Abstract
White spot syndrome virus (WSSV) is one of the major pathogens in shrimp aquaculture. Four proteins of WSSV are predicted to encode a RING H2 domain, which in presence of ubiquitin conjugating enzyme (E2) in shrimps can function as viral E3 ligase and modulate the host ubiquitin proteasome pathway. Modulation of host ubiquitin proteasome pathway by viral proteins is implicated in viral pathogenesis. In the present study, expression profile of Penaeus monodon Ubiquitin conjugating enzyme (PmUbc) was studied at protein level in WSSV challenged shrimp. A time point analysis of the expression of PmUbc was carried out at 0, 3, 6, 12, 24, 48 and 72 h post WSSV challenge in P. monodon. Recombinant PmUbc (rPmUbc) was produced in prokaryotic expression vector, BL21 (DE3) pLys S. The PmUbc expression pattern was studied by ELISA with rPmUbc antibodies raised in rabbit. A significant increase in PmUbc expression at 24 h post infection (hpi) was observed followed by a decline till 72 hpi. Since the up-regulation and a tremendous decline of PmUbc protein expression was observed at 24 and in 72 hpi respectively in ELISA, it can be speculated that these proteins might interact with host ubiquitination pathway for viral pathogenesis. Many findings have shown that viral infection can up-regulate expression of ubiquitin and that the ubiquitin system plays a key role in the course of viral infection. The present study reveals the expression patterns of PmUbc at protein level in WSSV infected P. monodon. However, further studies are to be carried out to unfold the molecular mechanism of interaction between host and virus to devise efficient control strategies for this major culprit in shrimp culture industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viruses are ubiquitous and extremely abundant in the marine environment. One of such marine viruses, white spot syndrome virus (WSSV), has emerged globally as one of the most prevalent, widespread and lethal for shrimp populations [1]. White spot syndrome virus is responsible for 100 % mortality within a few days after the onset of infection and is a serious threat to the shrimp culture industry worldwide [21]. The virus had, and still has the greatest impact in shrimp aquaculture. White spot syndrome virus, that causes “white spots” in the exomesoderm under the carapace, remains as a major pathogen in shrimp aquaculture industry. The causative agent, WSSV is enveloped, large circular double stranded DNA virus which has a wide host range among not only shrimp species but also many other crustaceans [4]. Due to the impact that WSSV has caused to shrimp cultures all over the world, several approaches have been used for the management of the disease. To understand the pathogenesis of any disease, knowledge of interactions between virus and host is critical. Virus-host interactions may result in immune response against the invader, and also result in changes in the expression levels of host genes that favour virus replication [27].
Viruses employ many fascinating strategies to infiltrate the host line of defense. One of the interesting and relevant mechanisms is through the manipulation of the host’s own ubiquitination pathway, where the host proteins are redirected for degradation in the 26S proteasome. Viruses have evolved to use cellular pathways to their advantage, including the ubiquitin proteasome pathway of protein degradation. This specific process often involves an E3 ubiquitin ligase that is directly encoded either by the virus or the host genome [2]. In several cases, viruses synthesize proteins that highjack cellular E3 ligases to modify their substrate specificity in order to eliminate unwanted cellular proteins, in particular inhibitors of the cell cycle. They can also inhibit E3 ligases to prevent specific protein degradation or even use the system to control the level of expression of their own proteins [3]. Certain ubiquitin conjugating enzymes interact with the RING finger proteins that may play roles as E3 s in the ubiquitin–proteasome dependant pathway [17,20]. RING finger domain of certain viral proteins mimic E3 ubiquitin protein ligase of the infected animals. In WSSV four WSSV proteins, WSSV199, WSSV222, WSSV249, and WSSV403 are reported to be functioning as ubiquitin ligase of shrimp due to the presence of RING finger domain which helps in ubiquitination. WSSV 249, acting as an E3 ligase, sequesters the shrimp E2-ubiquitin-conjugating enzyme (PvUbc) for viral pathogenesis in Litopenaeus vannamei [26]. Fang et al. [11] reported that putative protein WSSV 222 has a RING finger domain and act as RING H2 E3 ligase. WSSV222 is a E3 ubiquitin protein ligase from WSSV that can specifically interact with an E2-conjugating enzyme and mediate transfer of ubiquitin to a specific substrate protein. WSSV 403 is a latency associated gene and possess C3H2C3-type RING finger which is involved in ubiquitination [10].
The mechanisms involved in the interaction between the virus and the host at molecular level, from viral entry, through replication, enhanced cell survival and finally viral release is yet to be explored. In this context the present study was taken up to see the expression profile of shrimp ubiquitin conjugating enzyme in WSSV infected P. monodon through a time course approach at protein level.
Materials and Methods
Shrimp Rearing
Penaeus monodon of 15 ± 2 g size were transported from Pancham Aqua farm, Maharashtra, India and maintained in 1,000 l Fibreglass reinforced plastic (FRP) tanks (25 shrimp/tank) in natural seawater of 35 ppt with continuous aeration. The shrimp were fed adlib with artificial pelleted feed (CP feeds) twice a day. Left over feed was siphoned daily and 30 % water exchange was done once in a week. Salinity was maintained at 35 ppt, temperature 22–25 °C and pH 7.8 throughout the experimental period and the health of the animals was monitored regularly. Shrimps were held for a minimum of 2 weeks prior to experimental use and feeding was stopped 24 h before treatment.
Preparation of Viral Inoculum
White spot syndrome virus infected P. monodon with prominent white spots were collected and head soft tissues were homogenized in chilled sterile 1X Phosphate Buffered Saline (PBS), pH 7.4. The homogenate was centrifuged at 2,460×g for 20 min at 4 °C. The supernatant was again subjected to centrifugation to remove the cell debris. The supernatant was filtered (0.22 μ-pore diameter-filter) and the presence of the viral particles in the inoculum was confirmed with WSSV detection kit (Bangalore Genei, India) following manufacturer’s instructions. Infectivity of the viral inoculum was confirmed by in vivo titration with healthy shrimp. The viral dose that caused 100 % mortality in 96 h was used for time course analysis.
Construction of Plasmid
The total RNA was isolated from the gills of P. monodon and cDNA was prepared. BamHI–HindIII fragments of PmUbc were obtained by PCR amplification of cDNA using the primers PmUbc-5 (5′-GCGGATCCTTGATGTCTGCCTCGCCTGCTG-3′) and PmUbc-3 (5′-CGAAGCTTACCCAAATTGGCATGGTGAAAG-3′). The PCR cycling conditions included an initial denaturation at 94 °C for 6 min followed by amplification for 30 cycles at 94 °C for 30 s, 64 °C for 30 s and 72 °C for 1 min. The final extension was done for 8 min. The PmUbc amplicon was introduced and cloned into prokaryotic expression vector pRSET A (Invitrogen, USA) plasmid downstream to the T7 promoter and in frame with N-terminal His tag with the help of BamHI and HindIII restriction enzymes and transformed into Escherichia coli (DH5α) cells. The plasmid was validated by DNA sequencing (Bioserve Technologies, Hyderabad). The plasmid was then isolated using QIAprep Spin Miniprep Kit (Qiagen, USA).
Protein Expression
The BL21 (DE3) pLysS cells were transformed with pRSET-PmUbc and the PmUbc expression was induced with 1 mM IPTG and confirmed by SDS-PADE on a 12 % acrylamide gel followed by western blot analysis using anti-his antibody following [25]. The expression of PmUbc protein was optimized by a time course study using different concentrations of IPTG. The rPmUbc protein was purified from the bacterial cells using Ni–NTA Agarose (Qiagen, Germany) following manufacturer’s instructions.
Production of Polyclonal Antibody Against rPmUbc Protein
For production of polyclonal antibody against rPmUbc, healthy New Zealand white rabbits were immunized with 200 μg of purified recombinant PmUbc protein mixed with Freund’s complete adjuvant (FCA) at 1:1 ratio. Initial dose was followed by booster doses on 7th, 14th, 21st and 28th day of immunization, subcutaneously with the same dose of purified rPmUbC emulsified with Freund’s incomplete adjuvant (FIA). The rabbit was bled on 35th day of immunization through marginal ear vein. The collected blood was allowed to clot at room temperature and left at 4 °C overnight. Blood samples were centrifuged at 2,460×g for 10 min and the serum was separated, aliquoted and preserved at −20 °C for further use. The titre value of the anti-rPmUbc serum was calculated by ELISA with serial dilutions. The antiserum was used to study the expression levels of the PmUbc protein in P. monodon on infection with WSSV.
Expression Profiling of PmUbc
Experimental Conditions
Four groups of 6 shrimps (15 ± 2 g) each in triplicates were maintained in plastic crates of 25 l capacity with adequate aeration. After acclimatization for 3 days, three groups of shrimps were injected with 100 μl of WSSV inoculum ventral to the second abdominal segment. The unchallenged fourth group served as control. Samples were collected at 0 h and six time points post challenge at 3, 6, 12, 24, 48 and 72 h to quantify the ubiquitin conjugating enzyme in comparison to unchallenged control and a total of 12 shrimps were collected at each time points from all groups including control.
Enzyme Linked Immuno Sorbent Assay
The expression pattern of PmUbc in WSSV challenged shrimp was quantified using indirect ELISA. For this, 50 μl of Ubc protein extracted from the gills of WSSV challenged shrimps was coated at the rate of 100 ng/well in an ELISA plate. The plate was incubated at room temperature for 2 h and washed three times with PBST (Phosphate Buffered Saline Tween 20). An equal volume of anti rPmUbc rabbit IgG (50 μl), (1:15,000 in PBS) was added to all the wells and incubated for 1 h. The wells were again washed thrice with PBST. Anti-rabbit HRPO conjugate, 50 μl, (1/10,000 in PBS) was added; the plate was wrapped and incubated for 1 h at room temperature. The solution was discarded and the plate was washed with PBST. A volume of 50 μl ABTS solution was added to the wells and incubated in dark for 10 min. After incubation, 100 μl of stopping solution (0.5 M oxalic acid) was added to each well and absorbance was read at 450 nm in ELISA reader (Bio-Rad, USA). The controls were antigen control (no rabbit serum) and antibody controls (only recombinant PmUbc) and control without secondary antibody.
Results
Expression of PmUbc recombinant protein in E. coli
The amplified PmUbc gene was 750 bp in length. To express the recombinant PmUbc protein, BL21 (DE3) pLys S cells were transformed with pRSET-PmUbc. The gene expressed was confirmed to be PmUbc of size 33 kDa by SDS-PAGE analysis (Fig. 1a). Western blot was performed using anti-his antibodies. Treatment of the membrane with HRPO specific substrate revealed the presence of recombinant protein in induced cells.
a SDS PAGE analysis of rPmUbc. Lane M: Protein ladder (Fermentas), C1: Uninduced control at 2 h, C2: Uninduced control at 4 h, l1: IPTG induced cells at 2 h, l2: IPTG induced cells at 4 h. b SDS PAGE of purified rPMUBC Lane M: Protein ladder (Fermentas), C:Control cells, I: Induced cells, F: Flowthrough, W1: 1st wash, W2: 2nd wash, W3: 3rd wash, E1: 1st elute, E2: 2nd elute, E3: 3rd elute, E4: 4th elute. c Western blot membrane showing the specificity of anti-rPmUbc to rPm Ubc injected in rabbit. Lane 1 and 2 PmUbc protein
Purification of rPmUbc Protein with Ni–NTA column
The rPmUbc protein expressed in BL21 (DE3) pLys S cells was purified using Ni–NTA column under native conditions. Different fractions collected during recombinant protein purification were run on a 12 % polyacrylamide gel to confirm the purification. The protein band of 33 kDa was observed on the gel in four different elute fractions after Coomassie brilliant blue staining (Fig. 1b). Among the four different elutes, although the elute 1 (E1) showed maximum recombinant protein, it also had several nonspecific bands. Elutes E3 and E4 were shown to have minimum number of nonspecific bands and were negligible, hence were selected for immunizing the rabbits. Protein concentration (A280) of elutes E3 and E4 was estimated as 0.877 and 0.331 μg/ml, respectively.
Production of Anti-rPmUbc Antibodies in Rabbit
The purified recombinant PmUbc protein was used to immunize New Zealand white rabbit at a concentration of 200 μg/ml. Following a starter dose, three booster doses with 200 μg protein each were injected with 7 days interval using FIA. The rabbit was bled on the 35th day of immunization; 10 ml blood was collected from which 6 ml of serum was separated.
The specificity of anti-rPmUbc antibodies produced in rabbit was estimated by Western blotting. For this, the purified rPmUbc protein was run on a 12 % SDS-PAGE gel and electro blotted on to a PVDF membrane (Fig. 1c).
Time Course Expression Analysis of PmUbc Protein
For the time course analysis of PmUbc protein in WSSV challenged shrimp, total protein was extracted from the gills of the WSSV challenged shrimps at different time intervals viz., 0, 3, 6, 12, 24, 48 and 72 hpi.
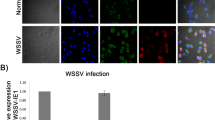
The level of PmUbc protein in shrimp at different time points post WSSV challenge was estimated using indirect ELISA. For this, 100 ng of protein from each sample was coated into the wells of an ELISA plate and probed with the rabbit anti-rPmUbc at 1:15,000 dilutions. The expression level of PmUbc protein was quantified at 405 nm wavelength in an ELISA reader. The quantity of the PmUbC protein in each samples were estimated with the help of a standard curve prepared with known quantities of rPmUbc protein. It was observed that the PmUbc levels increased at the 24 hpi followed by a decrease at 48 and 72 hpi (Fig. 2a).
Discussion
White Spot syndrome virus (WSSV), a large double-stranded DNA virus, is the most serious pathogen of shrimp causing 100 % mortality within 3–10 days from the onset of visible gross signs [6, 28, 22, 5]. Viruses employ many fascinating strategies to infiltrate the host line of defense. One of the interesting and relevant mechanisms is through manipulation of the host’s own ubiquitination pathway. In ubiquitin-dependent proteolytic pathway, ubiquitin is linked to substrates through a well-organized process involving the sequential action of an ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2), and a ubiquitin ligase (E3). Polyubiquitinated proteins are then targeted to the 26S proteasome for degradation [8, 9, 13, 24]. Many viruses are known to hijack the host ubiquitination pathway where the host proteins are redirected for degradation in the 26S proteasome [23, 15]. This specific process often involves an E3 ubiquitin ligase that is directly encoded by either the virus or the host genome [2]. Since ubiquitin-mediated proteolysis of cellular proteins is an important process in many basic cellular processes, perturbations in this system often lead to pathogenesis of many diseases [7].
The PmUbc expression at protein level, at different time points in WSSV challenged P. monodon was studied in the present work. The antibodies raised in rabbit against purified recombinant PmUbc were used to quantify the PmUbc protein levels by ELISA. The results show a gradual increase in PmUbc levels from 12 to 24 hpi followed by a decline from 24 to 72 hpi. The protein data was found to be consistent with the transcript data obtained by both semi-quantitative RT-PCR and Real Time PCR (Fig. 2a, b). In our previous work the expression profiles of PmUbc was compared with the expression of WSSV 199 and WSSV 249. The expression patterns of PmUbc, in comparison with WSSV 199 and 249 speculated that the ubiquitin conjugating enzyme (PmUbc) is modulated by the WSSV proteins [16]. Four proteins of WSSV namely WSSV 199, WSSV 222, WSSV 249 and WSSV 403 are known to contain RING H2 domains [29, 26] which in presence of ubiquitin conjugating enzyme (E2) in shrimp can function as E3 ligase can modulate the ubiquitin proteosome pathway. Wang et al. [26] detected an increased expression of ubiquitin conjugating enzyme in P. vannamei (PvUbc) in a time course study by Real time PCR as well as RT PCR and reported that RING H2 protein WSSV 249 from WSSV may function as E3 ligase by sequestering PvUbc for viral pathogenesis in shrimp. Their study also revealed a weak expression of PvUbc in uninfected shrimp suggesting that PvUbc expression is induced in WSSV infection.
Wang et al. [26] studied the expression pattern of ubiquitin conjugating enzyme till 48 hpi in P. vannamei (PvUbc) while in the present study the expression was studied till 72 hpi in P. monodon (PmUbc). The ubiquitin conjugating enzyme expression is seen not only in the infected animal but also in the normal healthy animal. This is quite expected because ubiquitin-dependent proteolysis regulates protein abundance and serves as a central regulatory function in many biological processes such as cell cycle regulation, signal transduction, transcriptional regulation, DNA repair, inflammatory response, and antigen presentation in eukaryotic cells [12, 14].
Time course expression analysis of PmUbc in WSSV infected shrimp at different time points in P. monodon post WSSV challenge revealed its up regulation of PmUbc at 24 hpi. On comparing the expression pattern with the increasing time point of WSSV infection it can be said that the up-regulation in PmUbc expression might be correlated with WSSV infection. Subsequent decrease in PmUbc expression at 48 hpi could be because the WSSV proteins modulate its expression for efficient pathogenesis. It can be speculated from the above observations that the host machinery tries to defend the viral multiplication by ubiquitin mediated protein degradation, which is evident with an increase in PmUbc expression at 24 hpi. However, soon after 48 hpi the expression of PmUbc was modulated for viral pathogenesis as observed by a decline in PmUbc expression. This is supported by [26] who reported that the RING-H2 protein WSSV249 from WSSV may function as an E3 ligase by sequestering PvUbc for viral pathogenesis in shrimp [26]. The upregulation of PmUbc at 24 hpi in WSSV challenged shrimp was revealed using semi-quantitative and real time PCR [16]. Many findings have shown that viral infection can up-regulate expression of ubiquitin [19, 18], suggesting that the ubiquitin system may play a key role in the course of viral infection. Therefore, the present study reveals the expression patterns of PmUbc at protein level in WSSV infected P. monodon.
References
Arturo. White spot syndrome virus: an overview on an emergent concern. Vet Res. 2010;41:43.
Banks L, Pim D, Thomas M. Viruses and the 26S proteosome: hacking into destruction. Trends Biochem Sci. 2003;28:452–9.
Blanchette P, Branton EP. Manipulation of the ubiquitin–proteasome pathway by small DNA tumor viruses. Virology. 2009;384:317–23.
Bonilla EC, Alday-Sanz V, Wille M, Sorgeloos P, Pensaert MB, Nauwynck HJ. A review on the morphology, molecular characterization, morphogenesis and pathogenesis of white spot syndrome virus. J Fish Dis. 2008;31:1–18.
Chen XF, Chen C, Wu DH, Huai H, Chi XC. A new baculovirus of cultured shrimp. Sci China C Life Sci. 1997;40:630–5.
Chou HY, Huang CY, Wang CH, Chiang HC, Lo CF. Pathogenicity of a Baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis Aquat Organ. 1995;23:165–73.
Ciechanover A. The ubiquitin proteolytic system and pathogenesis of human diseases: a novel platform for mechanism-based drug targeting. Biochem Soc Trans. 2003;31:474–81.
Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. BioEssays. 2000;22:442–51.
Deshaies RJ. SCF and Cullin/ringH2 based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999;15:435–67.
Fang H, Kwang J. Identification and characterization of a new E3 ubiquitin ligase in white spot syndrome virus involved in virus latency. Virology. 2008;5:151.
Fang H, Fenner BJ, Godwin KA, Kwang J. White spot syndrome virus open reading frame 222 encodes a viral E3 ligase and mediates degradation of a host tumor suppressor via ubiquitination. J Virol. 2006;80:3884–92.
Haas AL, Rose IA. Hemm inhibits ATP-dependent ubiquitin-dependent proteolysis: role of hemin in regulating ubiquitin conjugate degradation. Proc Natl Acad Sci USA. 1981;78:6845–8.
Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79.
Hershko A, Ciechanover A, Heller H, Haas AL, Rose IA. Proposed role of ATP in protein breakdown: conjugation of proteins with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci USA. 1980;77:1783–6.
Huang J, Huang Q, Zhou X, Shen MM, Yen A, Yu SX, Dong GQ, Qu K, Huang P, Anderson EM, Daniel-Issakani S, Buller RML, Payan DG, Lu HH. The poxvirus p28 virulence factor is an E3 ubiquitin ligase. J Biol Chem. 2004;279:54110–9.
Jeena K, Pani Prasad K, Mujahid Khan P, Gireesh Babu P. Expression profiling of WSSV ORF 199 and shrimp ubiquitin conjugating enzyme in WSSV infected P. monodon. Asian-Aust J Anim Sci. 2012;8:1184–9.
Joazeiro CA, Wing SS, Huang H, Leverson JD, Hunter T, Liu YC. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–12.
Kemp LM, Latchman DS. The herpes simplex virus type I immediate-early protein ICP 4 specifically induces increased transcription of human ubiquitin B gene without affecting the ubiquitin A and C genes. Virology. 1988;166:258–61.
Latchman DS, Estridge JK, Kemp LM. Transcriptional induction of the ubiquitin gene during herpes simplex virus infection is dependent upon the viral immediate-early protein ICP4. Nucleic Acids Res. 1987;15:7283–93.
Leverson JD, Joazeiro CA, Page AM, Huang H, Hieter P, Hunter T. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol Biol Cell. 2000;11:2315–25.
Lightner DV. A handbook of shrimp pathology and diagnostic procedures for diseases of cultured penaeid shrimp. Baton Rouge: World Aquatic Society; 1996.
Lo CF, Ho CH, Peng SE, Chen CH, Hsu HE, Chiu YL, et al. White spot syndrome associated virus (WSBV) detected in cultured and captured shrimp, crabs and other arthropods. Dis Aquat Organ. 1996;27:215–25.
Okubo K, Yamano K, Qin QW, Aoyagi K, Ototake M, Nakanishi T, Fukuda H, Dijkstra JM. Ubiquitin gene in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2002;12:335–51.
Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–33.
Sambrook SJ, Russel DW, Janseen KA, Irwuin NJ. Molecular cloning—a laboratory manual. 3rd ed. Cold Spring Harbour: Cold Spring Harbour Laboratory Press; 2001.
Wang Z, Chua HK, Gusti AA, He F, Fenner B, Manopo I, Wang H, Kwang J. RING-H2 protein WSSV 249 from white spot syndrome virus sequesters a shrimp ubiquitin-conjugating enzyme, PvUbc, for viral pathogenesis. J Virology. 2005;79:8764–72.
Wang HC, Wang HC, Leu JH, Kou GH, Wang AHJ, Lo CF. Protein expression profiling of shrimp cellular response to white spot syndrome virus infection. Dev Comp Immunol. 2007;31:672–86.
Wongteerasupaya C, Wongwisansri S, Boonsaeng V, Panyim S, Pratanpipat P, Nash GL. DNA fragment of Penaeus monodon baculovirus PmNOBII gives positive in situ hybridization with white-spot viral infections in six Penaeid shrimp species. Aquaculture. 1996;143:23–32.
Yang F, He J, Lin X, Li Q, Pan D, Zhang X. Complete genome sequence of the shrimp white spot bacilliform virus. J Virol. 2001;75:11811–20.
Acknowledgments
The authors acknowledge the support of Dr. W. S. Lakra, Director/Vice Chancellor, Central Institute of Fisheries Education, Mumbai, India for providing all necessary facilities and Indian Council of Agricultural Research, New Delhi for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keezhedath, J., Kurcheti, P.P., Pathan, M.K. et al. Expression Profile of Penaeus monodon Ubiquitin Conjugating Enzyme (PmUbc) at Protein Level in White spot syndrome virus Challenged Shrimp. Indian J. Virol. 24, 48–53 (2013). https://doi.org/10.1007/s13337-013-0131-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-013-0131-6