Abstract
Aims
Whether the titer of glutamic acid decarboxylase antibodies (GADAs), especially a low titer, is a marker of progression of beta cell dysfunction in patients with slowly progressive insulin-dependent (type 1) diabetes (SPIDDM) is unclear.
Materials and methods
Patients were subdivided as follows: patients with high GADA titers [≥10 U/ml (≥180 WHO U/ml): high GADA] (group 1, n = 37); those with low GADA titers [<10 U/ml (<180 WHO U/ml): low GADA] (group 2, n = 33); those without GADA and with islet cell antibodies (ICA) (group 3, n = 8); those without both GADA and ICA and with insulinoma-associated antigen 2 antibodies (IA-2A) (group 4, n = 6). We also allocated 198 type 2 diabetic patients without any GADA, ICA or IA-2A as group 5. Serum C-peptide responses to annual oral glucose tolerance tests (OGTTs) were followed up for a mean of 107 months from entry.
Results
The proportion of patients progressing to an insulin-dependent state in groups 1, 2, 3 and 4 was significantly higher than in group 5. C-peptide responses in OGTTs of patients in groups 1 and 2 were decreased at a significantly higher rate than in group 5. Multivariate Cox proportional hazard analysis revealed that factors including high GADA, low GADA, onset age <45 years, duration of diabetes <24 months, body mass index (BMI) <22.0 kg/m2, low degree of preserved beta cell function and ICA were independent risk factors for progression to an insulin-dependent state.
Conclusions
SPIDDM patients with low GADA titers have a significantly higher risk of progression to an insulin-dependent state than type 2 diabetic patients, suggesting that the presence of GADA, irrespective of the titer, is a hallmark of beta cell failure. Other risk factors for further progression to an insulin-dependent state in SPIDDM patients were ICA, onset age, duration of diabetes, BMI and residual beta cell function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distinct serological markers for the autoimmune process against islet beta cells, the most important of which is glutamic acid decarboxylase antibody (GADA), are available to discriminate slowly progressive insulin-dependent (type 1) diabetes (SPIDDM) from type 2 diabetes [1–3]. However, determination of an association between the GADA titer and progression of beta cell failure is inconclusive [4–6]. Some studies indicated that the GADA titer was negatively correlated with beta cell function. Other studies reported no association between the GADA titer and progression of beta cell function. This confusion results from (1) the small number of studied patients, (2) short observation periods, and (3) use of different units to measure GADA and different thresholds for positivity. Hence, we studied a sufficient number of patients for a long observation period of up to 15 years using a well-standardized GADA assay and GADA standard samples provided by the Diabetes Antibody Standardization Program 2005.
Research design and methods
Patient eligibility
Diabetic patients treated without insulin were screened for GADA and insulinoma-associated antigen 2 antibodies (IA-2A) between 1995 and 2005 in our hospitals. Inclusion criteria were (1) a diagnosis of diabetes according to the American Diabetes Association [7], (2) no insulin requirement, (3) no evidence of ketosis from diagnosis to screening time, and (4) disease duration between 6 months and 5 years. Exclusion criteria included prior insulin therapy, pregnancy and the presence of any other severe diseases (e.g., malignant diseases, systemic inflammatory diseases, renal or liver disorders, or malabsorption). Patients without either GADA or IA-2A were also randomly allocated to this study. The presence of islet cell antibodies (ICAs) is included in the recent Japan Diabetes Society diagnostic criteria for SPIDDM [8]; therefore, all enrolled patients were examined for ICA using serum samples kept at −80 °C until the assay. According to previous reports [3], patients with GADA were divided into two groups based on GADA titers as follows: group 1 included patients with high GADA titers [≥10 U/ml (≥180 WHO U/ml)], and group 2 included patients with low GADA titers [<10 U/ml (<180 WHO U/ml)]. Among patients without GADA, those with ICA were included in group 3. Patients with neither GADA nor ICA were divided into two groups based on the finding of IA-2A as follows: group 4 included patients with IA-2A, and group 5 included patients without IA-2A. Group 5 patients were defined as having type 2 diabetes.
Study protocol
All enrolled participants received diet therapy according to food exchange lists [9]. Target glycemic levels were defined as fasting blood glucose (FBG) less than 120 mg/dl (6.7 mmol/l), postprandial blood glucose levels less than 200 mg/dl (11.1 mmol/l) and HbA1c levels less than 7.4 % (57 mmol/mol). If patients could not obtain target glycemic levels using oral hypoglycemic agents (OHA), insulin therapy was started. Dosages of OHA or insulin in each patient were adjusted to obtain target glycemic levels during the study.
Follow-up assessments and end point
Residual beta cell function was evaluated by annual 100-g oral glucose tolerance tests (OGTTs). Patients underwent an OGTT after a 12-h fast without receiving their morning injection of insulin or dose of oral hypoglycemic agents. Residual beta cell function was assessed by the sum of serum C-peptide values at 0, 30, 60, 90 and 120 min during the OGTT (sigma C-peptide). Patients whose sigma C-peptide levels fell below 5 ng/ml (1.67 nmol/l) were defined as being in an insulin-dependent state because studies on the natural history of C-peptide levels in SPIDDM have demonstrated that all patients whose sigma C-peptide levels reach less than 5 ng/ml require insulin and are prone to ketosis [1, 2, 10]. The primary end point was the time when the sigma C-peptide levels of patients indicated an insulin-dependent state (sigma C-peptide <5 ng/ml).
Laboratory measures
GADA, ICA and IA-2A were assayed as previously described [11]. The sensitivity and specificity of the GADA assay were both 100 % in the First International GADA Workshop [12]. Our laboratory participated in the second through the fifth International Workshop on Standardization of the ICA Assay, in which we established the quality of our ICA assay as follows: a cutoff point of 5 Juvenile Diabetes Foundation units, sensitivity of 90 % and specificity of 92 % [10]. The IA-2Ab assay was evaluated in the Third Proficiency IA-2Ab test organized by the Research Institute for Children, and the results showed 100 % sensitivity and 100 % specificity [13]. Anti-thyroid peroxidase antibody (TPOA) and anti-thyroglobulin antibody (TgA) were measured using a commercial RIA kit as described previously [14]. Autoimmune thyroid disease (AITD) was defined as Graves’ disease, Hashimoto’s thyroiditis or positive findings of TPOA and/or TgAb [14]. Graves’ disease and Hashimoto’s thyroiditis were diagnosed by endocrinologists clinically and confirmed by abnormal levels of thyroid hormones and positive findings of TPOA, TgA and/or antibodies against TSH receptor [14]. HLA-DRB1-DQB1 haplotype analysis was performed as previously described [15–18]. HLA-DR and -DQ alleles were typed by the previously described PCR-restriction fragment-length polymorphism (RFLP) methods [15–18]. The most probable DRB1-DQB1 haplotypes were deduced from known linkage disequilibria [19]. In the Japanese population, the DRB1*04:05-DQB1*04:01, DRB1*09:01-DQB1*03:03 and DRB1*08:02-DQB1*03:02 haplotypes are positively associated with type 1 diabetes (susceptible HLA haplotype), whereas the DRB1*15:01-DQB1*06:02 and DRB1*15:02-DQB1*06:01 haplotypes have a negative association (protective HLA haplotype) [20, 21].
Ethics
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and later revisions. Ethical committees of the University of Yamanashi approved this study, and informed consent was obtained from all patients included in the study.
Statistical analysis
Data were analyzed according to the intention-to-treat principle. Categorical variables were compared using Fisher’s exact test. Differences in continuous variables between the two groups were compared using the Mann-Whitney U test. Kaplan-Meier life tables were constructed and compared using the log-rank chi-square statistic. Longitudinal data on sigma C-peptide values were analyzed using a generalized linear mixed model. When comparing the rates of change in sigma C-peptide values among or between several groups, we added the time, groups and interaction between time and groups as fixed effect factors, and random effects of the case and interaction between the case and time were also added. In order to obtain an estimated linear equation between sigma C-peptide values and time for each group, time was added as a fixed effect factor to the model, and random effects of the case and interaction between the case and time were also added. We estimated multiple hazard ratios of covariates for progression to an insulin-dependent state using a Cox proportional-hazards regression analysis with stepwise selection. A stringency level (P value) of 0.05 was used to both include and exclude variables in the stepwise selection. Tests of significance were two tailed. Statistical analyses were performed using JMP. Values are expressed as mean ± SD except as otherwise described.
Results
Patients
Among 1547 patients with diabetes treated without insulin, 105 (6.8 %) had either GADA or IA-2A in two consecutive serum samples. Of these, 94 (6.1 %) had GADA, 53 had IA-2A (3.4 %), and 42 (2.7 %) had both. IA-2A in the absence of GADA was found 11 patients (0.7 %). Among 105 patients with either GADA or IA-2A, 81 gave us their written informed consent. Two patients were lost before the initiation of evaluation of residual beta cell function using OGTT. Therefore, a total of 79 patients with GADA and/or IA-2A underwent annual OGTTs. We obtained written informed consent from 203 patients with neither GADA nor IA-2A. They were randomly allocated and included in this study. Study patients were followed up for 105 ± 50 months.
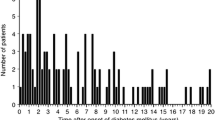
ICAs were detected in 28 (40.0 %) patients with GADA, 3 (33.3 %) GADA-negative patients with IA-2A and 5 (2.5 %) with neither GADA nor IA-2A. Based on these antibody findings, study subjects were subdivided into five groups (group 1: n = 37; group 2: n = 33; group 3: n = 8; group 4: n = 6; group 5 = 198). The distribution of GADA titers in patients with GADA was expressed on Fig. 1.
When compared to patients in group 5 at baseline, those in groups 1 and 2 had significantly lower values of fasting C-peptide and sigma C-peptide, significantly higher levels of blood glucose at 120 min during OGTT and significantly higher frequencies of ICA and IA-2A (Table 1). Furthermore, patients in group 1 had a significantly shorter duration of diabetes and significantly higher frequencies of AITD, TgA, TPOA, OHA therapy, sulfonylurea (SU) agent therapy and risk HLA haplotype than those in group 5. Group 1 patients also showed significantly lower BMI levels and significantly higher HbA1C levels than group 5 patients. When compared with patients in group 1, those in group 2 had significantly higher BMI levels, significantly lower AITD, TgA, TPOA, ICA and IA-2A frequencies, and susceptible HLA haplotypes. When compared with patients in group 1, those in group 3 had significantly higher levels of fasting C-peptide, and those in group 4 had significantly lower frequencies of ICA and risk HLA. Group 3 patients had a significantly higher frequency of ICA than groups 2, 4 and 5 and had a significantly higher frequency of IA-2A than group 5. Group 4 patients had a significantly higher frequency of IA-2A than groups 2, 3 and 5.
Progression rate to an insulin-dependent state
Twenty-seven (73 %), 9 (27 %), 3 (38 %), 2 (33 %) and 3 (2 %) patients in groups 1, 2, 3, 4 and 5, respectively, progressed to an insulin-dependent state. The proportions of patients with an insulin-dependent state in groups 1, 2, 3 and 4 were significantly higher than that in group 5 (all p values <0.0001, log-rank test) (Fig. 2a). This proportion in group 1 was also significantly higher than those in groups 2 and 3 (p < 0.0001 and p = 0.0494, respectively). Excluding patients with AITD, the proportions of patients with an insulin-dependent state in groups 1, 2, 3 and 4 were significantly higher than in group 5 (all p values <0.0001), and the proportion in group 1 was significantly higher than that in group 2 (p = 0.0075) (Fig. 2b). Excluding patients with IA-2A, the proportions of patients with an insulin-dependent state in groups 1, 2 and 3 were significantly higher than that in group 5 (all p values <0.0001), and the proportion in group 1 was significantly higher than that in group 2 (p = 0.0117) (Fig. 2c).
Frequency of progression to an insulin-dependent state. a All study patients; b patients excluding those with autoimmune thyroid disease; c patients excluding those with insulinoma-associated antigen 2 antibodies (IA-2A). Group 1: patients with glutamic acid decarboxylase antibodies (GADA) titer ≥10 U/ml (≥180 WHO U/ml); group 2: patients with GADA titer <10 U/ml (<180 WHO U/ml); group 3: patients without GADA and with islet cell antibodies (ICA); group 4: patients without GADA or ICA and with IA-2A; group 5: type 2 diabetic patients without GADA, ICA or IA-2A
Longitudinal changes in C-peptide response to OGTT
A generalized linear mixed model showed a significant interaction between time and groups (groups 1, 2, 3, 4 and 5) (F 13.7931, df 4, p < 0.0001). Using the generalized linear mixed model, the reduction rate of sigma C-peptide in each group was 1.45 ± 0.18 (ng/ml/year, mean ± SEM) (Figs. 3a, 4), 0.95 ± 0.23 (ng/ml/year) (Figs. 3b, 4), 0.85 ± 0.29 (ng/ml/year) (Figs. 3c, 4), 0.67 ± 0.40 (ng/ml/year) (Figs. 3d, 4) and 0.07 ± 0.07 (ng/ml/year) (Fig. 3e, 4) in groups 1, 2, 3, 4 and 5, respectively. Significant associations between sigma C-peptide and time were recognized in groups 1, 2 and 3 [p < 0.0001 (Fig. 3a), p = 0.0004 (Fig. 3B), and p = 0.0283 (Fig. 3C), respectively], but not recognized in groups 4 and 5 (Fig. 3d, e). In order to compare the reduction rates of sigma C-peptide between two different groups, we analyzed the interaction between time and two groups selected among the groups and obtained significant interactions not only between groups 1 and 5 (F 43.8040, df 1, p < 0.0001), but also between groups 2 and 5 (F 12.1114, df 1, p = 0.0006) (Fig. 4). This interaction between groups 3 and 5 was not significant, although we observed a trend (F 3.5124, df 1, p = 0.0612) (Fig. 4). The interactions between the following groups were not significant: groups 1 and 2, groups 1 and 3, groups 1 and 4, groups 2 and 3, groups 2 and 4, groups 3 and 4, and groups 4 and 5.
Longitudinal changes of the integrated value of serum C-peptide during the 100-g oral glucose tolerance test (sigma C-peptide) of patients in groups 1 (a), 2 (b), 3 (c), 4 (d) and 5 (e). Open circle indicates each values of sigma C-peptide. Black line indicates the estimated linear equation of each patient. Red line indicates the estimated linear equation of each group. Green line indicates the upper or lower limit of the 95 % confidence interval of an estimated linear equation of each group
Reduction rate of sigma C-peptide in group 1 [patients with glutamic acid decarboxylase antibodies (GADA) titer ≥10 U/ml (≥180 WHO U/ml)], group 2 [patients with GADA titer <10 U/ml (<180 WHO U/ml)], group 3 [patients without GADA and with islet cell antibodies (ICA)], group 4 [patients without GADA or ICA and with insulinoma-associated antigen 2 antibodies (IA-2A)] and group 5 (type 2 diabetic patients, those without GADA, ICA or IA-2A)
Risk factors for progression to an insulin-dependent state
Multivariate Cox proportional hazard analysis revealed that progression to an insulin-dependent state occurred when patients had low GADA titers (hazard ratio 4.69) as well as high GADA titers (hazard ratio 11.21) (Table 2). Furthermore, onset age of <45 years (hazard ratio 2.18), short duration of diabetes (<24 months, hazard ratio 2.54), low BMI value (<22.0 kg/m2, hazard ratio 2.40), low degree of preserved beta cell function (fasting C-peptide <1.0 ng/ml, hazard ratio 4.61) and patients with ICA (hazard ratio 7.74) were also independent risk factors for progression to an insulin-dependent state (Table 2). To assess which variables are significant independent ones in the absence of ICA, which are sometimes hard to obtain in clinical practice, a further multivariate Cox proportional hazard analysis was done after excluding ICA. In this case, the following factors were recognized as independent risk factors: high GADA titers (hazard ratio 20.98), low GADA titers (5.35), onset age of <45 years (2.18), fasting C-peptide <1.0 ng/ml (3.05) and IA-2A (3.90).
Discussion
We demonstrated that non-insulin-treated diabetic patients with low as well as high GADA titers have a significantly higher frequency of progression to an insulin-dependent state than type 2 diabetic patients. The reduction rate of residual beta cell functions of non-insulin-treated diabetic patients with low GADA titers was significantly larger than that of type 2 diabetic patients. Multivariate Cox regression analysis indicated low GADA titers as well as high GADA titers were independent risk factors for progression to an insulin-dependent state. Indeed, Suzuki et al. [22] reported that GAD-reactive interferon-γ producing CD4+ cells in the peripheral blood were recognized in not only patients with high GADA titers but also those with low GADA titers. Hence, non-insulin-treated diabetic patients with low GADA titers as well as those with high GADA titers might be candidates for an intervention strategy to prevent progression of beta cell failure (Tokyo Study) [3]. We previously reported that non-insulin-treated diabetic patients with low GADA titers had low-grade insulitis and thus should be classified as SPIDDM patients [23].
For clinical characteristics at baseline, non-insulin-treated diabetic patients with low GADA titers (SPIDDM patients with low GADA titers) have a significantly higher degree of decreased residual beta cell function than type 2 diabetic patients. Blood glucose levels at 120 min during the OGTT in SPIDDM patients with low GADA titers were significantly higher than in patients with type 2 diabetes. However, many SPIDDM patients with low GADA titers and type 2 diabetic patients had the same levels of residual beta cell function and blood glucose at the onset (or diagnosis) of diabetes mellitus. Therefore, it is difficult to distinguish these two clinical subtypes of diabetes without GADA. Hence, all patients who seem to have type 2 diabetes should be measured for GADA.
Mean BMI at baseline of type 2 diabetes patients (group 5) was 22.2 kg/m2 in this study. Sone et al. [24] reported that the mean BMI was 23.1 kg/m2 in thousands of type 2 diabetic patients in the Japan Diabetes Complications Study. Hence, there is a possibility that type 2 diabetic patients in this study might not represent typical type 2 diabetic patients of Japanese populations, because our study started in 1995 and the clinical features of type 2 diabetes (i.e., BMI) might have changed with time.
Multivariate Cox proportional hazard analysis indicated that findings of islet-related autoantibodies (GADA and ICA), onset age, duration of diabetes, BMI and residual beta cell function were independent risk factors for progression to an insulin-dependent state. An SU agent was not recognized as an independent risk factor, although our previous report (Tokyo study) indicated that SU agents were an independent risk factor for an insulin-dependent state [3]. In the present study, as much as 70 % subjects with type 2 diabetes was also included in the analysis as controls. In such a situation, the sensitivity to identify risk factors for progression to an insulin-dependent state in terms of mode of treatment (i.e., SU agents) becomes low, because the patients with type 2 diabetes rarely progress to an insulin-dependent state.
Turner et al. [25] reported that newly diagnosed diabetic patients treated without insulin aged 44 years or younger had different clinical features on future insulin treatment from those aged older than 45 years (UKPDS 25). They reported that both GADA and ICA were independent predictors of an insulin requirement in patients older than 45 years, although only GADA, but not ICA, was included in the independent predictive factors for an insulin requirement in patients aged 44 years or younger [25]. Hence, we set the cutoff age as 45 years of age for multivariate Cox’s proportional hazard analysis. Younger onset age (onset age <45 years) was demostrated to be an independent predictive factor for future progression to an insulin-dependent state.
Murao et al. [26] reported that the presence of either TgA or TPOA, markers of AITD, was an independent factor for beta cell failure in patients with latent onset diabetes in adults. In their analysis, the presence of a high titer of GADA (≥10 U/ml) was excluded from the risk factors for beta cell failure. In our present study, we demonstrated that not only the presence of a high titer of GADA but also the presence of a low titer of GADA (<10 U/ml) was an independent risk factor for progression to an insulin-dependent state, although the presence of either TgA or TPOA was excluded from the risk factors. We also analyzed the situation in which AITD patients were excluded from the analysis of progression to an insulin-dependent state. The results were similar to the situations in which AITD-patients were not excluded. These differences between our study and that of Murao et al. [26] might be caused by the differences in analyzed subjects. Our study analyzed both GADA-positive and -negative subjects, although the study of Murao et al. analyzed a small number of GADA-positive subjects.
We evaluated the effects of positivity of GADA, ICA and IA-2A in the present study (Fig. 2a–c). However, this might be not enough because some additional islet-related antibodies, except for GADA, ICA and IA-2A, are detected in SPIDDM patients [e.g., insulin autoantibody (IAA) and zinc transporter 8 autoantibody (ZnT8A)]. The effects of IAA or ZnT8A on progression to an insulin-dependent state in patients with SPIDDM remain unclear.
In the present study, we also demonstrated that both GADA- and ICA-negative patients with IA-2A had a significantly higher frequency of progression to an insulin-dependent state than type 2 diabetic patients (Table 2). This might mean that we should also distinguish non-insulin-treated diabetic patients with IA-2A from type 2 diabetic patients, suggesting that non-insulin-treated diabetic patients with IA-2A are candidates for an intervention strategy for progressive beta cell dysfunction as well.
References
Kobayashi T. Subtype of insulin-dependent diabetes mellitus (IDDM) in Japan: slowly progressive IDDM—the clinical characteristics and pathogenesis of the syndrome. Diabetes Res Clin Pract. 1994;24(Suppl):S95–9.
Kobayashi T, Tamemoto K, Nakanishi K, et al. Immunogenetic and clinical characterization of slowly progressive IDDM. Diabetes Care. 1993;16:780–8.
Maruyama T, Tanaka S, Shimada A, et al. Insulin intervention in slowly progressive insulin-dependent (type 1) diabetes mellitus. J Clin Endocrinol Metab. 2008;93:2115–21.
Desai M, Cull CA, Horton VA, et al. GAD autoantibodies and epitope reactivities persist after diagnosis in latent autoimmune diabetes in adults but do not predict disease progression: UKPDS 77. Diabetologia. 2007;50:2052–60.
Lohmann T, Kellner K, Verlohren HJ, et al. Titre and combination of ICA and autoantibodies to glutamic acid decarboxylase discriminate two clinically distinct types of latent autoimmune diabetes in adults (LADA). Diabetologia. 2001;44:1005–10.
Buzzetti R, Di Pietro S, Giaccari A, Non Insulin Requiring Autoimmune Diabetes Study Group, et al. High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care. 2007;30:932–8.
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–97.
Tanaka S, Ohmori M, Awata T, et al. Diagnostic criteria for slowly progressive insulin-dependent (type 1) diabetes mellitus (SPIDDM) (2012)—report by the committee on slowly progressive insulin-dependent (type 1) diabetes mellitus of the Japan Diabetes Society. Diabetol Int. 2015;6:1–7.
Japan Diabetes Society. Food exchange lists—dietary guidance for persons with diabetes. Tokyo: Bunkodo; 2003.
Kobayashi T, Nakanishi K, Murase T, et al. Small doses of subcutaneous insulin as a strategy for preventing slowly progressive β-cell failure in islet cell antibody-positive patients with clinical features of NIDDM. Diabetes. 1996;45:622–6.
Tanaka S, Endo T, Aida K, et al. Distinct diagnostic criteria of fulminant type 1 diabetes based on serum C-peptide response and HbA1c levels at onset. Diabetes Care. 2004;27:1936–41.
Schmidli RS, Colman PG, Bonifacio E, et al. High level of concordance between assays for glutamic acid decarboxylase antibodies: the first international glutamic acid decarboxylase antibody workshop. Diabetes. 1994;43:1005–9.
Masuda M, Powell M, Chen S, et al. Autoantibodies to IA-2 in insulin-dependent diabetes mellitus: measurements with a new immunoprecipitation assay. Clin Chim Acta. 2000;291:53–66.
Awata T, Kawasaki E, Tanaka S, Japanese Study Group on Type 1 Diabetes Genetics, et al. Association of type 1 diabetes with two Loci on 12q13 and 16p13 and the influence coexisting thyroid autoimmunity in Japanese. J Clin Endocrinol Metab. 2009;94:231–5.
Nomura N, Ota M, Tsuji K, et al. HLA-DQB1 genotyping by a modified PCR-RFLP method combined with group-specific primers. Tissue Antigens. 1991;38:53–9.
Ota M, Seki T, Fukushima H, et al. HLA-DRB1 genotyping by modified PCR-RFLP method combined with group-specific primers. Tissue Antigens. 1992;39:187–202.
Tanaka S, Kobayashi T, Nakanishi K, et al. Association of HLA-DQ genotype in autoantibody-negative and rapid-onset type 1 diabetes. Diabetes Care. 2002;25:2302–7.
Kobayashi T, Tanaka S, Okubo M, et al. Unique epitopes of glutamic acid decarboxylase autoantibodies in slowly progressive type 1 diabetes. J Clin Endocrinol Metab. 2003;88:4768–75.
Imanishi T, Akaza T, Kimura A, et al. Allele frequencies and haplotype frequencies for HLA and complement loci in various ethnic groups. In: Tsuji K, Aizawa M, Sasazuki T, editors. HLA 1991. Oxford: Oxford University Press; 1992. p. 1065–220.
Rønningen KS, Spurkland A, Tait BD, et al. HLA class II associations in insulin-dependent diabetes mellitus among blacks, Caucasoids, and Japanese. In: Tsuji K, Aizawa M, Sasazuki T, (Eds) HLA 1991. Proceedings of the 11th international histocompatibility workshop and conference, Yokohoma, Japan, 6–13 November 1991. Oxford University Press, Oxford, 1992, p. 713 –22.
Yasunaga S, Kimura A, Hamaguchi K, et al. Different contribution of HLA-DR and genes in susceptibility and resistance to insulin-dependent diabetes mellitus (IDDM). Tissue Antigens. 1996;47:37–48.
Suzuki R, Shimada A, Maruyama T, et al. T-cell function in anti-GAD65(+)diabetes with residual beta-cell function. J Autoimmun. 2003;20:83–90.
Kobayashi T, Nishida Y, Tanaka S, et al. Pathological changes in the pancreas of fulminant type 1 diabetes and slowly progressive insulin-dependent diabetes mellitus (SPIDDM): innate immunity in fulminant type 1 diabetes and SPIDDM. Diabetes Metab Res Rev. 2011;27:965–70.
Sone H, Ito H, Ohashi Y, et al. Obesity and type 2 diabetes in Japanese patients. Lancet. 2003;361:85.
Turner R, Stratton I, Horton V, UK Prospective Diabetes Study Group, et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet. 1997;350:1288–93.
Murao S, Kondo S, Ohashi J, et al. Anti-thyroid peroxidase antibody, IA-2 antibody, and fasting C-peptide levels predict beta cell failure in patients with latent autoimmune diabetes in adults (LADA)–a 5-year follow-up of the Ehime study. Diabetes Res Clin Pract. 2008;80:114–21.
Acknowledgments
We thank Prof. Kazuhiko Kobayashi (Department of Global Agricultural Sciences, Graduate School of Agricultural and Life Sciences, University of Tokyo) for statistical advice and also thank Ms. Kaori Hosaka, Ms. Chihiro Imai and Ms. Sachiko Osada (Third Department of Internal Medicine, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi) and Ms. Fumie Takano (Department of Endocrinology and Metabolism, Toranomon Hospital) for secretarial work.
Conflict of interest
Tetsuro Kobayashi received honoraria for lectures from Sanofi K.K. and research grants from Sanofi K.K., Tanabe-Mitsubishi K.K., Kowa-Souyaku Co. and Eli Lilly Japan K.K. Shoichiro Tanaka, Minoru Okubo, Kaoru Nagasawa, Shouichi Takizawa, Masashi Ichijo, Sayaka Ichijyo, Masahiro Kaneshige, Kaoru Aida, Hiroki Shimura and Yasumichi Mori declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions. Informed consent or a substitute for it was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tanaka, S., Okubo, M., Nagasawa, K. et al. Predictive value of titer of GAD antibodies for further progression of beta cell dysfunction in slowly progressive insulin-dependent (type 1) diabetes (SPIDDM). Diabetol Int 7, 42–52 (2016). https://doi.org/10.1007/s13340-015-0211-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-015-0211-5