, Ravi S, Martinez GV, Chinnasamy V, Raulji P, Howell M, Davis Y, Mallela J, Seehra MS, Mohapatra S. Dual-purpose magnetic micelles for MRI and gene delivery. J Control Rel. 2012. doi:10.1016/j.jconrel.2012.04.030
Cheong SJ, Lee CM, Kim SL, Jeong HJ, Kim EM, Park EH, Kim DW, Lim ST, Sohn MH. Superparamagnetic iron oxide nanoparticles-loaded chitosan–linoleic acid nanoparticles as an effective hepatocyte-targeted gene delivery system. Int J Pharm. 2009;372:169–76.
Geinguenaud F, Souissi I, Fagard R, Motte L, Lalatonne L. Electrostatic assembly of a DNA superparamagnetic nano-tool for simultaneous intracellular delivery and in situ monitoring. Nanomed: Nanotechnol Biol Med. 2012. doi:10.1016/j.nano.2011.12.010
Lee JH, Lee K, Moon SH, Lee Y, Park TG, Cheon J. All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew Chem Int Ed Engl. 2009;48:4174–9.
Kam NW, Liu Z, Dai H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J Am Chem Soc. 2005;127:12492–3.
Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, Stone V, Brown S, MacNee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–8.
Zhao X, Liu R. Recent progress and perspectives on the toxicity of carbon nanotubes at organism, organ, cell, and biomacromolecule levels. Environ Int. 2012;40:244–56.
Alpatova AL, Shan W, Babica P, Upham BL, Rogensues AR, Masten SJ, Drown E, Mohanty AK, Alocilja EC, Tarabara VV. Single-walled carbon nanotubes dispersed in aqueous media via non-covalent functionalization: effect of dispersant on the stability, cytotoxicity, and epigenetic toxicity of nanotube suspensions. Water Res. 2010;44:505–20.
Firme CP, Bandaru PR. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed: Nanotechnol Biol Med. 2010;6:245–56.
Kayat J, Gajbhiye V, Tekade RK, Jain NK. Pulmonary toxicity of carbon nanotubes: a systematic report. Nanotechnol Biol Med. 2011;7:40–9.
Boczkowski J, Lanone S. Respiratory toxicities of nanomaterials—a focus on carbon nanotubes. Adv Drug Deliv. Rev. 2012. doi:10.1016/j.addr.2012.05.011
Petushkov A, Ndiege N, Salem AK, Larsen SC. Toxicity of silica nanomaterials: zeolites, mesoporous silica, and amorphous silica nanoparticles. Advances in molecular toxicology. Amsterdam: Elsevier, 2010. p. 223–66.
Liu X, Sun J. Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-kB pathways. Biomater. 2010;31:8198–209.
Ahmad J, Ahamed M, Akhtar MJ, Alrokayan SA, Siddiqui MA, Musarrat J, Al-Khedhairy AA. Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol Appl Pharmacol. 2012;259:160–8.
Gonzalez L, Thomassen LC, Plas G, Rabolli V, Napierska D, Decordier I, Roelants M, Hoet PH, Kirschhock CE, Martens JA, Lison D, Kirsch-Volders M. Exploring the aneugenic and clastogenic potential in the nanosize range: A549 human lung carcinoma cells and amorphous monodisperse silica nanoparticles as models. Nanotoxicol. 2010;4:382–95.
Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62:1052–63.
Lee HA, Imran M, Monteiro-Riviere NA, Colvin VL, Yu WW, Riviere JE. Biodistribution of quantum dot nanoparticles in perfused skin: evidence of coating dependency and periodicity in arterial extraction. Nano Lett. 2007;7:2865–70.
Yan M, Zhang Y, Xu K, Fu T, Qin H, Zheng X. An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicol. 2011;282:94–103.
Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles: perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol. 2009;238:280–8.
Hoshino A, Fujioka K, Oku T, Suga M, Sasaki T, Ohta M, Yasuhara K, Suzuki K, Yamamoto K. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modifications. Nano Lett. 2004;4:2163–88.
Li JJ, Hartono D, Ong CN, Bay BH, Yung LYL. Autophagy and oxidative stress associated with gold nanoparticles. Biomater. 2010;31:5996–6003.
Mahmoudi M, Simchi A, Imani M, Milani AS, Stroeve P. Optimal design and characterization of superparamagnetic iron oxide nanoparticles coated with polyvinyl alcohol for targeted delivery and imaging. J Phys Chem B. 2008;112:14470–81.
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63:24–46.
Winer JL, Liu CY, Apuzzo MLJ. The use of nanoparticles as contrast media in neuroimaging: a statement on toxicity. World Neurosurg. 2011. doi:10.1016/j.wneu.2011.08.013.
Gil PR, Huhn D, del Mercato LL, Sasse D, Parak WJ. Nanopharmacy: inorganic nanoscale devices as vectors and active compounds. Pharmacol Res. 2010;62:115.
Petri-Fink A, Steitz B, Finka A, Salaklang J, Hofmann H. Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (SPIONs): colloidal stability, cytotoxicity, and cellular uptake studies. Eur J Pharm Biopharm. 2008;68:129–37.
Yildirimer L, Thanh NTK, Loizidou M, Seifalian AM. Toxicological considerations of clinically applicable nanoparticles. Nano Today. 2011;6:585–607.
Bharali DJ, Lucey DW, Jayakumar H, Pudavar HE, Prasad PN. Folate-receptor-mediated delivery of InP quantum dots for bioimaging using confocal and two-photon microscopy. J Am Chem Soc. 2005;127:11364–7.
Zimmer JP, Kim SW, Ohnishi S, Tanaka E, Frangioni JV, Bawendi MG. Size series of small indium arsenide–zinc selenide core–shell nanocrystals and their application to in vivo imaging. J Am Chem Soc. 2006;128:2526–7.
Rosenthal SJ, Chang JC, Kovtun O, McBride JR, Tomlinson ID. Biocompatible quantum dots for biological applications. Chem Biol. 2011;18:10–24.
Huang P, Bao L, Zhang C, Lin J, Luo T, Yang D, He M, Li Z, Gao G, Gao B, Fu S, Cui D. Folic acid-conjugated silica modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomater. 2011;32:9796–809.
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pranatharthiharan, S., Patel, M.D., D’Souza, A.A. et al. Inorganic nanovectors for nucleic acid delivery. Drug Deliv. and Transl. Res. 3, 446–470 (2013). https://doi.org/10.1007/s13346-012-0116-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-012-0116-9