Abstract
Rabies virus (RABV) stimulates nitric oxide (NO) production, which either triggers T cell differentiation or suppresses T cell function depending on its concentration. Herein, we assessed the potential role of NO in regulation of immune responses during RABV infection in mice model. The experimental animals were divided into four groups and 100LD50 of challenge virus standard (CVS) strain of RABV was inoculated intracerebrally on day 0 and subsequently aminoguanidine (AG; inducible nitric oxide synthase inhibitor) was injected intraperitoneally twice a day, up to 6 days. The samples were collected at 2, 4, 6, 8, 9, 10 and 12 days post infection (DPI). The immune cells including CD4+, CD8+ T lymphocytes and natural killer (NK) cells were estimated from peripheral blood mononuclear cells (PBMCs) and splenocytes. Serum total NO concentration, histopathology, immunohistochemistry, direct fluorescent antibody technique and TUNEL assay was performed. Infection with CVS resulted in significant early increase in CD4+, CD8+ and NK cells in blood and spleen until 2 DPI. From 4 DPI onwards significant reduction was noticed in these parameters which coincided with increased NO on 4 DPI, rising to maximum on 8 DPI, until their death on 10 DPI. Conversely, the CVS-AG treated group showed lower levels of NO and increased number of CD4+, CD8+ and NK cells. Increased number of cells in blood and spleen coincided with increased survival time, delayed development of clinical signs, reduced viral load and less apoptotic cells. NO played important role in regulation of immune responses during RABV infection. The findings of present study confirmed the role of NO and/or iNOS using iNOS inhibitor (aminoguanidine) in immune response during RABV infection, which would further help in understanding the virus immunopathogenesis with adoption of newer antiviral strategies to counter the progression of disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rabies is virtually a fatal viral disease of central nervous system (CNS) causing non suppurative encephalitis. Rabies virus (RABV), the causative agent of rabies, is a member of the genus Lyssavirus and family Rhabdoviridae [1–3]. It is a major zoonotic disease of worldwide occurrence and only few countries are free of rabies that includes the islands like Australia, New Zealand and Scandinavian countries [1, 2]. The disease affects all warm-blooded animals, including man. More than 99 % of all human deaths from rabies occur in the developing countries and it is still a great public health problem in Asia, Africa and Latin America.
Even though, CNS is naturally sequestered from the immune system, RABV has the capacity to induce a protective antiviral immune response [1, 4]. Innate and acquired immunity play a vital role in RABV infection. According to previous reports that type 1 T helper (Th1) cell responses are important for RABV clearance from the CNS. Induction of specific cell mediated immunity (CMI) is a crucial factor in the protection of hosts from rabies [5, 6]. The CD8+ T cells are helpful by producing non-cytolytic cytokines through IFN-γ pathway [4]. Studies showed that infection of CNS with antigen results in transport of CNS antigen to lymphoid organs by interstitial and cerebrospinal fluid circulation. This stimulates antigen-specific activation of naive lymphocytes in lymphoid organs and reactivation of lymphoblasts that have migrated into the CNS. The initial peripheral immune response to CNS-derived antigen is characterized by strong antibody response and cytotoxic T cell responses [7, 8]. Activated T cells routinely cross the blood brain barrier (BBB) as part of the normal immunological surveillance of all tissues [9]. Infiltration of natural killer (NK) cells, antigen specific CD4+ and CD8+ T cells, B cells and monocyte/macrophages into the CNS begins 3 to 4 days after infection [9, 10]. The peripheral CMI activity of the spleen cells of mice infected with street RABV increased transiently until 4 DPI, but declined rapidly thereafter until their death [6, 11]. However, in rabies the T cell response was found inefficient being inactivated by the virus [2, 12]. Further, cells undergoing death were leukocytes and not neurons, which were confirmed by immunohistochemical studies [2, 3, 12–14]. The CVS strain of RABV by intracerebral route in mice produces encephalitis with marked neuronal apoptosis [15, 16], whereas peripheral routes of inoculation produces severe and fatal encephalitis without prominent neuronal apoptosis [3, 13, 17–19].
Nitric oxide (NO) is a gaseous and inorganic free radical, which has pleiotropic activities. Nitric oxide synthase in the presence of O2 and NADPH converts l-arginine (l-Arg) into NO [20]. Inflammatory phagocytic cells, epithelial and neuronal cells express inducible NOS (iNOS), which produces larger volume of NO for longer time (10–100 times more). The iNOS has a protective function against bacteria, viruses, fungi and parasites [21]. Macrophages (microglia cells) and NK cells are mainly responsible for the production of NO in brain.
Nitric oxide has been reported to be involved in pathogenesis of neurodegenerative diseases, including rabies [22]. Reduced state of NO (NO−) in RABV infected brains [17], reacted with superoxide (O2 −) to form peroxynitrate, which damage BBB by lipid peroxidation [22, 23]. It also participates in immune response regulation, particularly in the adaptive response by controlling T lymphocyte proliferation [24–26]. Further, NO exerts either proliferative or anti-proliferative response on T cells depending on its concentration. Higher concentrations of NO exert immunosuppressive action and anti-proliferative effect on T cells. Increased concentrations of NO disturbs IL-2R signalling with inhibition of Janus kinases (JAK1 and JAK2) and transcription factor 5 (STAT5) activation [27, 28], and induction of apoptosis in T cells by stimulating p53, caspase and Fas ligand expression [29] or by controlling Bcl-2 members expression [20]. NO from iNOS-expressing cells suppressed mouse T cell proliferation and ameliorated T cell-mediated murine autoimmune diseases such as graft vs-host disease [30]. Low levels of NO promote the differentiation of Th1 (both CD4+ and CD8+ T cells) but not Th2 cells [31]. Low-to-moderate levels of NO were shown to protect T cells from apoptosis [25].
The iNOS is inhibited by l-Arg analogues or chemical aminoguanidine AG [16, 17, 32]. NOS is induced by various neurotrophic viruses like RABV, Borna disease virus, herpes simplex virus type 1 (HSV-1), and others [33]. The NO inhibition and lack of NO biosynthesis significantly reduces the pathological consequences of various virus infections viz., influenza induced viral pneumonia in mice, Sendai virus and HSV-1 induced encephalitis in rats, flavivirus induced murine encephalitis, and encephalomyocarditis virus induced carditis and diabetes [16, 17, 32, 34]. The action of NO in the immunopathogenesis of RABVis not very clear, and the present study was designed to understand the role of NO and/or iNOS using iNOS inhibitor (aminoguanidine) in immune response during rabies virus infection.
Materials and methods
Virus
CVS strain of RABV (laboratory fixed) was obtained from Biological Products Division, ICAR-IVRI, Izatnagar. The mouse inoculation test (MIT) was used for calculation of virus dose using Reed and Muench method and lethal dose 50 (LD50) titre. The aliquots of 100 LD50 titre were stored at −80 °C for future use.
Experimental animals
Swiss albino mice of either sex were obtained from Laboratory Animal Resource (LAR) Section of ICAR-IVRI, Izatnagar. The mice were housed in polypropylene cages, and feed and ad libitum water provided. Controlled environmental conditions like temperature 27 ± 2 °C, relative humidity 30–55 % and 12/12 h light/dark cycle were provided to animals. The mice were maintained as per the guidelines of Institute Animal Ethics Committee (IAEC).
Experimental design and assessment of clinical signs
The mice were divided into 4 groups namely; CVS, CVS-AG, AG control and PBS control group, each consisting of 20 mice. In CVS group, 20 healthy mice were inoculated intracerebrally (i.c.) with 0.03 ml of 100 LD50 mouse dose of RABV on day 0. Aminoguanidine hydrochloride (AG; Sigma-Aldrich, USA) was injected in 40 mice intraperitoneally (i.p.) at a dose rate of 600 mg/kg b. wt twice a day, up to 6 days. Out of these 40 AG treated mice, 20 mice were inoculated with RABV by i.c. route on day 0 and named as CVS-AG group, and 20 mice were kept as AG control. In PBS control group, 20 mice were inoculated by i.c. route with sterile phosphate buffered saline (PBS, pH 7.2). The mice were observed for clinical signs like ruffled fur, tremors, hunchback appearance, in-coordination, paralysis and prostration till 12 days post infection (DPI). Anti-coagulated blood and spleen for estimation of immune cells, serum for total NO estimation and brain for antigen detection, histopathology and TUNEL assay were collected either from dead/sacrificed mice at 2, 4, 6, 8, 9, 10 and 12 DPI. Before inoculation of virus and during collection of blood, mice were anesthetized with ether inhalation.
Peripheral blood mononuclear cell (PBMC) isolation
Anti-coagulated blood was pooled according to experimental groups for isolation of PBMCs as per the method of Stachowiak et al. [35] and diluted with Dulbecco’s Phosphate Buffered Saline (D-PBS; 1x; pH 7.2; ratio 1:1) without calcium and magnesium. The diluted blood was subjected to density gradient separation by gently layering of Histopaque® (Sigma-Aldrich, USA) with density of 1.083 g/ml (ratio 1:1) and centrifuged at 1800 rpm for 10 min at room temperature (RT) which resulted in the separation of PBMCs at the plasma-histopaque interface. The PBMC layer was collected and washed in isotonic PBS (250 g for 10 min) and then re-suspended in 2 ml of RPMI-1640 medium. The cell viability was determined immediately by using trypan blue dye exclusion method and PBS as the diluent.
Separation of splenocytes
Splenocytes were separated from spleen as described by Hirai et al. [11] with slight modifications. Spleen was minced into small pieces and splenocytes were squeezed from the splenic capsule in PBS (pH 7.4) through 70 µm nylon mesh cell strainer in a Petri dish to create single cell suspension by gently mashing spleen pieces with the rubber end of a plunger from a 1 ml tuberculin syringe. The suspensions of dispersed cells were again filtered through cell strainer into a sterile centrifuge tube on ice. Then, cells were centrifuged at 1500 rpm for 10 min at 4 °C and supernatant was discarded. The cell pellet was re-suspended in chilled red blood cell (RBC) lysis buffer and centrifuged at 1500 rpm for 5 min at 4 °C. Supernatant was discarded and pellet was re-suspended in sterile PBS and cell viability was determined immediately by using trypan blue dye exclusion.
Antibodies
Monoclonal antibodies used in the study were mouse anti-CD4+ FITC labelled (Cat no. C2255-28L), mouse anti-CD8+ PE labelled (Cat no. C2259-14Q), mouse anti-CD56 FITC labelled (Cat no. C2410-57E) and mouse anti-CD16 FITC labelled (Cat no. C2267-31 M) procured from US Biological Life Sciences, USA.
Fluorescence-activated cell sorting (FACS)
PBMCs and splenocytes were processed for FACS for estimation of CD4+, CD8+ T lymphocytes and NK cells (CD56−CD16+) percentage in various experimental groups at 2, 4, 6, 8, 9, 10 and 12 DPI using BD FACSCalibur™ Instrument, USA as per the method described by Perry & Lodmell [36]. The cells were incubated with 10 µl of fluorescein isothiocyanate (FITC) and phycoerythrin (PE) conjugated antibodies (1:10 dilution) at 37 °C in dark for 45 min. The tubes were held in icebox in dark and taken for FACS analysis. Ten thousand cells were counted and analysis was performed in the flow cytometer using the cell quest pro software (BD Bioscience, USA).
Estimation of total nitric oxide in serum
Serum samples from various experimental groups at different time intervals were subjected to total NO estimation by colorimetric assay based on Griess reaction (EMSNOTOT, Pierce Biotech, USA) as per the method described by Guevara et al. [37]. Briefly, 200 μl of 1 × reagent diluent was pipetted into duplicate wells in a 96 well plate. 50 μl of diluted nitrate standard and samples were added to 96 well plate, followed by 25 μl of NADH and diluted nitrate reductase and incubated at 37 °C for 30 min. Later, 50 μl of Griess reagent I followed by 50 μl of Griess reagent II were added to all the wells, and incubated at room temperature for 10 min. Optical density (OD) of each well was taken using a micro-plate reader at 550 nm wavelength.
RNA extraction and reverse transcription
Total RNA was isolated from the brain using TRIZOL reagent (Invitrogen, USA). The integrity of the RNA was tested by electrophoresis and then subjected to the first strand cDNA synthesis by reverse transcriptase enzyme before being further amplified by polymerase chain reaction (PCR) using specific primers. Rabies virus glycoprotein (RABV-G) gene primers used were sense 5′ TAATCCCAGAGATGCAATCA 3′ and antisense 5′ CCTCACAGTCTGGTCTCACC 3′.
Histopathology
The mice of different experimental groups were necropsied at 2, 4, 6, 8, 9, 10 and 12 DPI and lesions were recorded. The coronal sections of cerebrum, cerebellum and hippocampus were collected in 10 % neutral buffered formalin. Paraffin embedded sections of 5 μm thickness were prepared and stained with haematoxylin and eosin (H&E). The remaining portions of the brain were collected in RNAlater (Qiagen, USA) and stored at −80 °C.
Direct fluorescent antibody technique (dFAT) and immunohistochemistry (IHC) for rabies antigen detection
The paraffin sections from brain at different DPI intervals were deparaffinised and rehydrated in descending grades of ethanol and then in distilled water. For dFAT, the sections were incubated with 50 μl of anti-nucleocapsid fluorescin conjugated rabies polyclonal antibody in a humidified chamber for 1 h in the dark. Slides were rinsed in PBS (pH 7.2) and mounted with aqueous mountant as per the method described by Baloul et al. [19]. The slides were viewed under fluorescent microscope (Leitz Leica, Germany) for the presence of specific apple green fluorescence signals. IHC was performed at different DPI intervals as per the method described by Hooper et al. [4]. The sections were microwaved in 10 mM tri-sodium citrate buffer (pH 6.0) for antigen retrieval. Endogenous peroxidase activity was quenched by incubating with 0.3 % hydrogen peroxide (H2O2) in 80 % methanol. Non-specific antigen binding sites were blocked with 5 % normal goat serum. Sections were incubated with primary anti-rabies monoclonal antibody for overnight at 4 °C in humidified chamber. Then, the sections were incubated with biotinylated anti-mouse secondary antibody and VECTASTAIN ABC reagent (Vector Biologicals, USA) for 30 min at RT. Sections were incubated with 3, 3′-Diaminobenzidine (DAB) chromogen until desired stain developed and then counter-stained with Meyer’s hematoxylin and mounted in aqueous medium. The distribution and intensity of positive signals were visualized under the light microscope. Quantitative analysis of immunopositive cells was done by counting both positive and negative cells (minimum of 1000 cells) in 15–20 representative high-power fields.
TUNEL staining for detection of apoptosis
Apoptotic cells in the brain sections were detected at different DPI intervals, using TUNEL assay (DeadEnd™ Colorimetric TUNEL System, Promega, USA) by keeping suitable controls as per the method described by Baloul et al. [19]. The sections were fixed in 4 % para-formaldehyde solution and permeabilized by proteinase K (20 μg/ml). The sections were equilibrated with equilibration buffer and rTdT reaction mix (1 μl rTdT enzyme, 98 μl of equilibration buffer and 1 μl of biotinylated nucleotide) was added and incubated for 60 min in humidified chamber to allow end-labelling reaction. The reaction was terminated by adding 2× SSC and washed in PBS to remove unincorporated biotinylated nucleotides. The endogenous peroxidase was blocked with 0.3 % H2O2 and incubated with streptavidin HRP (1:500). DAB was added for colour development, slides were mounted and apoptotic cells were observed under light microscope. Quantitative analysis of TUNEL positive cells was done by counting both positive and negative cells (minimum of 1000 cells) in 15–20 representative high-power fields
Statistical analysis
The data from different experimental groups in respect to various parameters were analysed statistically by one way ANOVA. The results were considered as significant at the confidence level of 95 % (P < 0.05).
Results
Clinical signs
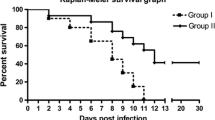
The CVS infected mice exhibited prominent clinical signs like ruffled fur and tremors as early as on 3 DPI, incordination on 4 DPI, paralysis, prostration, mortality and less survival time. In contrast, the CVS-AG treated mice showed delayed onset of less severe clinical signs, which started on 4 DPI. Three mice survived longer in CVS-AG treated group and were sacrificed on 30th day to terminate the experiment. The aminoguanidine and PBS control mice did not show any clinical signs throughout the experiment period.
Kinetics of CD4+ T cells in blood and spleen
The CVS infected and CVS-AG treated groups of mice showed significantly increased number of CD4+ T cells in blood and spleen up to 2 DPI when compared to control groups. The aminoguanidine and PBS control groups showed normal levels and non-significant differences during all time intervals. From 4 DPI onwards, the number of CD4+ T cells started to decrease in CVS alone and CVS-AG treated groups up to 12 DPI. However, CVS alone infected group showed significant decline when compared to CVS-AG treated group. On 9 and 10 DPI, number of CD4+ T cells in blood and spleen was significantly low in CVS alone infected group when compared to CVS-AG treated and control groups. On 12 DPI, the number of CD4+ T cells in blood and spleen was same in AG treated group and both control groups (Fig. 1a, b).
Percentage of CD4+ and CD8+ T lymphocytes in blood and spleen of adult Swiss albino mice inoculated with challenge virus standard (CVS) strain of rabies virus and aminoguanidine (AG) were analysed by FACS using monoclonal antibodies. CVS: mice inoculated intracerebrally (i.c.) with CVS strain of rabies virus. CVS + AG: mice were injected with AG intraperitoneally (i.p.) at 600 mg/kg body weight twice a day up to 6 days and CVS strain of rabies by i.c. route. AG: mice were injected with only AG by i.p. route. PBS: mice were inoculated with sterile phosphate buffer saline (PBS, pH 7.2) by i.c. route and sacrificed at 2, 4, 6, 8, 9, 10 and 12 days post infection (DPI). a Percentage of CD4+ T lymphocytes in blood. b Percentage of CD4+ T lymphocytes in spleen. c Percentage of CD8+ T lymphocytes in blood. d Percentage of CD8+ T lymphocytes in spleen. e Mean CD4+/CD8+ ratio of T lymphocytes in blood. f Mean CD4+/CD8+ ratio of T lymphocytes in spleen. The data are expressed as mean ± SD at each time point. Error bars indicate the SD
Kinetics of CD8+ T cells in blood and spleen
The CD8+ subset of T lymphocytes behaved in a similar way to that of CD4+ T cells in blood and spleen. The CVS alone and CVS-AG treated groups showed significantly increased number of CD8+ T cells in blood and spleen up to 2 DPI, when compared to both control groups. The aminoguanidine and PBS control groups showed normal levels and non-significant differences on all intervals. From 4 DPI onwards, the number of CD8+ T cells started to decrease in CVS alone and CVS-AG treated groups up to 12 DPI. However, CVS alone infected group showed significant decline, when compared to CVS-AG treated group. On 9 and 10 DPI, number of CD8+ T cells in blood and spleen was significantly low in CVS alone infected group when compared to CVS-AG treated and control groups. On 12 DPI, the number of CD8+ T cells in blood and spleen was same in both AG treated groups and PBS control group (Fig. 1c, d).
CD4+/CD8+ ratio in blood and spleen
The CD4+/CD8+ ratio was found to be significantly increased in CVS alone infected group, whereas CVS-AG treated group showed decreased ratio until 8 DPI in blood. The CD4+/CD8+ ratio decreased from 9 DPI onwards in CVS alone infected group in blood, whereas CVS-AG treated group showed increased ratio. The CD4+/CD8+ ratio was found to be decreased in CVS alone infected group, whereas CVS-AG treated group showed increased ratio until 8 DPI in spleen. The CD4+/CD8+ ratio increased from 9 DPI onwards in CVS alone infected group in spleen, whereas CVS-AG treated group showed decreased ratio (Fig. 1e, f).
Kinetics of natural killer cells in blood and spleen
The CVS infected and CVS-AG treated groups showed significantly increased number of natural killer cells in blood and spleen up to 2 DPI, when compared to both control groups. The aminoguanidine and PBS control groups showed normal levels and non-significant differences on all intervals. From 4 DPI onwards, the number of NK cells started to decrease in CVS infected and CVS-AG treated groups up to 12 DPI. However, CVS alone infected group showed significant decline, when compared to CVS-AG treated group. On 9 and 10 DPI, number of NK cells in blood and spleen was significantly low in CVS alone infected group when compared to CVS-AG treated and control groups. On 12 DPI, the number of NK cells in blood and spleen was same in both AG treated groups and PBS control group (Fig. 2a, b).
Percentage of natural killer cells in blood and spleen of adult Swiss albino mice inoculated with challenge virus standard (CVS) strain of rabies virus and aminoguanidine (AG) were analysed by FACS using monoclonal antibodies. CVS: mice inoculated intracerebrally (i.c.) with CVS strain of rabies virus. CVS + AG: mice were injected with AG intraperitoneally (i.p.) at 600 mg/kg body weight twice a day up to 6 days and CVS strain of rabies by i.c. route. AG: mice were injected with only AG by i.p. route. PBS: mice were inoculated with sterile phosphate buffer saline (PBS, pH 7.2) by i.c. route and sacrificed at 2, 4, 6, 8, 9, 10 and 12 days post infection (DPI). a Percentage of natural killer cells in blood. b Percentage of natural killer cells in spleen. The data are expressed as mean ± SD at each time point. Error bars indicate the SD
Nitric oxide assay and detection of rabies virus
The CVS infected mice had significantly increasing levels of serum nitrate from 2 to 12 DPI when compared to both AG treated groups and PBS control group. CVS-AG treated groups had low levels of serum nitrate throughout the experimental period. No significant variations were noticed among control groups (Fig. 3). Rabies viral glycoprotein gene (RABV-G) was detected in brain samples of CVS infected and CVS-AG treated groups by amplification of a 406 bp gene product by RT-PCR and visualized in 1 % agarose gel (Fig. 4).
Nitric oxide concentration (μM/L) in serum of various groups of adult Swiss albino mice inoculated with challenge virus standard (CVS) strain of rabies virus and aminoguanidine (AG) were analysed using Griess reagent. CVS: mice inoculated intracerebrally (i.c.) with CVS strain of rabies virus. CVS + AG: mice were injected with AG intraperitoneally (i.p.) at 600 mg/kg body weight twice a day up to 6 days and CVS strain of rabies by i.c. route. AG: mice were injected with only AG by i.p. route. PBS: mice were inoculated with sterile phosphate buffer saline (PBS, pH 7.2) by i.c. route and sacrificed at 2, 4, 6, 8, 9, 10 and 12 days post infection (DPI). The data are expressed as mean ± SD at each time point. Error bars indicate the SD. The mean with common letters at a given DPI does not differ significantly (p < 0.05). The mean with different letters (a, b, c, d) at a given DPI differs significantly (p < 0.05) from each other
Histopathology
The CVS infected mice showed perivascular cuffing with mononuclear cells and mild meningitis on 2 DPI. Moderate degree of perivascular cuffing, meningitis and foci of glial nodules were noticed on 4 DPI. Diffuse microgliosis, severe perivascular cuffing and more degree of neuronal damage in hippocampus (CA1 and CA2 regions) on 6 and 8 DPI (Fig. 5a). More gitter cells, pyknosis and karyorhexis of the neurons and many apoptotic bodies were noticed in cerebral hemisphere and hippocampus on 10 DPI. Perivascular cuffing and gliosis were noticed on all intervals in cerebellum.
Histopathological findings and direct fluorescent antibody technique (dFAT) in CVS alone infected (a and c) and aminoguanidine treated (b and d) groups on 8 DPI. a Perivascular cuffing with mononuclear cells and diffuse gliosis with neuronal degeneration in cerebral cortex. H&E × 200. b Mild perivascular cuffing with mononuclear cells in cerebral cortex. H&E ×200. c Brain showing strong and diffuse positive signals with bright apple green fluorescence of rabies antigen in parenchyma. dFAT ×200. d AG treated mice brain showing mild, weak and focal positive signals. dFAT ×200
The CVS-AG treated mice showed mild degree of meningitis and perivascular cuffing in the cerebral hemisphere and hippocampus on 2, 4, 6 and 8 DPI (Fig. 5b). Mild changes like slight degeneration of neurons and moderate degree of gliosis were noticed in different parts of the brain including CA1 and CA2 regions of hippocampus on 10 and 12 DPI. The aminoguanidine and PBS control groups did not show any histopathological lesions in the brain.
dFAT, IHC and TUNEL assay
The CVS infected and CVS-AG treated brain sections showed positive signals like apple green fluorescence for dFAT and brown signals for IHC as early as on 4 DPI in cerebral cortex, cornua ammonis (CA1 and CA2 regions) of hippocampus and cerebellum. However, the intensity of positive signals was more in CVS alone infected group (Figs. 5c, 6a) when compared to CVS-AG treated group till 12 DPI (Figs. 5d, 6b). The IHC signals were more intense and significantly higher number of positive neurons were observed in cerebral cortex of CVS infected mice (Fig. 3a) than CVS-AG treated mice, which showed mild signals and less number of positive neurons (Fig. 3b). The quantification of immunopositive neurons in brain of CVS infected and CVS-AG treated mice has been presented in Fig. 6c. Sections from CVS infected brain showed more TUNEL positive apoptotic cells in cerebrum (Fig. 7a), but the TUNEL positive apoptotic cells were few in CVS-AG treated brain sections (Fig. 7b). The quantification of TUNEL positive apoptotic cells in brain of CVS infected and CVS-AG treated mice has been presented in Fig. 7c.
Immunohistochemistry in CVS infected and CVS-AG treated groups. a Cerebral cortex showing strong positive signals for rabies antigen in neuronal cytoplasm on 8 DPI. IHC-DAB-MH ×200. b Cerebral cortex showing weak positive signals of rabies antigen on 8 DPI. IHC-DAB-MH ×200. c Quantification of immunopositive cells in CVS infected and CVS-AG treated groups. Mean with *indicates statistically significant (p < 0.05). Error bars indicate the SD
TUNEL assay in CVS infected and aminoguanidine treated groups. a Brain showing brown colour more TUNEL positive apoptotic cells on 8 DPI. ×200. b Brain showing few TUNEL positive apoptotic cells on 8 DPI. ×200. c Quantification of TUNEL positive apoptotic cells in CVS infected and CVS-AG treated groups. Mean with *indicates statistically significant (p < 0.05). Error bars indicate the SD
Discussion
The present study was aimed to understand the role of NO and/or iNOS using aminoguanidine (iNOS inhibitor) in the regulation of immune responses during rabies virus infection. CVS alone infected group showed more clinical signs, histopathological lesions and mortality when compared to CVS-AG treated group. Recently, we reported that severe clinical signs and histopathological lesions in CVS alone infected group could be due to increased NO levels, pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) and iNOS mRNA levels in brain [16]. CVS-AG treated group showed increased survival time, delay in development and decreased intensity of clinical signs and histopathological lesions in mice with decreased NO, iNOS and TNF-α levels [16, 17]. Prolonged survival time was correlated with reduced viral load, which was evident by real-time PCR, reduced fluorescent and IHC signals in brain, suggesting the role of NO in rabies virus replication. NO has been reported to be involved in the pathogenesis of neurodegenerative diseases by damaging the BBB [22, 38]. CVS strain of fixed RABV in suckling, neonatal and adult mice produces encephalitis with marked neuronal apoptosis in various regions of brain when inoculated intracerebrally [15, 16]. In contrast, peripheral routes of inoculation of CVS in adult mice showed severe and fatal encephalitis without prominent neuronal apoptosis [3, 13, 17–19].
Even though CNS is an immunoprivileged site, CMI plays vital role in limiting the viral pathogens targeting the brain. The role of both CD4+ and CD8+ T cells in antiviral immunity against rabies in brain has been studied [1, 4, 6, 19, 36]. In the present study, CVS strain of RABV infection resulted in significant early increase in CD4+, CD8+ T lymphocytes and NK cells in blood and spleen until 2 DPI, but thereafter significant reductions in level of these cells were noticed until their death on 10 to 12 DPI, when compared to CVS-AG treated group. The early increase in peripheral cell mediated immunity in blood and spleen but rapid declining of CMI thereafter until their death on 10 to 12 DPI were similar to the observations of earlier workers [11, 19, 39]. The early increase in CD4+, CD8+ T lymphocytes and NK cells in blood and spleen might be due to immune response directed against rabies virus by the host after exposure to prevent viral replication [39]. The activation of naive T and B cells in peripheral secondary lymphoid organs occurs during viral infections of CNS and activated T cells will cross the BBB to reach the CNS [7–9]. Further, nucleocapsid of RABV acted as super antigen in mice and resulted in stimulation of T cell proliferation, and also decreased nitric oxide levels up to 4 DPI could be the reasons for early increased immune cells in blood and spleen [16, 23].
The CD4+/CD8+ ratio was found to be significantly increased in blood of CVS alone infected group between 4 to 8 DPI, which indicates cell mediated immune response plays major role during rabies virus infection. In spleen, increased CD4+/CD8+ ratio (normal 2:1) in CVS alone infected group indicated cell mediated immune response, whereas CVS-AG treated group showed cell mediated immune response up to 8 DPI and there after humoral immune response.
In this study, less number of CD4+, CD8+ T lymphocytes and NK cells in blood and spleen from 4 DPI onwards were likely due to the action of nitric oxide. It is proved that infection with CVS strain of RABV in mice resulted in 30-fold increase in NO levels [16, 23], iNOS mRNA expression [16, 40, 41] and iNOS enzyme activity [16, 17, 33]. Also, it was well established that high concentration of NO showed immunosuppressive action by its anti-proliferative effects on T cells [24, 26, 30, 42], blocking of IL-2R signaling [27, 30] and induced T cell apoptosis by caspase or Fas or p53-dependent mechanisms [25, 26, 29, 43]. Recently, we reported that CVS strain of RABV up regulates caspase 1 and FasL mRNA expressions and more TUNEL positive apoptotic bodies in brain, which was positively correlated with intensity of clinical signs and mortality [16]. These findings were corroborated with the observation of Ubol et al. [17] and Baloul et al. [19] reported that CVS strain of RABV infection triggered capsae-1 and Fas ligand (FasL) mRNA and protein expression in brain results in destruction of migratory T cells by Fas/FasL apoptosis pathway, which is an immuno-subversive strategy for RABV.
The CVS strain of RABV had the ability to kill protective migrating CD8+ T cells [2, 3, 12–14, 18, 19] in brain by up regulation of immunoevasive proteins such as B7-H1 and activation of Fas molecules on their surface, after binding to FasL. Recently, we reported that NO inhibition resulted in reduced rabies viral load in brain and decreased expression of apoptotic genes like caspase 1 and FasL and less apoptotic bodies in brain [16]. Ubol et al. [17] demonstrated that iNOS inhibition resulted significant reduction in caspase 1 expression. Nitric oxide can prevent or induce apoptosis depending on its concentration, cell type, and the oxidative milieu. NO plays a signalling role in Fas/Fas ligand-mediated apoptotic death in mature T lymphocytes [44]. These findings suggest that NO-mediated apoptosis play major role in pathogenesis and regulating immune responses during rabies virus infection. The increased number of CD4+, CD8+ T lymphocytes and NK cells in blood and spleen in CVS-AG treated group might be due to reduced rabies viral load in brain, which resulted in less killing of protective migrating T cells by rabies virus due to low expression of caspase 1 and FasL.
Aminoguanidine reduces the NO production and it is well established that low concentration of NO enhances Th1 response causing proliferation of CD4+ and CD8+ T lymphocytes and stimulating the production of secondary messenger cGMP with increased expression of IL-12Rβ2 expression and IFN-γ secretion by T cells [28]. It was found that increased numbers of CD4+, CD8+ T lymphocytes and NK cells significantly increased the survival time of mice and delay in development of clinical signs in rabies virus infection. Low concentrations of NO also exhibit anti-apoptotic effects, which was evident by low caspase-1 and FasL mRNA expressions in brain and lower number of apoptotic cells [16]. Kiang et al. [45] reported that inhibition of inducible nitric-oxide synthase prevents T cell apoptosis induced by hypoxia. These findings were similar to the observations of Lane et al. [32] reported that inhibition of nitric oxide synthase-2 (NOS2) by AG resulted in significant decrease in the severity of clinical signs of mouse hepatitis virus and reduced severity of virus-induced demyelination. Infection with more virulent strains of influenza (Spanish flu) stimulates higher production of NO and blocking of iNOS with NG-Monomethyl-l-arginine increased the survival of mice and delayed the development of pneumonia [46]. Li et al. [47] reported that Ivabradine has therapeutic potential against acute coxsackievirus B3-induced myocarditis due to its inhibitory effect on inducible nitric oxide synthase. Based on these findings, the role of nitric oxide in the regulation of innate and adaptive immune responses in rabies virus infection has become evident.
The RABV infection results in significant early increase in CD4+, CD8+ T lymphocytes and NK cells in blood and spleen, but thereafter significant reductions in level of these cells were noticed, when compared to aminoguanidine treated group. Less number of cells in CVS infected group was due to increased NO. The aminoguanidine treated group showed increased number of cells, which was positively correlated with increased survival time, delay in development and decreased intensity of clinical signs and histopathological lesions in mice with decreased NO, iNOS and TNF-α levels. Low concentrations of NO also exhibit anti-apoptotic effects, which was evident by low caspase-1 and FasL mRNA expressions and lower number of apoptotic cells in brain. The results of this study have confirmed the potential role of NO in the regulation of immune responses during RABV infection. However, more studies including gene knock-out and immunohistochemical demonstration of cells are required to clarify the mechanism of action of NO in the regulation of immune responses during RABV infection.
References
Consales CA, Bolzan VL. Rabies review: immunopathology, clinical aspects and treatment. J Venom Anim Toxins incl Trop Dis. 2007;13(1):5–38.
Lafon M. Evasive strategies in Rabies virus infection. In: Jackson AC, editor. Advances in virus research. Burlington, MA: Academic Press; 2011. p. 3355.
Kojima D, Park CH, Satoh Y, Inoue S, Noguchi A, Oyamada T. Pathology of the spinal cord of C57BL/6 J mice infected with rabies virus (CVS-11 strain). J Vet Med Sci. 2009;71(3):319–24.
Hooper DC, Morimoto K, Bette M, Weihe M, Koprowski H, Dietshchold B. Collaboration of antibody and inflammation in the clearance of rabies virus from the CNS. J Virol. 1998;72:3711–9.
Kawano H, Mifune K, Ohuchi M, Mannen K, Oho S, Hiramatsu K, Shichijo A. Protection against rabies in mice by a cytotoxic T cell clone recognizing the glycoprotein of rabies virus. J Gen Virol. 1990;71:281–7.
Venkataswamy MM, Madhusudana SN, Sanyal SS, Taj S, Belludi AY, Mani RS, Hazra N. Cellular immune response following pre-exposure and postexposure rabies vaccination by intradermal and intramuscular routes. Clin Exp Vaccine Res. 2015;4(1):68–74.
Harling-Berg CJ, Park TJ, Knof PM. Role of the cervical lymphatics in the Th2 type hierarchy of CNS immune regulation. J Neuroimmunol. 1999;101:111–27.
Louveau A, Harris TH, Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015;36(10):569–77.
Engelhardt B. Regulation of immune cell entry into the central nervous system. Results Probl Cell Differ. 2006;43:259–80.
Dörries R. The role of T cell mediated mechanisms in virus infections of the nervous system. Curr Top Microbiol Immunol. 2001;253:219–45.
Hirai K, Kawano H, Mifune K, Fujii H, Nishizono A, Shichijo A, Mannen K. Suppression of cell-mediated immunity by street rabies virus infection. Microbiol Immunol. 1992;36(12):1277–90.
Lafon M. Immune evasion, a critical strategy for rabies virus. Dev Biol. 2008;31:413–9.
Rossiter JP, Hsu L, Jackson AC. Selective vulnerability of dorsal root ganglia neurons in experimental rabies after peripheral inoculation of CVS-11 in adult mice. Acta Neuropathol. 2009;118(2):249–59.
Lafon M. Modulation of the immune response in the nervous system by rabies virus. Curr Top Microbiol Immunol. 2005;289:239–58.
Rutherford M, Jackson AC. Neuronal apoptosis in immunodeficient mice infected with the challenge virus standard strain of rabies virus by intracerebral inoculation. J Neurovirol. 2004;10(6):409–13.
Madhu BP, Singh KP, Saminathan M, Singh R, Tiwari AK, Manjunatha V, Harish C, Manjunathareddy GB. Correlation of inducible nitric oxide synthase (iNOS) inhibition with TNF-α, caspase-1, FasL and TLR-3 in pathogenesis of rabies in mouse model. Virus Genes. 2016;52(1):61–70.
Ubol S, Sukwattanapan C, Maneerat Y. Inducible nitric oxicide synthase inhibition delays death of rabies virus-infected mice. J Med Microbiol. 2001;50:238–42.
Baloul L, Lafon M. Apoptosis and rabies virus neuroinvasion. Biochimie. 2003;85(8):777–88.
Baloul L, Camelo S, Lafon M. Up-regulation of Fas ligand (FasL) in the central nervous system: a mechanism of immune evasion by rabies virus. J Neurovirol. 2004;10:372–82.
Bosca L, Zeini M, Traves PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005;208:249–58.
Schairer DO, Chouake JS, Nosanchuk JD, Friedman AJ. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence. 2012;3(3):271–9.
Yuste JE, Tarragon E, Campuzano CM, Ros-Bernal F. Implications of glial nitric oxide in neurodegenerative diseases. Front Cell Neurosci. 2015;9:322.
Hooper DC, Ohnishi ST, Kean R, Numagami Y, Dietzschold B, Koprowski H. Local nitric oxide production in viral and autoimmune diseases of the central nervous system. Proc Natl Acad Sci USA. 1995;92(12):5312–6.
Niedbala W, Cai B, Liew FY. Role of nitric oxide in the regulation of T cell functions. Ann Rheum Dis. 2006;65:iii37–40.
Ibiza S, Serrador JM. The role of nitric oxide in the regulation of adaptive immune responses. Inmunología. 2008;27(3):103–17.
Uehara EU, Shida BS, de Brito CA. Role of nitric oxide in immune responses against viruses: beyond microbicidal activity. Inflamm Res. 2015;64(11):845–52.
Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160:5729–34.
Niedbala W, Wei XQ, Campbell C, Thomson D, Komai-Koma M, Liew FY. Nitric oxide preferentially induces type 1 T cell differentiation by selectively up-regulating IL-12 receptor beta 2 expression via cGMP. Proc Natl Acad Sci USA. 2002;99:16186–91.
Li CQ, Trudel LJ, Wogan GN. Nitric oxide-induced genotoxicity, mitochondrial damage, and apoptosis in human lymphoblastoid cells expressing wild-type and mutant p53. Proc Natl Acad Sci USA. 2002;99:10364–9.
Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95.
Niedbala W, Wei XQ, Piedrafita D, Xu D, Liew FY. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur J Immunol. 1999;29:2498–505.
Lane TE, Fox HS, Buchmeier MJ. Inhibition of nitric oxide synthase-2 reduces the severity of mouse hepatitis virus-induced demyelination: implications for NOS2/NO regulation of chemokine expression and inflammation. J Neurovirol. 1999;5(1):48–54.
Akaike T, Weihe E, Schaefer MK-H, Fu ZF, Zheng YM, Vogel WH, Schmidt HHHW, Koprowski H, Dietzschold B. Effect of neurotropic virus infection on neuronal and inducible nitric oxide synthase activity in rat brain. J Neurovirol. 1995;1:118–25.
Hirasawa K, Jun HS, Hans HS, Zhang ML, Hollenberg MD, Yoon JW. Prevention of encephalomyocarditis virus-induced diabetes in mice by inhibition of the tyrosine kinase signalling pathway and subsequent suppression of nitric oxide production in macrophages. J Virol. 1999;73:8541–8.
Stachowiak R, Łyżniak M, Grabowska M, Roeske K, Jagielski T, Bielecki J, Budziszewska BK, Hoser G, Kawiak J. Cytotoxicity of purified listeriolysin O on mouse and human leukocytes and leukaemia cells. BMC Biotechnol. 2014;14:77.
Perry LL, Lodmell DL. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J Virol. 1991;65(7):3429–34.
Guevara I, Iwanejko J, Dembinska-Kiec A, Pankiewicz J, Wanat A, Anna P, Golabek I, Bartus S, Malczewska-Malec M, Szczudlik A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin Chim Acta. 1998;274:177–88.
Wang L, Hagemann TL, Kalwa H, Michel T, Messing A, Feany MB. Nitric oxide mediates glial-induced neurodegeneration in Alexander disease. Nat Commun. 2015;6:8966.
Wiktor TJ, Doherty PC, Koprowski H. Suppression of cell-mediated immunity by street rabies virus. J Exp Med. 1977;145(6):1617–22.
Koprowski H, Zheng YM, Heber-Katz E, Fraser N, Rorke L, Fu ZF, Hanlon C, Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci USA. 1993;90:3024–7.
Nakamichi K, Inoue S, Takasaki T, Morimoto K, Kurane I. Rabies virus stimulates nitric oxide production and CXC chemokine ligand 10 expression in macrophages through activation of extracellular signal-regulated kinases 1 and 2. J Virol. 2004;78(17):9376–88.
Vig M, Srivastava S, Kandpal U, Sade H, Lewis V, Sarin A, George A, Bal V, Durdik JM, Rath S. Inducible nitric oxide synthase in T cells regulates T cell death and immune memory. J Clin Invest. 2004;113(12):1734–42.
Kim PK, Kwon YG, Chung HT, Kim YM. Regulation of caspases by nitric oxide. Ann N Y Acad Sci. 2002;962:42–52.
Williams MS, Noguchi S, Henkart PA, Osawa Y. Nitric oxide synthase plays a signaling role in TCR-triggered apoptotic death. J Immunol. 1998;161:6526–31.
Kiang JG, Krishnan S, Lu X, Li Y. Inhibition of inducible nitric-oxide synthase protects human T cells from hypoxia-induced apoptosis. Mol Pharmacol. 2008;73(3):738–47.
Perrone LA, Belser JA, Wadford DA, Katz JM, Tumpey TM. Inducible nitric oxide contributes to viral pathogenesis following highly pathogenic influenza virus infection in mice. J Infect Dis. 2013;207:1576–84.
Li YC, Luo Q, Ge LS, Chen YH, Zhou ND, Zhang T, Guan XQ, Lin JF. Ivabradine inhibits the production of proinflammatory cytokines and inducible nitric oxide synthase in acute coxsackievirus B3-induced myocarditis. Biochem Biophys Res Commun. 2013;431(3):450–5.
Acknowledgments
We thank the Director and Joint Directors, ICAR- Indian Veterinary Research Institute for providing all the facilities to carry out this research work. The financial support in the form of Junior Research Fellowship (JRF) from the ICAR, New Delhi, India, to first author is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Madhu, B.P., Singh, K.P., Saminathan, M. et al. Role of nitric oxide in the regulation of immune responses during rabies virus infection in mice. VirusDis. 27, 387–399 (2016). https://doi.org/10.1007/s13337-016-0343-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-016-0343-7