Abstract
To identify the circulating serotype(s) of dengue viruses in Bangladesh, a retrospective molecular identification was performed on stored serum samples of dengue surveillance during the period of 2013–2016. Real time RT-PCR was performed on serum samples collected from the patients with less than 5 days fever for detection of dengue virus nucleic acid. The samples, positive for dengue PCR were further analyzed for serotypes by real time RT-PCR. The overall prevalence of dengue virus infection was varied among 13–42% in study years with a single peak flanked by April to September. Among the four dengue serotypes DEN1 and DEN2 were in the circulation in three metropolitan cities with sequential emergence of DEN1 where DEN2 was persisted constantly during the study period. Persistence of all four serotypes in the neighboring country makes Bangladesh vulnerable for devastating secondary infection by introduction of new serotype(s) other than currently circulating viruses in the country. Thus continuous virological surveillance is crucial for early warning of emergence of new serotype in the circulation and public health preparedness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dengue, an acute systemic viral infection has gradually become one of the leading causes of morbidity and mortality in tropical and subtropical areas in the last few decades [8]. Global expansion due to international travel, climate change with distribution from urban to rural settings make the dengue virus as one of the prioritized neglected tropical diseases (NTDs) (http://www.who.int/neglected_diseases/diseases/en/). Most of the time the actual numbers of dengue cases remain under reported and/or are misclassified. However World Health Organization (WHO) estimated about 50–100 million dengue cases in more than 100 countries per year, with upward trend of severe infections in Southeast Asia, Africa, South America and Western Pacific countries [1]. Dengue virus infection in humans usually remains asymptomatic, although ranging widely in severity from a mild fever with spontaneous remission to life-threatening hemorrhagic fever and/or shock syndrome. Dengue virus (DENV), the etiological agent of dengue fever (DF) belongs to the genus Flavivirus of the family Flaviviridae transmitted between humans by infected mosquitoes Aedes aegypti [6]. It is an envelope virus with a single-stranded positive sense RNA genome, contains single open reading frame encoding three structural proteins (including capsid protein, premembrane/membrane protein and envelope protein) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) [5]. There are four antigenically distinct serotypes of DENV (DENV-1 to DENV 4) and all of them are found in the tropical and subtropical regions across the globe [3]. Human infection with one serotype is believed to confer long-lived serotype-specific immunity [15]. But pre-existing antibody cannot confer protection against another serotype, rather secondary infection with heterologous type is frequently associated with severe clinical manifestations e.g. dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) which is thought to be linked with Antibody dependent enhancement [4].
Dengue hemorrhagic fever occurs more frequently with DENV-2 or DENV-3 infections in DENV-1 exposed individuals [2]. Another study in Thailand revealed that risk factors for dengue shock syndrome were associated with secondary infections caused by DEN-2 following primary infections with DEN-1, DEN-3, or DEN-4 serotypes [13].
Dengue is an emerging public health problem in Bangladesh since the first outbreak in 2000 (http://apps.who.int/disasters/repo/13755.pdf). From 2000 to 2002 there was a sudden surge of DF cases with on an average 65 deaths [11]. All four serotypes of dengue virus with predominance of DEN-3 were seen during 2000 outbreak in Bangladesh [10]. After that every year there is an outbreak with different amplitude and severity. But there is scarcity of data regarding circulating serotypes of dengue virus in Bangladesh. It is important to identify the circulating serotype of dengue virus at the beginning of every season for prediction of disease amplitude and severity of the disease in coming season. This attempt may contribute in early preparedness plan regarding management and containment of the disease at policy making level of the country.
Materials and methods
Study population and sample collection
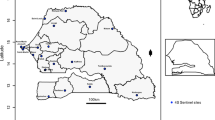
Institute of Epidemiology, Disease Control and Research (IEDCR), the premier national institute for disease surveillance in the country initiated surveillance on dengue under “mitigating the impact of climate change to reduce the burden of climate- sensitive illness through strengthening of health systems, collaborative networking and enhanced disease surveillance” protocol. One of the objectives of that protocol was to build a body of research that examines the impact of climate change on specific conditions including dengue. Under this objective IEDCR started dengue surveillance at four tertiary level hospitals in three metropolitan cities (Dhaka, Chittagong and Khulna) of Bangladesh. Two sentinel sites were selected in Dhaka city considering rapidly growing periurban region and population density.
With all aseptic precaution blood samples (5 ml) were collected from febrile patients enrolled from 2013 to December 2016 following WHO case definition (http://bestpractice.bmj.com/best-practice/monograph/1197/diagnosis/criteria.html) under dengue surveillance.
According to the case definition, a surveillance physician enrolled randomly 10 cases from each site in each month round the year.
Laboratory testing for dengue of IgM
Sera were separated from whole blood and preserved at the sentinel sites till transfer to IEDCR once in a month. Upon receiving the samples, every sample was tested for dengue IgM (DENV Detect IgM, InBios International, Inc, Seattle, USA) by ELISA according to the manufacturer instructions.
RNA extraction
Samples collected from patients with onset of fever within 5 days were taken for total RNA extraction using the RNeasy Mini Kit (Qiagen, Crawley, UK) and stored at − 70 °C till real time RT-PCR was performed.
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Dengue nucleic acid was detected by real-time RT-PCR (Dengue virus genesig detection kit, UK) and all PCR positive samples were further analyzed for DENV serotypes by multiplex real time RT-PCR. The nucleotide sequence for primers and probes (Table 1) were determined as previously described [7].
In multiplex reaction mixtures, 50 pmol (each) of DEN-1- and DEN-3-specific primers, 25 pmol (each) of DEN-2- and DEN-4-specific primers, and 9 pmol of each probe were combined in a 25-μl volume total reaction mixture. Reverse transcription of 10 min at 50 °C was followed by 45 cycles of amplification in an ABI 7500 FastDx real-time Detection System according to superscript III One-Step qRT-PCR kit (Invitrogen, USA) instructions for real-time RT-PCR conditions and using a 60 °C annealing temperature. In case of PCR result interpretation, Cycle Threshold (Ct) < 38 was evaluated as positive and Ct value > 38 was negative.
Ethics approval and consent to participate
Ethical approval was obtained from the “Institutional Review Board (IRB) of IEDCR for secondary analysis of preserved samples. Under the above mentioned original study all patients were informed on the purpose and intent of the study and procedures involved. Written informed consent from all participants (and assent from minor’s parents) were taken. Participation in the study was voluntary. A semi structured questionnaire was used to document personal details and other relevant medical data by the physicians of sentinel surveillance sites. In the main database, every patient was coded with a unique identifier (anonymous, numerical) and their blood samples were sent to the laboratory and the test results were recorded by de-coded the ID(s) by the Principal Investigator (PI) and entered into main database.
Results and Discussion
Prevalence of dengue in three major cities of Bangladesh
Dengue virus infection is considered as a major public health concern due to rapidly progressing geographic distribution of vector infestation. Monitoring of circulating serotypes in past and current infections is crucial for prevention of fatal outcomes in secondary dengue infections and also for patient management. Persistent circulation of four dengue virus serotypes is responsible for frequent epidemics with substantial number of hospitalized patients with DF/ DSS (http://apps.who.int/disasters/repo/13755.pdf). During the period of 2013 to 2016, a total of 1380 blood samples were collected from suspected dengue cases in four tertiary-level health care facilities of three metropolitan cities. All samples were initially tested for dengue IgM by ELISA. The overall dengue IgM positive was 42%, 21%, 16% and 13% in 2013, 2014, 2015 and 2016, respectively (Fig. 1). The result also showed that around 70% of dengue IgM positive cases were reported from Dhaka metropolitan city where two tertiary-level hospitals were included considering the population density and current circumstances. Since the first epidemic of dengue fever in 2000 till 2009, 91% of all reported cases were from Dhaka City [9], ranking the city as highest jeopardy for dengue infection.
Seasonal variation in dengue virus infection
The temporal distribution showed that the dengue infection occurred throughout the year with a single peak persisting for 6 months from April to September (pre-monsoon, monsoon and post-monsoon) (Fig. 2). Despite seasonal peak, dengue cases were detected round the year with sustained transmission (Fig. 2), similar to other dengue endemic countries of Southeast Asia [10]. The abundance of vector Aedes aegypti is proportionate to rainfall, which provides suitable condition for breeding and egg hatching. Similarly, temperature also has influence on mosquito survival, reproduction and virus transmissibility [11]. A study showed that the rainfall provides sufficient breeding habitats of Aedes aegypti, leading to high vector densities [12]. In addition, the extrinsic incubation period (EIP) is critical for viral transmission dynamics which is related to temperature variation; for instance, a rise of temperature from 17 to 30° C increases dengue transmission fourfold [13, 14]. Moreover, Aedes aegypti is a hydrophilic species, so humidity in association with rainfall and temperature increases the epidemic potential of dengue virus [15]. In Bangladesh, though the monsoon season comprises June to August with an average rainfall of 450 mm, considerable amount (~250 mm) of rainfall was also recorded in pre-monsoon (April and May) and post-monsoon (September and October). Similarly, the average monthly temperature was recorded around 28° C from April to October. The relative humidity remains 74%, 86% and 80% during pre-monsoon, monsoon and post-monsoon season, respectively, in the country http://sdwebx.worldbank.org/climateportal/index.cfm?page=country_historical_climate&ThisCCode=BGD. The climatic conditions of Bangladesh clearly demonstrate the appropriateness of vector breeding and viral transmission which makes the country vulnerable for vector-borne diseases including dengue. The vector breeds in household water containers such as those for water storage or for indoor plants and in the disposed water holding vessels like discarded cans, used tires, etc., as well as in the under-constructed buildings; these are abundant in Bangladesh.
Circulating dengue virus serotypes in three major cities of Bangladesh
Among 1380 collected blood samples, 395 samples were tested for dengue by real-time RT-PCR, who had onset of fever within five days. Of them, 141 samples were positive for dengue virus nucleic acid by real-time RT-PCR. The PCR-positive samples were further tested for all 4 dengue serotypes. In 2013, DEN2 was the only serotype that was in circulation in Chittagong and Khulna metropolitan cities, whereas in Dhaka, along with DEN2, DEN1 (31%) was also found to be circulated. In the next year (2014), it was observed that DEN1 began to spread outside Dhaka and emerge in Chittagong, but still Khulna was free from DEN1. In 2015, DEN1 appeared in the circulation along with DEN2. In 2016, DEN2 became the predominant strain with existence of DEN1 in all three cities under surveillance (Table 2). Beside the devastating outcome of dengue fever due to secondary infection with other dengue serotype, individual serotype can cause severe disease in primary infection. For instance, primary infection with DEN1 and DEN3 owing to severe clinical manifestation compared to other serotypes has been reported [16].
In Bangladesh, overall distribution of DEN1 and DEN2 serotype was observed 33.33% and 66.67%, respectively, in all three metropolitan cities during the study period. None of them were positive for either DEN3 or DEN4 during that period (Table 2). However, existence of all four serotypes with predominance of DEN3 (70.5%) was observed in 2000 dengue outbreak in Bangladesh [17], and it was in the circulation till 2002 [17, http://bestpractice.bmj.com/best-practice/monograph/1197/diagnosis/criteria.html]. After that, no DEN3 was reported from Bangladesh. Individuals infected with a particular serotype develop immunity against the same serotype, and if that particular serotype is not in circulation for a period of time, the populations are at risk of developing secondary infection (http://www.who.int/neglected_diseases/diseases/en/). At present, all four serotypes exist in the circulation in the neighboring country India [18]. As in the last fifteen years, no DEN3 was reported, however, the presence of DEN3 and DEN4 in the neighboring country makes Bangladesh vulnerable for an impending secondary dengue outbreak. Vector control is very difficult because of the suitable climatic condition as well as domestic and peridomestic atmosphere. Thus, considering the consequences of secondary infection by other serotypes, there is a need for virological surveillance especially in pre-monsoon and post-monsoon period to prevent large-scale, severe dengue outbreak in the country.
References
Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7.
Guzman MG, et al. Sequential infection as risk factor for dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) during the 1981 dengue hemorrhagic Cuban epidemic. Mem Inst Oswaldo Cruz. 1991;86(3):367.
Guzman MG, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8(12 Suppl):S7–16.
Halstead SB. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11(Supplement 4):S830–9.
Halstead SB. Dengue. The Lancet. 2007;370(9599):1644–52.
Henchal EA, Putnak JR. The dengue viruses. Clin Microbiol Rev. 1990;3(4):376–96.
Johnson BW, Russell BJ, Lanciotti RS. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J Clin Microbiol. 2005;43(10):4977–83.
Mackey TK, Liang BA. Threats from emerging and re-emerging neglected tropical diseases (NTDs). Infect Ecol Epidemiol. 2012;2:18667. https://doi.org/10.3402/iee.v2i0.18667
Pervin M, Tabassum S, Islam M. Isolation and serotyping of dengue viruses by mosquito inoculation technique from clinically suspected cases of dengue fever. Bangladesh Med Res Council Bull. 2002;28(3):104–11.
Pervin M, et al. Isolation and serotyping of dengue viruses by mosquito inoculation and cell culture technique: an experience in Bangladesh. Dengue Bull. 2003;27:81–90.
Raheel U, et al. Dengue fever in the Indian subcontinent: an overview. J Infect Dev Ctries. 2010;5(04):239–47.
Rahman M, et al. First outbreak of dengue hemorrhagic fever, Bangladesh. Emerg Infect Dis. 2002;8(7):738.
Sangkawibha N, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand I. The 1980 outbreak. Am J Epidemiol. 1984;120(5):653–69.
Sharmin S, et al. The emergence of dengue in Bangladesh: epidemiology, challenges and future disease risk. Trans R Soc Trop Med Hyg. 2015;109(10):619–27.
Simmons CP, et al. Dengue. N Engl J Med. 2012;366(15):1423–32.
Tjaden NB, et al. Extrinsic incubation period of dengue: knowledge, backlog, and applications of temperature dependence. PLoS Negl Trop Dis. 2013;7(6):e2207.
Van Kleef E, Bambrick H, Hales S. The geographic distribution of dengue fever and the potential influence of global climate change. TropIKA. net. [serial on the Internet]. Available from: http://journal.tropika.net/scielo.php?script=sci_arttext&pid=S2078-86062010005000001&lng=en (2010). [cited 2018 June 07]
Xuan LTT, et al. Estimates of meteorological variability in association with dengue cases in a coastal city in northern Vietnam: an ecological study. Global Health Action. 2014;7(1):23119.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muraduzzaman, A.K.M., Alam, A.N., Sultana, S. et al. Circulating dengue virus serotypes in Bangladesh from 2013 to 2016. VirusDis. 29, 303–307 (2018). https://doi.org/10.1007/s13337-018-0469-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-018-0469-x