Abstract
Apixaban is a potent, highly selective, reversible, oral, direct factor Xa (fXa) inhibitor in development for thrombosis prevention and treatment. The preclinical pharmacokinetic (PK) attributes of apixaban feature small volume of distribution (Vd), low systemic clearance (CL), and good oral bioavailability. Apixaban is well absorbed in rat, dog, and chimpanzee, with absolute oral bioavailability of approximately 50% or greater. The steady-state Vd of apixaban is approximately 0.5, 0.2, and 0.17 l/kg in rats, dogs, and chimpanzees, while CL is approximately 0.9, 0.04, and 0.018 l/h/kg, respectively. In vitro metabolic clearance of apixaban is also low. Renal clearance comprises approximately 10–30% of systemic clearance in rat, dog, and chimpanzee. Anti-fXa activity, prothrombin time (PT), and HEPTEST® clotting time (HCT) prolongation correlated well with plasma apixaban concentration in rat, dog and chimpanzee. There was no lag time between apixaban plasma concentration and the pharmacodynamic (PD) markers, suggesting a rapid onset of action of apixaban. The PK/PD analyses were performed using an inhibitory E max model for anti-fXa assay and a linear model for PT and HCT assays. The IC50 values for anti-fXa activity were 0.73 ± 0.03 and 1.5 ± 0.15 μM for rat and dog, respectively. The apparent K i values for PT were approximately 1.7, 6.6, and 4.8 μM for rat, dog and chimpanzee, respectively. The apparent K i for HCT was approximately 1.3 μM for dog. Apixaban exhibits desirable PK and PD properties for clinical development with good oral bioavailability, small Vd, low CL, and direct, predictable, concentration-dependent PD responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anticoagulant therapy is the cornerstone of treatment for venous thromboembolism and stroke prevention in atrial fibrillation (Weitz 2006). Currently available anticoagulants include vitamin K antagonists such as warfarin, unfractionated heparin, low-molecular weight heparins, and fondaparinux. Warfarin has been widely used as the oral anticoagulant in the clinic. However, warfarin therapy requires frequent monitoring and dose adjustment because of narrow therapeutic window, drug–drug interactions, food effects, and genetic polymorphic metabolism and action (Kimmel 2008). Warfarin also has a slow onset of pharmacological action, which limits its utility in providing rapid initial anticoagulation for patients after surgery to minimize the risk of thrombus extension and fatal pulmonary embolism (Weitz 2006; Kimmel 2008). Low-molecular weight heparins and fondaparinux overcome some of the limitations of vitamin K antagonists. However, they must be administered parenterally (Weitz 2006). Therefore, there is a great need for safe and efficacious oral anticoagulants that can overcome the various limitations of the current anticoagulant therapy for prevention and treatment of thromboembolic diseases.
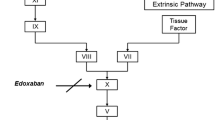
Coagulation factor Xa (fXa) catalyzes the conversion of prothrombin to thrombin and is crucial for both intrinsic and extrinsic coagulation pathways (Weitz 2006). Inhibition of fXa activity should attenuate the generation of thrombin, thus inhibit blood coagulation. Another approach is to directly inhibit thrombin activity (Weitz 2006). Experimental evidence suggests that fXa inhibitors have antithrombotic efficacy with lower bleeding risk in animal models compared with thrombin inhibitors (Hauptmann and Stürzebecher 1999; Leadley 2001; Wong et al. 2002). Moreover, in clinical trials in total hip and knee replacement patients, oral fXa inhibitors have demonstrated superior efficacy compared to enoxaparin, whereas oral direct thrombin inhibitors were found to be non-inferior to enoxaparin in these patient populations (Eriksson et al. 2008; Eriksson et al. 2007a, b; Kakkar et al. 2008; Lassen et al. 2008). In the past decade we have focused on discovery and development of fXa inhibitors, with continuous efforts on optimization of pharmacokinetic (PK), pharmacodynamic (PD), and safety properties (Pinto et al. 2001; Quan and Wexler 2001; Lam et al. 2003; Quan et al. 2005; Qiao et al. 2007). Recently we have discovered apixaban (BMS-562247), 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxo-1-piperidinyl)phenyl-4,5,6,7-tetrahydro-1H-pyrazole-[3,4-c]pyridine-3-carboxamide (Fig. 1), which is a potent and highly selective, oral, direct fXa inhibitor (Pinto et al. 2007). The human fXa K i is approximately 80 pM at 25°C, with greater than 30,000-fold selectivity over other coagulation and related serine proteases. Apixaban has demonstrated antithrombotic activity in preclinical animal models and clinical efficacy and safety for the prevention of venous thromboembolism in patients who underwent total knee or hip replacement surgery (Lassen et al. 2010a, b; Wong et al. 2008). Apixaban is currently in late-stage clinical development for prevention and treatment of thromboembolic diseases. In this study, we report the preclinical PK and PD properties of apixaban.
2 Materials and methods
2.1 Materials
Apixaban was prepared by the Discovery Chemistry Department, Bristol-Myers Squibb Co. (Princeton, NJ). Pooled human (from at least five individuals), rat and dog liver microsomes (from at least five animals) were purchased from XenoTech, LLC (Lenexa, KS), and cryopreserved human hepatocytes were obtained from Celsis In Vitro Technologies (Baltimore, MD). Chimpanzee liver microsomes, prepared from liver collected at surgical resection, were pooled from three animals before the experiment. Factor X Kit was purchased from Diapharma (West Chester, OH), Prothrombin time (PT) reagents Thromboplastin C-Plus from Dade-Behring (Deerfield, IL) and HEPTEST® clotting time (HCT) reagents from American Diagnostica (Stamford, CT). HemosIL reagents for PT were also purchased from Beckman Coulter (Fullerton, CA). Individual human serum was obtained from four healthy males and three healthy females. Pooled rabbit serum was collected by the Thrombosis Biology group at Bristol-Myers Squibb Co. Serum from other species was purchased from Biological Specialty Corp. (Colmar, PA) or Bioreclamation (Hicksville, NY). Human serum albumin (HSA) and α1-acid glycoprotein (α1-AGP) were purchased from Sigma-Aldrich Co. (St. Louis, MO).
2.2 Animal dosing and sample collection
Animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the regulations of the Animal Care and Use Committees of the Bristol-Myers Squibb Company and the New Iberia Research Center.
Apixaban was administered i.v. over 10 min to four rats at 0.5 mg/kg, and p.o. to four rats at 5 mg/kg. In dogs, apixaban was administered in three cases by i.v. infusion over 10 min at 0.5 mg/kg (0.17 mg/ml, 3 ml/kg), and p.o. in three cases at 0.5 mg/kg (0.25 mg/ml, 2 ml/kg). The p.o. dose was formulated in 0.5% methylcellulose containing 1% Tween™ 80, and the i.v. dose in 10% dimethylacetamide, 20% propylene glycol, and 70% deionized water. Blood samples for both rats and dogs were collected into sodium citrate tubes predose, at 10, 15, 30, and 45 min, and at 1, 2, 4, 6, 8, and 24 h relative to the start of the infusion. For the p.o. dosing groups, blood samples were collected predose and at 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, and 24 h postdose.
Apixaban was administered i.v. over 60 min to one chimpanzee (sedated during infusion) at 0.2 mg/kg, and p.o. to two alert chimpanzees at 0.5 mg/kg in the New Iberia Research Center (New Iberia, LA). Blood samples were collected into sodium citrate tubes predose and at 2, 4, 8, 12, 16, and 24 h postdose for the p.o. group. For the i.v. group, blood samples were collected predose and at 0.5, 1, 1.08, 1.25, 1.5, 2, 4, 8, 12, 16, and 24 h relative to the start of the infusion. Plasma and urine were collected and stored at −20°C until analysis. The p.o. dosing solution was a 50–50 (v/v) mixture of 0.5% methylcellulose and Tang™ orange juice containing 1% Tween™ 80. The i.v. dose was formulated in 10% dimethylacetamide, 20% propylene glycol, and 70% deionized water.
2.3 Determination of protein binding
The protein binding of apixaban in rat, dog, chimpanzee, and human serum, HSA, and α1-AGP was determined by equilibrium dialysis using a Dianorm dialysis system and Diachema dialysis membrane (MW cut-off, 10,000) (Lin et al. 1987). All sera were thawed at room temperature and centrifuged at 3,000 rpm for 10 min before the experiments to remove clotted proteins. HSA and α1-AGP were dissolved in 0.133 M potassium phosphate buffer (pH 7.4) to obtain final protein concentration of 40 mg/ml and 1 mg/ml, respectively. Prior to the experiments, the dialysis membranes were preconditioned with water and, subsequently, with 0.133 M potassium phosphate buffer (pH 7.4). Serum containing 1, 3, or 10 μM apixaban (0.75 ml) was added to one side of the cell and an equal volume of buffer (0.133 M potassium phosphate buffer, pH 7.4) was added to the opposite side of the cell. Equilibrium was achieved by rotating the cells at 3–5 rpm at 37°C for 3 h. After incubation, aliquots of the protein and the buffer sides were collected separately and mixed with an equal volume of the opposite matrix. Apixaban concentrations were determined by liquid chromatography–tandem mass spectrometry (LC–MS/MS).
2.4 Determination of in vitro intrinsic clearance of apixaban
Apixaban (1 μM) was incubated with rat, dog, chimpanzee, and human liver microsomes (0.5–2 mg/ml) in 50 mM potassium phosphate buffer (pH 7.4) or cryopreserved human hepatocytes in Krebs–Hensleit buffer (0.5–2 million cells/ml) for 0, 10, 20, and 30 min at 37°C with shaking. Reactions with liver microsomes were started with addition of NADPH (final concentration 1 mM) and stopped with acetonitrile. Reactions with cryopreserved human hepatocytes were started with addition of apixaban and stopped with acetonitrile. The concentrations of apixaban were determined using LC–MS/MS. Intrinsic clearance (CLint) was calculated as described previously (Obach et al. 1997).
2.5 Determination of apixaban by LC–MS/MS
For chimpanzee study and all the in vitro studies, the samples for analysis spiked with the internal standard DPC-A38702 were extracted using a solid phase extraction (SPE) system on a 3 M Empore C8 disk SPE cartridge (St. Paul, MN). The SPE cartridge was preconditioned with 250 μl of methanol and, subsequently, with 250 μl of 2 mM ammonium acetate (pH 5.5). Each sample (100 μl) was mixed with 50 μl of 200 nM DPC-A38702 (internal standard) and 500 μl of 2 mM ammonium acetate (pH 5.5) and loaded onto the cartridge. The cartridge was washed with 425 μl of water and, subsequently, with 425 μl of 15% methanol. The analytes were eluted with 75% acetonitrile containing 25% ammonium acetate (100 mM) and 0.2% formic acid. The eluate was diluted with 100 μl water before injection on the LC–MS/MS system for analysis. Apixaban concentrations were determined with multiple reaction monitoring (MRM) in positive electrospray ionization mode on a Micromass Quattro Ultima mass spectrometer (Manchester, UK) coupled with a Shimadzu HPLC systems (Columbia, MD) and a HTC PAL autosampler (Leap Technologies, Carrboro, NC). HPLC separation was achieved on a Supelco (Bellefonte, PA) Discovery C18 column, and eluted with water containing 0.1% formic acid (A) and acetonitrile containing 0.1% formic acid (B) in a gradient from 5 to 90% B in 2.5 min, 90% B for 1 min, from 90 to 5% B in 0.05 min and 5% B for 1.45 min. The parent-to-daughter ion transitions were 460.17 to 443.10 and 537.20 to 492.10 for apixaban and DPC-A38702, respectively. The quantitation limit for both compounds was 1 nM.
For rat and dog in vivo studies, the samples for the analysis were prepared using protein precipitation procedure. An aliquot (50 μl) of sample, standard, quality control (QC) sample or blank was transferred into a 96-well plate using a Packard MultiPROBE II liquid handler (Perkin Elmer, Boston, MA). Dilutions were made at this stage when appropriate. The protein precipitation process was performed using a Janus Mini liquid handler (Perkin Elmer, Boston, MA). An aliquot (150 μl) of acetonitrile containing 250 nM internal standard was added to the 96-well sample plate. A stable isotope-labeled (13C,32H) apixaban (BMS-562247-03) was used as internal standard for the rat and dog in vivo studies. The plate was first vortex mixed for 5 min and then centrifuged at 3,600 rpm for 6 min. An aliquot (100 μl) of supernatant was transferred into a clean collection plate and an aliquot (200 μl) of water containing 0.1% formic acid was then added to the collection plate and followed by vortex mixing for 2 min. Samples were analyzed with an LC–MS/MS system consisting of a Shimadzu HPLC (Columbia, MD), an HTC PAL autosampler (Leap Technologies, Carrboro, NC) and an API4000 Q-Trap mass spectrometer (Sciex, Toronto, Canada) equipped with the TurboIonSpray interface. Samples were separated on a Phenomenex Onyx Monolithic C18 column, 4.6 × 50 mm (Torrance, CA), with a flow rate of 2.0 ml/min and were detected using multiple reaction monitoring (MRM) in the positive electrospray ionization mode. The MRM transitions monitored were 460.15–443.10 for apixaban, 464.19–447.30 for the isotope-labeled apixaban or 537.20–492.10 for DPC-A38702. The quantitation limit was 1 nM.
2.6 Determination of fXa activity
FXa activity was measured using a commercially available Factor X Kit (Diapharma, West Chester, OH). All reagents were prepared and used according to the package insert directions. The buffer was 50 mM Tris buffer (pH 7.8) containing 20 mg/l polybrene (hexadimethrine bromide). For ex vivo samples, rat or dog plasma at each time point was diluted 21-fold (10 μl + 200 μl buffer). For in vitro samples, pooled normal rat, dog, chimpanzee, or human plasma was spiked to achieve final concentrations of apixaban from 0.1 to 12.8 μM, then diluted 21-fold with buffer. The kit substrate is Z-(d)Arg-Gly-Arg-para-nitroanalide at a concentration of 1,750 μM when reconstituted in 20 ml water (Diapharma, West Chester, OH; personal communication). To establish enzyme kinetic parameters for the Diapharma Factor X substrate separate experiments were conducted with substrate serially diluted with assay buffer to obtain 875, 438, 219 μM substrate. In a 96-well microtiter plate (Corning CoStar 3474), 50 μl diluted plasma was added and warmed to 37°C for 4 min in the plate reader (SpectraMax 384 Plus, Molecular Devices, Sunnydale, CA). Reactions were initiated by adding 50 μl substrate and 50 μl RVV/calcium, both warmed to 37°C in a water bath. Absorbance at 405 nm was measured every 15 s for up to 20 min. Initial rates of substrate hydrolysis (first 90 s) were used for analysis of enzyme kinetic parameters and steady-state rates of substrate hydrolysis (0–3 min for rat and dog; 2–5 min after initiation for human and chimpanzee) were used for apixaban inhibition analysis. The assay was not available for chimpanzee ex vivo samples when the chimpanzee study was carried out.
2.7 Determination of anticoagulant activity
PT and HCT assays were performed according to the reagent manufacturer directions using an automated coagulation analyzer (Sysmex CA-6000, Dade-Behring, Newark, DE). For the chimpanzee study, the PT assay was carried out with ACL 3000 (Beckman Coulter, Fuller, CA) using HemosIL reagents at the New Iberia Research Center (New Iberia, LA). For PT assay, rat plasma samples at each time point were first pooled. Warmed plasma (50 μl 37°C) was then combined with 100 μl PT reagent and clotting time was recorded by the automated analyzer. For HCT assay of dog plasma samples, warmed plasma (50 μl 37°C) was combined with 50 μl HEPTEST® bovine fXa for 2 min at 37°C. Clotting was initiated by adding 50 μl HEPTEST® RECALMIX® and clotting time was recorded by the automated analyzer. HCT assays were not completed for the rat ex vivo samples and were not available for chimpanzee ex vivo samples when the chimpanzee study was carried out.
2.8 Data analysis
Noncompartmental analysis was used to calculate the PK parameters. Area under the plasma drug concentration–time curve (AUC) and total area under the first moment–time curve were obtained using Lagrange polynomial integration from time zero to the last measured sample time, with extrapolation to time infinity using the least squares terminal slope by means of WinNonlin® software (V.1 to V3; Pharsight Corp., Mountain View, CA). With AUC, total area under the first moment–time curve, and terminal slopes, systemic clearance (CL), volume of distribution at steady state (Vdss), half-life (t 1/2), and mean residence time (MRT) were calculated. Time to maximum concentration (T max) and maximum concentration (C max) were obtained from the observed data. The absolute bioavailability (F) was estimated from the plasma AUC data after intravenous and oral administrations.
For anti-fXa activity, the IC50 values were determined using XLFit for Excel (version 2.0, IDBS, Bridgewater, NJ). The following equation was used to describe the PK/PD relationship:
For clotting times (PT and HCT), a linear model was used to calculate the apparent K i values. From an enzyme kinetics perspective, the one-stage clotting time represents 1/v, where v is the apparent velocity of overall coagulation reaction (Hemker et al. 1977). The plot of clotting time (reaction velocity) as a function of inhibitor concentration is a Dixon plot as follows for noncompetitive inhibition (Segel 1993; Luettgen et al. 2011):
which has the slope–intercept form, y = mx + b, where 1/v (y) is the clotting time, \( {\frac{{\left( {1 + {\raise0.5ex\hbox{$\scriptstyle K_{\rm m}$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle {S}$}}} \right)}}{{V_{\max } \times K_{i} }}} \) is the slope (m), and \( {\frac{{\left( {1 + {\raise0.5ex\hbox{$\scriptstyle K_{\text{m}}$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle {S}$}}} \right)}}{{V_{\max } }}} \) is the y-axis intercept (b), the clotting time when inhibitor is not present. Using the slope–intercept formula equivalence between negative x-intercept (−K i ) and the x-coordinate at y = 2-times the y-intercept can be established as follows. The x-intercept equals −b/m when y = 0. The x-intercept equals b/m when y = 2-times b. Thus, the apparent K i for inhibition of the clot formation by apixaban, which is the x-axis intercept, could be determined by Dixon plotting.
3 Results
3.1 Apixaban PK parameters in rat, dog, and chimpanzee
The PK parameters of apixaban in rat, dog, and chimpanzee are summarized in Table 1. The Vdss was small and ranged from approximately 0.2 to 0.5 l/kg in rat, dog, and chimpanzee. The CL ranged from approximately 0.02 to 0.9 l/(h kg), with the lowest value in chimpanzee and highest value in rat. The terminal t 1/2 ranged from 4 to 7 h in chimpanzee and dog, and was approximately 3 h in rats. The absolute oral bioavailability ranged from approximately 50% in both rat and chimpanzee to 80% in dog. The renal clearance ranged from 0.002 to 0.3 l/(h kg), representing approximately 10–30% of the systemic plasma clearance, with the lowest values in chimpanzee and the highest values in rat.
3.2 Serum protein binding of apixaban
The unbound fraction of apixaban in rat, dog, chimpanzee, and human serum are summarized in Table 2. The unbound fraction ranged from approximately 4–13% in rat, dog, chimpanzee, and human sera. The protein binding was not concentration dependent over the range from 1 to 10 μM in rat, dog, and chimpanzee sera (human serum was tested only at 1 μM). There was no gender difference in protein binding for rat, dog, and human. The mean unbound fraction of apixaban to HSA and human α1-AGP were 34 ± 5% (n = 3) and 91 ± 7% (n = 3), respectively.
3.3 In vitro intrinsic clearance
Apixaban is metabolically stable in dog, chimpanzee, and human liver microsomes, and human hepatocytes. There was no appreciable disappearance of apixaban in the liver microsomal incubation at 0.5–2 mg/ml microsomal protein for 60 min, nor in the hepatocyte incubation at 0.5–2 million cells/ml for 120 min. The intrinsic clearance could not be accurately determined in the in vitro systems. Compared with dog, chimpanzee, and human, there was small but appreciable disappearance of apixaban in rat liver microsomal incubation, with a calculated intrinsic clearance value of 0.02 ml/(min mg).
3.4 Effect of apixaban on fXa activity in vitro
Apixaban inhibits human, chimpanzee, rat, and dog fXa activity in a concentration-dependent manner (Fig. 2). The substrate Michaelis–Menten constant (K m) was determined for each species and, assuming competitive inhibition, this K m was used to calculate a K i of apixaban for each species fXa after correcting for the total plasma dilution (Table 3). Apixaban has higher affinity for human and chimpanzee fXa as compared to rat and dog fXa similar to what was observed using purified fXa from human, rat, and dog (Wong et al. 2008). The human fXa K i (0.48 nM) is comparable to what was recently reported for apixaban at 37°C (Luettgen et al. 2011).
3.5 Effect of apixaban on fXa activity and PK/PD relationship in rat and dog
Activity of fXa changed as a function of time in rat and dog following a single oral dose of apixaban (Fig. 3). The fXa activity showed good inverse correlation with the plasma apixaban concentration. There was no lag time between fXa inhibition and plasma apixaban concentrations for both species. The maximum effect occurred at approximately 1–2 h, and the fXa activity returned to approximately the baseline at 24 h postdose. Results from samples obtained from rats and dogs following i.v. administration were not included because of potential interferences of the dosing vehicles on the assay performance.
Factor Xa activity decreased as the plasma apixaban concentrations increased in both rat and dog (Fig. 4). The PK/PD relationship was modeled with a direct inhibitory E max model. The IC50 values were 0.73 and 1.5 μM for rat and dog, respectively (Table 3).
The in vitro and ex vivo results were comparable for both dog and rat. Ex vivo anti-fXa activity assay for chimpanzee and human were not performed in this study.
3.6 Effect of apixaban on plasma clotting times and PK/PD relationship in rat, dog, and chimpanzee
The PT changed as a function of time postdose in rat, dog, and chimpanzee following a single p.o. dose administration of apixaban (Fig. 5). The PT prolongation correlated well with the plasma apixaban concentration. There was no lag time between the PT prolongation and plasma apixaban concentration. Similarly, the HCT, which was only assayed for the dog study, changed as a function of time. There was no lag time between HCT prolongation and plasma apixaban concentrations. The maximum effect on clotting times occurred at 1, 2, and 2 h postdose in rat, dog, and chimpanzee (the earliest time point), respectively. The clotting times returned to baseline values at 24 h postdose.
Plasma apixaban concentration (mean ± SD) and ex vivo clotting times (mean ± SD) as a function of time in rats (upper panel), dogs (middle panel), and chimpanzees (lower panel) following a single oral administration of apixaban. The doses were 5, 0.5, and 0.5 mg/kg for rat, dog, and chimpanzee, respectively. Upper panel plasma samples from four rats were pooled and assayed for prothrombin time (PT), middle panel dog plasma samples were assays for both PT and HEPTEST® clotting time (HCT), lower panel two chimpanzees (X133, A008) were involved in the study
The PK/PD relationship was analyzed with a linear model as described in Sect. 2. The apparent K i values derived from PT were 1.7, 6.6, and 4.8 μM for rat, dog, and chimpanzee, respectively. The apparent K i value derived from HCT assay was 1.3 μM for dog. The HCT assay is more sensitive than PT as observed in dog. The concentration–PT response curve is steeper for rat than for chimpanzee and dog. The concentration–HCT curve is steeper than the concentration–PT response curve in dog plasma.
The baseline PT value was higher in dog than in rat. The baseline PT value in chimpanzee is between that in dog and that in rat, however, the reagent used for chimpanzee study is different from the one used in rat and dog studies.
4 Discussion
The results of this study show that apixaban has the key properties for an optimal oral anticoagulant with good oral bioavailability, small Vd, low CL, and a direct, predictable, concentration-dependent PK/PD response. Apixaban is well absorbed in rat, dog, and chimpanzee, with absolute oral bioavailability of approximately 50% or greater. The Vd value of apixaban is consistently small in rat, dog, and chimpanzee, ranging from approximately 0.2 to 0.5 l/kg, which is less than the total body water volume in the corresponding species (Davies and Morris 1993). In addition, apixaban appears to be equally distributed between plasma and blood (data not shown). Compared to the average blood volumes, approximately 20, 40, and 50% of apixaban remains in blood in rat, dog, and chimpanzee, respectively (Davies and Morris 1993). The small Vd is unlikely related to protein binding since the protein binding is not high in these species. A small Vd of apixaban suggests that it largely remains in blood where fXa resides, instead of extensively distributing to other tissues. Drug distribution primarily to the same compartment as the drug target leads to high potency and reduces the potential for off-target effects.
Low clearance is essential for a drug with a low volume of distribution to maintain adequate t 1/2. The CL value of apixaban is very low in dog and chimpanzee, accounting for less than 2% of the hepatic blood flow. Consistently, the intrinsic metabolic CLs are very low, with no appreciable disappearance in dog and chimpanzee liver microsomes. The renal clearances are also very low, and less than the protein binding-adjusted glomerular filtration rate. Compared with dog and chimpanzee, the rat CL value is higher, with a greater in vitro metabolic rate and a greater renal CL, though apixaban is still categorized as a low clearance compound in rat. Apixaban was the result of lead optimization efforts to achieve and maintain small Vd and low CL for a direct fXa inhibitor (Lam et al. 2003; Pinto et al. 2001, 2007; Quan et al. 2005; Wong et al. 2004). To compare to our previous clinical development candidates, the chimpanzee Vdss is 0.17, 2.6, and 2.4 l/kg, and the chimpanzee CL is 0.018, 0.21, and 0.55 l/h/kg for apixaban, DPC-423, and razaxaban, respectively (Wong et al. 2004).
The in vitro metabolic CL is very low in both human liver microsomes and hepatocytes, with no appreciable intrinsic CL, which is similar to dog and chimpanzee. Collectively, these results suggest that dog and chimpanzee are more relevant preclinical models than rat for human CL prediction. Furthermore, the chimpanzee model has been evaluated as an appropriate surrogate for human PK prediction for certain compounds because of generic, physiological, and biochemical similarities between chimpanzee and human (Wong et al. 2004). Based on the preclinical profiles, we predicted that apixaban would have a desirable PK profile in humans with good oral bioavailability, small Vd and low CL. Moreover, the chimpanzee PK predicts twice-daily or once-daily dosing regimen in human with an acceptable peak/trough ratio.
Primary biotransformation reactions of apixaban include O-demethylation and mono-oxidation (Zhang et al. 2009; Raghavan et al. 2009). Combinations of these reactions were also observed as sulfation of O-demethyl apixaban, sulfation of hydroxylated O-demethyl apixaban and glucuronidation of O-demethyl apixaban. No glutathione adduct of apixaban was detected in microsomes or hepatocytes, indicating that the formation of reactive metabolites with apixaban is unlikely. The in vitro metabolism of apixaban was primarily mediated by CYP3A4/5, with relatively minor contributions from CYP1A2 and CYP2J2 towards the formation of O-demethyl apixaban. In addition, low levels of O-demethyl apixaban formation were catalyzed by CYP2C8, CYP2C9 and CYP2C19 (Wang et al. 2010). The elimination of apixaban involves multiple pathways, including hepatic metabolism, renal excretion and intestinal/biliary secretion, each responsible for elimination of approximately one third of dose (Wang et al. 2010; Zhang et al. 2009). Given that apixaban has multiple routes of elimination and an oral bioavailability of approximately 50%, any such drug–drug interaction effects are likely to be of relatively low magnitude. The potential of apixaban to inhibit or induce CYP is minimal, suggesting that apixaban is unlikely to affect the metabolism of co-administered medications that are dependent on CYP-mediated clearance (Wang et al. 2010).
Apixaban shows relatively low plasma protein binding in humans, with a free fraction of approximately 13%. This level of protein binding indicates a low binding affinity of apixaban to plasma proteins. The low binding affinity in nature implies a low potential for apixaban to cause drug–drug interactions through displacement of plasma protein binding. It is particularly relevant for anticoagulants because the free concentration is considered to correlate with anticoagulant activity and, theoretically, with bleeding potential. This attribute partially explains the difficulty in using warfarin since clinically significant drug–drug interactions of warfarin are associated with plasma protein binding displacement (Harder and Thürmann 1996). Furthermore, it appears that HSA accounts for the plasma protein binding with minimal contribution from α1-AGP. Compared with α1-AGP, HSA has higher capacity to bind drugs, and its plasma level is less affected by physiological and pathological conditions (Svensson et al. 1986). Therefore, these plasma protein binding characteristics suggest that apixaban has a low potential for any significant drug–drug interactions due to plasma protein binding displacement.
To evaluate the PD response, anti-fXa activity, PT, and HCT were used as biomarkers in the PK/PD studies. The traditional clotting time-based PT assay measures the overall coagulation cascade reactions triggered through the extrinsic pathway. The PT assay has been widely used to monitor anticoagulant activity of warfarin (Kimmel 2008). However, it has been shown that PT is not a sensitive assay for Xa inhibition (Tobu et al. 2002; Walenga and Hoppensteadt 2004; Paccaly et al. 2006). The commercially available HCT employs exogenous bovine fXa as the activator. As shown in Fig. 6, HCT is more sensitive than PT to changes in apixaban concentrations in dog. A similar observation was found in human plasma (Wong et al. 2008). Compared with PT and HCT, the chromogenic fXa assay, which measures the fXa activity generated by activation of fX in plasma directly, appears to be a sensitive and specific assay for anti-fXa activity. The assay has been used to monitor the anticoagulant activity of the low-molecular weight heparin fondaparinux (Turpie 2004; Walenga and Hoppensteadt 2004). It is noteworthy that the assay sensitivity may be compound dependent even with the same mechanism of action (Walenga and Hoppensteadt 2004). Collectively, the results indicate that fXa activity assay could be used as PD markers for apixaban-induced anticoagulant activity.
Clotting time as a function of apixaban concentrations in plasma from rats, dogs, and chimpanzees following a single oral administration of apixaban. The doses were 5, 0.5, and 0.5 mg/kg for rat, dog, and chimpanzee, respectively. Upper panel prothrombin time (PT) for rat, dog, and chimpanzee; reagents used for chimpanzee was different from those for rat and dog; lower panel HEPTEST® clotting time (HCT) for dog only
The PD markers correlate well with apixaban plasma concentrations. This finding is expected since fXa resides in plasma and plasma apixaban should exhibit a direct pharmacological effect. Indeed, no lag time was observed between the PD markers and plasma level of apixaban, suggesting a rapid onset of action. The fXa activity was inhibited by apixaban in a concentration-dependent manner, with a saturation observed at higher apixaban concentrations. The ex vivo profile is consistent with the mechanism of action involving direct and reversible enzyme inhibition of fXa. The degree of the inhibition was estimated with a direct inhibitory E max model. Rat appears to be more sensitive than dog with IC50 of 0.73 versus 1.5 μM. The clotting times were prolonged in a concentration-dependent manner in rat, dog, and chimpanzee. The concentration:response relationship is linear as observed with other direct anticoagulants, including direct thrombin inhibitors and fXa inhibitors (Kubitza et al. 2005; Paccaly et al. 2006; Stangier et al. 2007). Theoretically, clotting time is equivalent to 1/v, where v represents the velocity of the overall enzyme reactions leading to clot formation. The plot of clotting time as a function of anticoagulant concentrations is therefore equivalent to a Dixon plot, depicting the overall inhibitory effect on enzymes involved in coagulation. In the case of apixaban, the inhibitory effect is attributed to the inhibition of the enzyme, fXa. Theoretically, as depicted by Dixon plotting, the concentration:response for clotting time assays should be linear, which was indeed observed in several reports (Kubitza et al. 2005; Paccaly et al. 2006). However, the linearity could be limited by other factors in the reaction system such as test reagents, enzyme stability, and substrate availability. Based on the enzymatic equation for Dixon plotting, the slope of the concentration–response curve would depend on the potency of direct anticoagulant and predisease or treatment conditions where the fXa activity and/or the prothrombin concentration are altered, which in turn affect the baseline clotting time. Apixaban has been found to cause noncompetitive inhibition of prothrombinase activity when the natural substrate prothrombin was used (Luettgen et al. 2011). Therefore, the apparent K i values could be estimated using Dixon plotting (Segel 1993). The apparent K i values are 1.7, 6.6, and 4.8 μM for rat, dog, and chimpanzee, respectively. As described in Sect. 2, mathematically, the apparent K i value is equal to the concentration doubling the clotting time, which is a parameter commonly reported (Pinto et al. 2001, 2007; Wong et al. 2002, 2008; Quan et al. 2005). Consistent with the anti-fXa assay, rat appears to be more sensitive than dog to PT prolongation. The species difference may be mainly due to the susceptibility of fXa to small molecular inhibitors (Hara et al. 1995). The baseline coagulation activity in species, which determines the baseline PT value (the y-axis intercept), would also affect the slope of concentration response curve. It is difficult to have a direct comparison for the PT results between chimpanzee and rat or dog because the assays were performed using different reagents and instruments which may affect the results. Overall, apixaban shows a predictable and concentration-dependent PD effect in the animal models tested.
In conclusion, apixaban is a potent, highly selective and reversible, oral, direct fXa inhibitor. Importantly, apixaban exhibits attributes consistent with an optimal oral anticoagulant exhibiting good oral bioavailability, small Vd, low CL, rapid onset of action, and a direct, predictable, concentration-dependent PD responses.
Abbreviations
- α1-AGP:
-
α1-Acid glycoprotein
- AUCinf :
-
Area under concentration–time curve from 0 to infinity
- AUC(0–24h) :
-
Area under concentration–time curve from 0 to 24 h
- CL:
-
Clearance
- CLint :
-
Intrinsic clearance
- C max :
-
Maximum concentration
- F :
-
Absolute bioavailability
- fXa:
-
Factor Xa
- HCT:
-
HEPTEST® clotting time
- HSA:
-
Human serum albumin
- IC50 :
-
Concentration required for 50% inhibition
- K i :
-
Inhibition constant
- LC–MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- MRT:
-
Mean residence time
- PD:
-
Pharmacodynamics
- PK:
-
Pharmacokinetics
- PT:
-
Prothrombin time
- t 1/2 :
-
Half-life
- T max :
-
Time to maximum concentration
- Vdss :
-
Volume of distribution at steady state
References
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10(7):1093–1095
Eriksson BI, Dahl OE, Rosencher N, Kurth AA, Van Dijk CN, Frostick SP, Kälebo P, Christiansen AV, Hantel S, Hettiarachchi R, Schnee J, Büller HR, for the Re-Model Study Group (2007a) Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 5(11):2178–2185
Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Büller HR (2007b) Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 370(9591):949–956
Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 358(26):2765–2775
Hara T, Yokoyama A, Morishima Y, Kunitada S (1995) Species differences in anticoagulant and anti-Xa activity of DX-9065a, a highly selective factor Xa inhibitor. Thromb Res 80(1):99–104
Harder S, Thürmann P (1996) Clinically important drug interactions with anticoagulants: an update. Clin Pharmacokinet 30(6):416–444
Hauptmann J, Stürzebecher J (1999) Synthetic inhibitors of thrombin and factor Xa: from bench to bedside. Thromb Res 93(5):203–241
Hemker H, Vermeer C, Govers-Riemslag J (1977) Kinetic aspects of the interaction of blood clotting enzymes. VII. The relation between clotting time and prothrombin concentration. Thromb Haemost 37(1):81–85
Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap ÁF, Misselwitz F, Haas S (2008) Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 372(9632):31–39
Kimmel SE (2008) Warfarin therapy: in need of improvement after all these years. Expert Opin Pharmacother 9(5):677–686
Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G (2005) Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59–7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther 78(4):412–421
Lam PYS, Clark CG, Li R, Pinto DJP, Orwat MJ, Galemmo RA, Fevig JM, Teleha CA, Alexander RS, Smallwood AM, Rossi KA, Wright MR, Bai SA, He K, Luettgen JM, Wong PC, Knabb RM, Wexler RR (2003) Structure-based design of novel guanidine/benzamidine mimics: potent and orally bioavailable factor Xa inhibitors as novel anticoagulants. J Med Chem 46(21):4405–4418
Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AGG (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 358(26):2776–2786
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P (2010a) Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 375(9717):807–815
Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM (2010b) Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 363(26):2487–2498
Leadley R (2001) Coagulation factor Xa inhibition: biological background and rationale. Curr Top Med Chem 1(2):151–159
Lin JH, Cocchetto DM, Duggan DE (1987) Protein binding as a primary determinant of the clinical pharmacokinetic properties of non-steroidal anti-inflammatory drugs. Clin Pharmacokinet 12(6):402–432
Luettgen JM, Knabb RM, He K, Pinto DJP, Rendina AR (2011) Apixaban inhibition of factor Xa: microscopic rate constants and inhibition mechanism in purified protein systems and in human plasma. J Enzyme Inhib Med Chem. doi:10.3109/14756366.2010.535793
Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, Macintyre F, Rance DJ, Wastall P (1997) The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther 283(1):46–58
Paccaly A, Frick A, Ozoux M-L, Chu V, Rosenburg R, Hinder M, Shukla U, Jensen BK (2006) Pharmacokinetic/pharmacodynamic relationships for otamixaban, a direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol 46(1):45–51
Pinto DJP, Orwat MJ, Wang S, Fevig JM, Quan ML, Amparo E, Cacciola J, Rossi KA, Alexander RS, Smallwood AM, Luettgen JM, Liang L, Aungst BJ, Wright MR, Knabb RM, Wong PC, Wexler RR, Lam PYS (2001) Discovery of 1-[3-(aminomethyl)phenyl]-N-[3-fluoro-2′-(methylsulfonyl)-[1,1′-biphenyl]-4-yl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide (DPC423), a highly potent, selective, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem 44(4):566–578
Pinto DJP, Orwat MJ, Koch S, Rossi KA, Alexander RS, Smallwood A, Wong PC, Rendina AR, Luettgen JM, Knabb RM, He K, Xin B, Wexler RR, Lam PYS (2007) Discovery of 1-(4-Methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem 50(22):5339–5356
Qiao JX, Wang TC, Wang GZ, Cheney DL, He K, Rendina AR, Xin B, Luettgen JM, Knabb RM, Wexler RR, Lam PYS (2007) Enantiopure five-membered cyclicdiamine derivatives as potent and selective inhibitors of factor Xa. Improving in vitro metabolic stability via core modifications. Bioorg Med Chem Lett 17(18):5041–5048
Quan ML, Wexler RR (2001) The design and synthesis of noncovalent factor Xa inhibitors. Curr Top Med Chem 1(2):137–149
Quan ML, Lam PYS, Han Q, Pinto DJP, He MY, Li R, Ellis CD, Clark CG, Teleha CA, Sun J-H, Alexander RS, Bai S, Luettgen JM, Knabb RM, Wong PC, Wexler RR (2005) Discovery of 1-(3′-aminobenzisoxazol-5′-yl)-3-trifluoromethyl-N-[2-fluoro-4-[(2′-dimethylaminomethyl)imidazol-1-yl]phenyl]-1H-pyrazole-5-carboxyamide hydrochloride (razaxaban), a highly potent, selective, and orally bioavailable factor Xa inhibitor. J Med Chem 48(6):1729–1744
Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, Pinto D, Chen S, Bonacorsi S, Wong PC, Zhang D (2009) Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Disp 37(1):74–81
Segel IH (1993) Enzyme kinetics: behavior and analysis of rapid equilibrium and steady-state enzyme systems. Wiley and Sons, New York
Stangier J, Rathgen K, Stähle H, Gansser D, Roth W (2007) The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 64(3):292–303
Svensson CK, Woodruff MN, Baxter JG, Lalka D (1986) Free drug concentration monitoring in clinical practice: rationale and current status. Clin Pharmacokinet 11(6):450–469
Tobu M, Iqbal O, Hoppensteadt DA, Shultz C, Jeske W, Fareed J (2002) Effects of a synthetic factor Xa inhibitor (JTV-803) on various laboratory tests. Clin Appl Thromb Hemost 8(4):325–336
Turpie AGG (2004) Fondaparinux: a Factor Xa inhibitor for antithrombotic therapy. Expert Opin Pharmacother 5(6):1373–1384
Walenga JM, Hoppensteadt DA (2004) Monitoring the new antithrombotic drugs. Semin Thromb Hemost 30(6):683–695
Wang L, Zhang D, Raghavan N, Yao M, Ma L, Frost CA, Maxwell BD, Chen S-Y, He K, Goosen TC, Griffith WH, Grossman SJ (2010) In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab Disp 38(3):448–458
Weitz JI (2006) Emerging anticoagulants for the treatment of venous thromboembolism. Thromb Haemost 96(3):274–284
Wong PC, Crain EJ, Watson CA, Zaspel AM, Wright MR, Lam PY, Pinto DJP, Wexler RR, Knabb RM (2002) Nonpeptide factor Xa inhibitors III: effects of DPC423, an orally-active pyrazole antithrombotic agent, on arterial thrombosis in rabbits. J Pharmacol Exp Ther 303(3):993–1000
Wong H, Grossman SJ, Bai SA, Diamond S, Wright MR, Grace JE, Qian M, He K, Yeleswaram K, Christ DD (2004) The chimpanzee (Pan troglodytes) as a pharmacokinetic model for selection of drug candidates: model characterization and application. Drug Metab Disp 32(12):1359–1369
Wong PC, Crain EJ, Xin B, Wexler RR, Lam PYS, Pinto DJ, Luettgen JM, Knabb RM (2008) Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost 6(5):820–829
Zhang D, He K, Raghavan N, Wang L, Mitroka J, Maxwell BD, Knabb RM, Frost C, Schuster A, Hao F, Gu Z, Humphreys WG, Grossman SJ (2009) Comparative metabolism of 14C-labeled apixaban in mice, rats, rabbits, dogs, and humans. Drug Metab Disp 37(8):1738–1748
Conflict of interest
The authors are current or former employees of Bristol-Myers Squibb. This study was sponsored by Bristol-Myers Squibb and Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, K., Luettgen, J.M., Zhang, D. et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor. Eur J Drug Metab Pharmacokinet 36, 129–139 (2011). https://doi.org/10.1007/s13318-011-0037-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-011-0037-x