Abstract
In Australia, Pythium soft rot (PSR) outbreaks caused by P. myriotylum were reported in 2009 and since then this disease has remained as a major concern for the ginger industry. From 2012 to 2015, a number of Pythium spp. were isolated from ginger rhizomes and soil from farms affected by PSR disease and assessed for their pathogenicity on ginger. In this study, 11 distinct Pythium spp. were recovered from ginger farms in Queensland, Australia and species identification and confirmation were based on morphology, growth rate and ITS sequences. These Pythium spp. when tested showed different levels of aggressiveness on excised ginger rhizome. P. aphanidemartum, P. deliense, P. myriotylum, P. splendens, P. spinosum and P. ultimum were the most pathogenic when assessed in vitro on an array of plant species. However, P. myriotylum was the only pathogen, which was capable of inducing PSR symptoms on ginger at a temperature range from 20 to 35 °C. Whereas, P. aphanidermatum only attacked and induced PSR on ginger at 30 to 35 °C in pot trials. This is the first report of P. aphanidermatum inducing PSR of ginger in Australia at high temperatures. Only P. oligandrum and P. perplexum, which had been recovered only from soils and not plant tissue, appeared non-pathogenic in all assays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ginger (Zingiber officinale Rosc.), belonging to the Zingiberaceae, is a cash crop for many growers in various countries including China, India, Indonesia, Fiji and Australia (Kavitha and Thomas 2008). In Australia, ginger was introduced as a crop in the early twentieth Century and has been cultivated on the Sunshine Coast, Queensland since 1916 (Hogarth 2000). Presently, production extends from Gatton to Bundaberg in southeast Queensland, but the Australian ginger industry is relatively small (less than 1 % contribution to global share) with an involvement of around 30 full time growers. Nevertheless, it was worth AUD 15.6 million at the farm gate in 2009 (Camacho and Brescia 2009) with an annual production estimated at about 8000 t. Almost half of this production is delivered to the domestic fresh market with the remainder going for processing inside the world’s largest ginger factory, Buderim Ginger Ltd. (Camacho and Brescia 2009).
Pythium soft rot (PSR) of ginger was first reported around a century ago in India by Butler (1907) and has since been problematic in most ginger growing regions worldwide (Dohroo 2005). The disease caused by any one of a number of Pythium spp. (Dohroo 2005) is of most concern due to the destructiveness and aggressiveness of the pathogens on ginger. Losses generally vary from 5 to 30 %, but in some cases they can be up to 100 % in fields where conditions conducive for disease development, such as water logging and high temperatures, are reached (Stirling et al. 2009). Once ginger fields have been infested with Pythium spp., the persistence of the pathogens leads to PSR in subsequent replanting of ginger in these fields, as reviewed in Le et al. (2014).
In Australia, Pythium spinosum was recorded on ginger as early as 1962 by Teakle (1962), but it was considered a secondary pathogen to Fusarium oxysporum, the causal agent of Fusarium yellows. During 2007, a PSR disease outbreak on ginger in the Sunshine Coast of Australia was attributed to Pythium myriotylum (Stirling et al. 2009) which at the time was considered to be a ubiquitous pathogen with a wide host range and consequently the relevance of the PSR outbreak may have been underestimated. However, PSR has since then continued to be a major constraint for the Australian ginger industry with up to 70 % of the growers admitting to have PSR infestation on their farms (T. Pattison pers. Comm.).
Following the first report of PSR on two of the oldest ginger farms in Australia (Stirling et al. 2009), PSR disease has since been observed on at least 11 other nearby farms. Therefore, it was questioned if there was probably more than one Pythium species/strains associated with PSR on ginger in Australia; and also if the pathogen has been spread from the original infested farms. In this study, a large number of PSR diseased ginger samples and soils from around infected ginger were collected from 13 infested farms for an assessment of the Pythium spp. diversity associated with PSR of ginger in Queensland, Australia.
Materials and methods
Pythium isolation and cultures
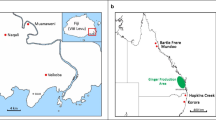
Diseased ginger rhizomes were sampled from 13 farms in Queensland (Fig. 1). Isolations were undertaken by excising sections (5 mm2) of rhizome with PSR symptoms, quickly surface decontaminating (around 10 s) with 25 % bleach (1 % HOCl), then washing twice with sterilized distilled water (DW), and blotting dry with autoclaved paper towel. The sections were then transferred on to Petri plates with corn meal agar with an amendment of 50 μg/mL Penicillin, 50 μg/mL Polymyxin, and 25 μg/mL Pirmaricin (CMA + 3P). The Petri plates were incubated in the dark overnight at 27 °C, then sections taken from the growing edge of the colonies were transferred onto 1.5 % water agar (WA), incubated again under the same conditions for another night, after which hyphal tips of each of the isolates were excised out under an inverted compound microscope (Leica) and placed onto full strength potato dextrose agar (PDA, Difco).
In addition, a baiting technique based on descriptions of Stanghellini and Kronland (1985) modified by using excised ginger rhizomes was deployed in an attempt to recover Pythium spp. from soil collected from around ginger showing symptoms of PSR. A total 10 g of putatively infested soil was saturated with DW in a 9 cm Petri plate. Excised pieces (2 cm2) of apparent healthy ginger rhizomes were immersed in 25 % bleach for 1 min, rinsed twice with autoclaved DW, and blotted dry with autoclaved paper towels. Ten of these excised ginger pieces were then placed onto the soil surface in each plate. On the top of each ginger piece, a plug (5 mm2) of WA was placed. The plates were incubated at 27 °C for 2–4 days. Hyphal-colonised WA plugs were then transferred onto 1.5 % WA for single hyphal tip isolation.
In addition to representative isolates of each of Pythium spp. recovered in this study, reference specimens of P. myriotylum CBS254.70 and P. zingiberis NBRC30817, as well as P. myriotylum UQ5993 recovered from sudden wilt capsicum in Australia were also included in pot trials on ginger plants. Where the reference isolates were used for pot trials, the assays were conducted in a contained controlled environment facility, as required by quarantine for the overseas isolates. All cultures were placed on half strength corn meal agar (CMA) prepared from maize meal (polenta), based on the protocol of Dhingra and Sinclair (1995). The culture on the CMA was then submerged in autoclaved DW and stored at room temperature for future use.
Hyphal growth rate
For each Pythium spp. tested, daily mycelial growth rate under a temperature range from 5 to 45 °C was assessed on 9 cm Petri plates. The growth rate was assessed at 5 °C intervals and carried out as descriptions previously in Le et al. (2015). Generally, 0.5 cm2 plugs of tested cultures (one-week-old) were subcultured onto PDA contained in Petri plates. Two plates were used for isolate, representing two replicates. After subculturing, initial growth of the cultures were manipulated by leaving the plates at room temperature for at least 8 h. Growing edges were marked before the plates were incubated at each designated temperature for 24 h. Growing edges again were marked after 24 h of incubation; radial growth of the colonies was measured at four points in mm using two transecting lines from the first mark to the next. Daily growth rate was presented as a mean of the four measurements. The growth rates were assessed at each of the designated temperature twice.
Morphology
Sexual and asexual structures of Pythium spp. were produced in soil extract cultures amended with two pieces (1 cm2) of grass leaves (Pennisetum setaceum). To make the soil extract, 20 g of air-dried sandy soil was soaked in 1 L DW overnight. The filtrate sieved through 150 mm Whatman filter paper (No. 1) was made up to a volume of 1 L with DW and autoclaved at 121 °C for 20 min (McLeod et al. 2009). Active growing cultures of Pythium spp. on PDA were excised and the plugs (0.5 cm2) were submerged into the soil extract cultures contained in Petri plates. The plates were kept at 27 °C and checked daily for development of taxonomic characteristics. The resultant mycelial mats were mounted on microscopic slides, stained with cotton blue, and observed under a BH2 (Olympus) microscope where measurements were recorded of structures at 400× magnification. For each isolate, 20 randomly selected structures were measured.
DNA extraction and amplification
The CTAB protocol of Doyle and Doyle (1990) was employed to extract DNA from mycelia mats growing in potato dextrose broth. The ITS regions including the 5.8S rRNA subunit were amplified in a Mastercycler by using two universal primers: ITS 1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS 4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al. 1990). In every 20 μL PCR reaction, there was 1 μL of DNA template (25 ng/μL) and 19 μL of master mix, the latter which included 0.18 μL of Taq DNA polymerase (5 u/μL), 1 μL of primer ITS1 and primer ITS4 each (10 μM), 2.83 μL of 5X Green GoTaq flexi buffer, 0.72 μL of 25 mM MgCl2, 1.05 μL of 10 mM dNTPs and 13.2 μL of dH2O. The reaction cycle was first denatured for 5 min at 94 °C, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 90 s. The reaction was finally extended for 10 min at 68 °C.
The CoxI gene was amplified by using a primer set of OomCoxILevup (5′-TCAWCWMGATGGCTTTTTTCAAC-3′) and Fm85mod (5′-RRHWACKTGACTDATRATACCAAA-3′) (Robideau et al. 2011). The reaction cycle for CoxI included a first step at 95 °C for 2 min, followed by 35 cycles of 95 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min. The reaction was finally extended for 10 min at 72 °C.
The CoxII gene was amplified by using two primers: FM66 (5′ TAGGATTTCAAGATCCTGC 3′) and FM58 (5′ CCACAAATTTCACTACATTGA 3′) (Martin 2000). The reaction cycle was first denatured for 5 min at 94 °C, followed by 35 cycles of 94 °C for 30 s, 52 °C for 30 s, and 72 °C for 90 s. The reaction was finally extended for 10 min at 68 °C.
To amplify the β-tubulin gene, two primers TUBUF2 (5′ CGGTAACAACTGGGCCAAGG 3′) and TUBUR1 (5′ CCTGGTACTGCTGGTACTCAG 3′) were used (Kroon et al. 2004). The cycling program was based on descriptions of Mu et al. (1999) with some modifications. The reaction cycle was first denatured for 5 min at 94 °C, followed by eight cycles of 94 °C for 1 min, 52 °C for 1 min, and 72 °C for 2 min. The reaction was then continued with another 22 cycles of 94 °C for 1 min, 62 °C for 1 min, 72 °C for 2 min. The program was completed with a 7 min step at 72 °C. All PCR products were run at 110 V in a 1.5 % agarose gel for 40 min to confirm the presence of an amplified product.
Sequencing and analysing sequences
Both forward and reverse directions were sequenced directly from PCR products by Macrogen (Korea) and the consensus sequences were created after manual alignment and comparison using Clustal 2.0.2. The Geneious 7.1.4 was used to align the sequences of the different Pythium isolates collected from ginger fields. Comparisons were made among the sequences in this study with those of deposited sequences in Genbank to confirm species identification and determine whether there were any genetic variations. A maximum likelihood tree of the ITS region was drawn to assess the phylogenetic relationship among the isolates and species.
Pathogenicity tests
In vitro pathogenicity test on excised ginger sticks
The experiment was adapted from methods described in Le et al. (2015) to test for colonization and aggressiveness of Pythium spp. on ginger pieces at 27 ± 2 °C. Briefly, disease free ginger rhizomes were cut into sticks (5.5 cm long × 1 cm wide × 1 cm high), which were surface disinfected in 25 % bleach for 1 min, washed twice again with autoclaved DW for another min, and blotted dry on autoclaved paper towels. Three replicate sticks were prepared for each isolate by plating together in 9 cm Petri plates containing wet autoclaved filter paper. The sticks were inoculated by placing small squares (0.5 cm2) of active growing Pythium cultures on PDA at one end of each ginger stick. Three days after incubation, each stick was chopped up into five smaller pieces (1 × 1 × 1 cm) under sterile conditions which were then plated (except for the one end containing the culture) onto PDA in a Petri plate in order of distance from the inoculated end of the ginger sticks. These plates were then incubated at the same temperature in the dark for 24 h and observed for recovery of Pythium spp. from each excised piece. The negative control treatment included the same procedure but with uninoculated PDA squares (non-cultured). The data were recorded determining the colonization of Pythium spp.to 1, 2, 3, 4 or 5 cm sections on the ginger sticks. The assay was independently repeated.
In vitro pre-emerging damping off test
The pathogenicity of the Pythium spp. was assessed by screening seedlings of 13 different plant species in Petri plates against cultures of each isolate. For each isolate-plant species interaction assessed, a one-week-old culture that had been grown on PDA and was then sub-cultured onto three replicate 9 cm Petri plates of 1.5 % WA, and kept in an incubator set at 27 °C for a week. On each WA plate, ten surface disinfected seeds were placed one of the following 13 plant species: rye (Secalecereale), wheat (Triticum sp.), millet (Panicum miliaceum), barley (Hordeum vulgare), buck wheat (Fagopyrum esculentum), beet-root (Beta vulgaris), spring onion (Allium fistulosum), carrot (Daucus carota), cauliflower (Brassica oleracea botrytis), cucumber (Cucumis sativus), eggplant (Solanum melongena), lettuce (Lactuca sativa), and gypsophila (Gypsophila elegans). The plates were then incubated at 27 °C for 7 days before checking for disease indices. The disease index was calculated using the equation of Zhang and Yang (2000).
DI is disease index rating from 0 (all seedlings healthy after germination) to 1 (all seeds dead before germination).
Xi is disease rating of the i th replicate (from 1 to 10).
40 is equal to the number of replicates multiplying with the highest rating scale (from 0 to 4).
The rating scale used was as follows: 0 = seed germinated and healthy seedlings with no obvious symptoms; 1 = seed germinated and seedlings with light brown lesion on roots; 2 = seed germinated and seedlings with short and enlarging brown lesion on roots; 3 = seedlings died after germination; 4 = seed died with no apparent germination.
Glasshouse pathogenicity test on ginger plants
Ginger (cv ‘Queensland’) plants derived from tissue culture were used in pot trials to assess their reaction to a subset of Pythium spp. isolates. Inoculum for pot trials was prepared using sorghum seeds that had been soaked in water for 24 h in the dark and autoclaved twice at 121 °C for 20 min. The sorghum seeds were then plated onto 1 to 2-day-old Pythium cultures grown on PDA in Petri plates and kept at 27 °C for a week or until the seeds were fully covered with mycelia of Pythium. For each isolate of Pythium spp. tested, ginger plants (about 4-month-old after deflasking) that had been grown in 140 mL pots were inoculated by inserting the two sorghum seeds under the soil to a depth of 20–30 mm, which were fully colonized with Pythium spp. The pots of inoculated ginger were kept saturated with water from time of inoculation onwards by placing on saucers where the water was maintained. Plants were monitored daily for disease development based on aboveground symptoms applying the following scale: 0 = plants remain green and healthy; 1 = leaf sheath collar discoloured and lower leaves turned yellow; 2 = plants alive, but shoots either totally yellow or dead; and 3 = all shoots dead (Stirling et al. 2009). Attempts were made to re-isolate the Pythium spp. either from diseased rhizomes or from soil, in the latter case by baiting with carrot pieces placed on the soil. The pathogenicity assays were conducted in growth cabinets, which were assigned two temperature ranges 20/25 °C and 30/35 °C (night/day) both with 10 h photoperiod under fluorescent light. The assays were undertaken second time on cv ‘Canton’ due to the shortage of cv ‘Queensland’.
All data from pathogenicity assays and the growth rate experiments were subjected to analysis of variance (ANOVA) and the means were compared using Tukey’s least significant difference (LSD) test (P ≤ 0.05). Where stated, data from repeated assays were pooled and analyzed together if there were no significant difference between the two repetitions. The ANOVA and comparisons of means were performed with Minitab 16.
Results
Isolation and identification
A total of 173 isolates of Pythium spp. and 15 of Pythiogeton ramosum isolates were obtained either directly from PSR ginger or from baiting of soil from around infected ginger. Eleven different species were initially identified based on morphological characteristics by using the keys of van der Plaats-Niterink (1981) and Dick (1990). Of these 11 different Pythium spp. and one Pythiogeton ramosum, only three of the Pythium spp. as well as the Pythiogeton ramosum were recovered directly from PSR ginger tissue (Table 1). PSR ginger sampled from a single location only ever yielded one single species of Pythium sp. When using ginger as bait from the soil, P. spinosum and P. splendens were recovered most frequently followed by P. aphanidermatum, P. deliense, P. heterothallicum, P. oligandrum, P. perplexum, P. torulusum, and P. ultimum. However, using ginger baits from the soil failed to retrieve either P. graminicola or P. myriotylum.
All of the species recovered grew well at temperature ranges above 5 °C and below 40 °C, except for P. graminicola which grew poorly below 10 °C, and P. aphanidermatum which grew normally even at 40 °C and above (Table 2). P. aphanidermatum was exceptional in that it still grew at 45 °C.
All recovered isolates had the ability to be cultured on different media including PDA, CMA, WA, potato broth and all isolates were stored and kept in a collection at Plant Pathology lab, School of Agriculture and Food Sciences, The University of Queensland (UQ).
For species confirmation, the ITS sequences, which were deposited to GenBank (Table S1) were between 761 to 910 bp and once compared with sequences on the Genbank database, all sequences were above 99 to 100 % identical with reference species (CBS isolates) on the Genbank, so confirming the status of Pythium spp. in this study. A phylogenetic tree built on the ITS region showed very high bootstrap values (98–100 %) between the morphologically identified and the reference species; subsequently, the species identification was verified (Fig. 2). Of those UQ isolates considered to be P. myriotylum, sequences of nuclear and mitochondrial loci, including of ITS (57 sequences), CoxI (47 sequences), CoxII (67 sequences) and β-tubulin (19 sequences) were 99.8 to 100 % homologous to each other (Fig. S1-S4).
Pathogenicity tests
In vitro tests on excised ginger
The different Pythium spp. showed varying levels of aggressiveness when inoculated onto ginger sticks. Once colonized, the ginger became soft then discoloured and then white mycelia of pathogenic Pythium spp. covered the tissue. Most of the isolates identified as P. myriotylum showed a high level of aggressiveness as assessed by rate of development on the excised ginger rhizome the exception being P. myriotylum UQ5892 isolate (Fig. 3). Isolates of P. deliense and P. aphanidermatum also showed a high level of aggressiveness followed by P. spinosum, P. splendens, P. ultimum, P. graminicola. P. heterothallicum and P. torulosum were the least aggressive. Those isolates identified as P. perplexum and P. oligandrum were not able to grow on the ginger sticks and colonize the tissue in the conditions tested at 27 ± 2 °C; subsequently, neither of these species were recovered from the inoculated ginger sticks. None of the control sticks yielded Pythium spp. when samples were plated out onto PDA plates (Fig. 3).
Aggressiveness of selected Pythium spp. assessed based on mean colonization and recovery of Pythium spp. on 1 cm segments excised from 5.5 cm long ginger sticks at 27 ± 2 °C at 3 DAI (bars represent standard deviations, n = 6). Means were combined from two independent assays due to no significant difference between the two repetitions (P = 0.05)
In vitro pre-emerging damping off
The seeds of all tested plant species, except for beet-root germinated well in the control treatments (disease index, DI ≤ 0.2) and the subsequent seedlings remained healthy afterwards. The DIs in control treatments were significantly less (P = 0.05) than those recorded for seeds treated with Pythium spp., excluding P. oligandrum in which DIs on tested plant species were not different from those of the control. Similarly, DI recorded on millet infected with P. torulosum was as low as that of the control. P. aphanidermatum, P. myriotylum, P. graminicola, P. spinosum, P. splendens and P. ultimum appeared the most aggressive on the tested plant species, except on barley which was less vulnerable to attack by P. splendens and P. ultimum (DI < 0.5). All these pathogenic Pythium spp. attacked and killed the seeds before germination and also young seedlings of plant species tested in the assays (DI > 0.5–1) (Table 3). All eight tested P. myriotylum isolates were highly aggressive on the 12 different tested plant species (DI > 0.5), except for P. myriotylum UQ6152 and UQ6349 which were less aggressive on lettuce (DI = 0.42–0.43). The isolate P. myriotylum UQ5892 was least aggressive, compared to other P. myriotylum isolates on ginger sticks, but it was comparable to the others in attacking and killing seeds and young seedlings of tested plant species in this assay (Table 3). Where tested, the isolate of P. torulosum was only highly aggressive as classified by Zhang and Yang (2000) on wheat (DI > 0.5). P. heterothallicum and P. perplexum appeared to be least aggressive to those plant species on which they were tested.
Pathogenicity on ginger plants
Reference isolates of P. myriotylum CBS254.70 and P. zingiberis NBRC30817 were included in this pot trial after an additional permit was granted. Only P. myriotylum isolates, excluding P. myriotylum CBS254.70, all of which were obtained from PSR ginger, were able to infect and kill ginger plants at the two different temperature ranges, regardless of ginger cultivars tested; the only exception was P. myriotylum UQ5892 which showed a low disease severity level at both temperatures and more so at the lower of the two temperature regimes. None of the other Pythium spp. caused disease when assessed at the lower temperature. Where disease developed, initially above ground symptoms such as water soaking at collar regions and yellowing of lower leaves were observed as early as 5 and 10 DAI on ginger plants grown at 30/35 °C and 20/25 °C, respectively. However, by 21 DAI, disease severity eventually was not significantly different between the two temperature ranges, except for the one recorded on P. myriotylum UQ5892. P. myriotylum UQ5993 from capsicum, P. myriotylum CBS254.70, P. zingiberis NBRC30817, and P. aphanidermatum UQ6082 showed different levels of disease severity on ginger plants from the control only when the temperatures were higher than 30 °C (Fig. 4). All other Pythium spp. were non-pathogenic on ginger plants in the bioassays regardless of temperature. In all assays, Pythium spp. were re-isolated and identified based on morphology from infested potting mix by baiting confirming successful colonization of tested Pythium spp. in soil. Where applicable, the pathogenic Pythium spp. were also recovered from artificially induced PSR ginger in the bioassays.
Disease severity (0–3) which was recorded on ginger plants inoculated with Pythium spp. (only pathogenic Pythium spp. to ginger plants were presented) at 21DAI at two temperature ranges 20/25 °C and 30/35 °C with 10 h of photoperiods (bars represent standard errors, n = 6). Data presented the combination of two pot trials due to no significant difference in disease severity recorded on the two ginger cultivars tested (P = 0.05)
Discussion
Several oomycetes, namely Pythium spp. and Pythiogeton ramosum were isolated directly from PSR ginger in Queensland, Australia. P. myriotylum was isolated from most of the farms assessed. P. spinosum and P. graminicola were also occasionally recovered from PSR ginger. Unlike studies on other crops, including rooibos, bell pepper, common bean and soybean, of which more than one Pythium sp. was often recovered from diseased plants (Bahramisharif et al. 2013; Chellemi et al. 2000; Li et al. 2014b; Zitnick-Anderson and Nelson 2014), with PSR on ginger in this study only one particular Pythium species was isolated per site. In some cases, mixed infections of Fusarium sp. and Pythium sp. and Pythiogeton sp., were recorded although our findings indicated that PSR in ginger in Australia was not caused by such a disease complex.
Other Pythium spp., including P. aphanideramtum, P. deliense, P. splendens and P. ultimum have been reported as causal agents of PSR on ginger around the world (Dohroo 2005); in this study however, their recovery was only obtained from ginger baits. Ginger baits were quite effective in isolating Pythium spp. from soils around ginger, but the species of interest, P. myriotylum was not trapped from the field soils. This is possibly due to either the use of unsuitable baiting conditions or use of substrates which may have favoured the overgrowth of other more saprophytic species, namely P. splendens and P. spinosum. If this was the case, soil plating dilution with a Pythium selective medium will possibly be effective since P. myriotylum populations were assumed to be dominant in the soil habitat, and it will be important from a ginger farming system perspective to evaluate P. myriotylum population levels before planting ginger back into infested fields.
When potting mix was inoculated with a single Pythium species in an artificially inoculated pathogenicity assays in the pot trials, the same baiting technique successfully allowed recovery of P. myriotylum. Therefore in the absence of other competitors, recovery of P. myriotylum was obtained without much effort from the simple baiting technique. The success of recovering Pythium spp. from soil has been shown in the literature to be dependent on the target species and for P. myriotylum in particular, others have also reported difficulty (Lumsden et al. 1975; Wang and Chang 2003) and our experience with field baits support the assumption that P. myriotylum is often overgrown by other saprophytes and will prove difficult to isolate from soils.
In most cases, morphology and growth rate allowed us to identify to species status the Pythium spp. isolated in this study. All 11 Pythium spp. were identified using the keys of van der Plaats-Niterink (1981) and Dick (1990), although identification of some species, including P. perplexum, P. splendens and P. heterothallicum were at first not assured due to morphological similarities to others as well as a lack of sexual structures. The identified and putative species were further confirmed by sequences of the ITS region. The ITS sequence analysis is now used worldwide for Pythium identification, but for many cases, species boundaries could not be revealed by the ITS locus solely (Levesque and De Cock 2004; Robideau et al. 2011). In this study, a BLAST search from the Genbank database resulted in very high levels of identity, namely over 99 to 100 % homology to well-known reference isolates, so multiple gene sequencing was not required. The only exception was that species boundary of P. myriotylum and P. zingiberis was not clearly distinguished using only ITS sequence data. However, this was addressed by analyzing morphology, whole genome data and pathogenicity exclusively (unpublished data).
There have been many different Pythium spp. reported worldwide as major pathogens of PSR of ginger (Dohroo 2005). In Australia, P. myriotylum was reported in 2009 (Stirling et al. 2009) and in this study, the prevalence and importance of the pathogen were revealed from all infested farms from the Queensland, Australia. Moreover, P. myriotylum also caused major losses to ginger crops in many other countries that being China, India, Fiji, Korea and Taiwan (Kim et al. 1997; Kumar et al. 2008; Stirling et al. 2009; Tsai 1991; Yuan et al. 2013). P. myriotylum is well-known for its high temperature preferences and it is most pathogenic at about its temperature optimum (37 °C) (van der Plaats-Niterink 1981). This was supported by the pot trial results, from which PSR symptoms were only observed on ginger plants inoculated with P. myriotylum CBS254.70, P. zingiberis NBRC30817 and P. myriotylum UQ5993 (capsicum isolate) at 30–35 °C. However, isolates of P. myriotylum initially obtained from PSR ginger in Australia appeared more aggressive at a wide temperature range, 20–35 °C. Likewise, Perneel et al. (2006) found that P. myriotylum recovered from cocoyam was most pathogenic to cocoyam at 28 °C. Interestingly, cocoyam was also more vulnerable to P. myriotylum isolates, which were initially isolated from diseased cocoyam, than those recovered from other hosts (Perneel et al. 2006). In addition, from our unpublished pot trial data, P. myriotylum from capsicum and P. myriotylum from ginger were strongly pathogenic to capsicum and ginger, respectively regardless of testing temperatures, but they were much less pathogenic when cross inoculations were undertaken. This could be implied that the P. myriotylum isolated from PSR infected ginger in this study possibly exhibited some level of host preference, so more pot trail screenings for their pathogenicity on other crops is warranted. If the theory is supported by future results, it may have a significant contribution to PSR control programs through crop rotations.
In addition to the ITS region, sequences of CoxI, CoxII and β-tubulin regions of representatives of P. myriotylum obtained from PSR ginger revealed genetic uniformity of the population. Unlike the P. myriotylum population recovered also from PSR ginger in China, certain genetic differences were noticed within sequences of the ITS region (Yuan et al. 2013). Because of the genetically uniform population of P. myriotylum on ginger farms in Australia, it could be implied that P. myriotylum may have been spread from one original source. Stirling et al. (2009) argued that the incidence of PSR on ginger in 2007 was a sporadic event in a very wet year on two of the oldest farms under continuous ginger cultivation, and so specific control methods were not recommended at the time apart from paying better attention to drainage and soil health. However, PSR is now observed on many farms (up to 70 % of the ginger farmers in the Sunshine Coast region have admitted to having PSR on their farms according T. Pattison (pers. Comm.)) and is of major concern for the ginger industry. Although quarantine approaches have been deployed in the regions, stricter on farm biosecurity to prevent further spread is necessary.
In this study, P. aphanidermatum, P. deliense, P. graminicola, P. spinosum, P. splendens and P. ultimum, which were isolated from either soil or diseased ginger or both, have also been found associated with PSR on ginger elsewhere around the world (Dohroo 2005). Isolates of these species were able to colonize and cause soft rot on ginger sticks at a single incubation temperature (27 °C) and were also highly pathogenic on a wide host range in vitro assays. However, none of these listed species were able to induce symptoms on ginger plants, except for P. aphanideramtum which only attacked and induced PSR on ginger at 30/35 °C. This is also the first report of P. aphanidermatum attacking ginger in Australia. The results here are therefore in agreement with the literature that P. aphanidermatum is a high temperature preference species (van der Plaats-Niterink 1981). Nonetheless, Li et al. (2014a) found that P. aphanidermatum recovered initially from PSR ginger in China was capable of inducing symptoms of PSR on ginger at 24–26 °C. Therefore, it could be of interest to undertake population studies of P. aphanidermatum and ginger around major ginger growing regions since findings might lead to a significant impact for PSR control. The current study could not induce PSR symptoms on ginger plants inoculated with P. deliense, P. graminicola, P. spinosum, P. splendens and P. ultimum, but presence of these pathogenic species should not be underestimated. Except for P. spinosum and P. ultimum which were reported as secondary and postharvest pathogens on ginger, respectively (Dohroo 2001; Le et al., 2010; Teakle 1962), presence of other Pythium spp. on Australian ginger farms might pose additional threats to the ginger industry in Australia once favorable environmental conditions for these species to thrive are met.
The recovery of non-pathogenic P. oligandrum, which is in fact a well-known wide host range mycoparasite and capable of parasitizing species within the same genus, that being Pythium spp. (Gerbore et al. 2014), might be of interest for further interaction studies between the main PSR pathogen, P. myriotylum and the mycoparasite.
In conclusion, PSR disease has remained as a main constraint for ginger industry in Australia since the first outbreaks recorded in 2007. Although a number of Pythium spp. have been found to be associated with PSR in certain conditions, the PSR on ginger in Australia was mainly attributed to P. myriotylum. Pathogenicity of P. myriotylum across a wide temperature range might indicate for an occurrence of a new more aggressive strain of P. myriotylum. Therefore, it is worth practicing a stricter quarantine approach to contain the spread, as well as looking for alternative control strategies, including biocontrol.
References
Bahramisharif A, Lamprecht SC, Spies CFJ, Botha WJ, Calitz FJ, McLeod A (2013) Pythium spp. associated with rooibos seedlings, and their pathogenicity toward rooibos, lupin, and oat. Plant Dis 98:223–232
Butler EJ (1907) An account of the genus Pythium and some Chytridiaceae. Memoirs of the Department of Agriculture, India (Botanical Series) 1:70
Camacho HE, Brescia A (2009) The Australian ginger industry: overview of market trends and oppotunities. Department of Employment, Economic Development and Innovation, The State of Queensland, p. 54
Chellemi DO, Mitchell DJ, Kannwischer-Mitchell ME, Rayside PA, Rosskopf EN (2000) Pythium spp. associated with bell pepper production in Florida. Plant Dis 84:1271–1274
Dhingra OD, Sinclair JB (1995) Basic plant pathology methods, 2nd edn. CRC Press, United States of America
Dick MW (1990) Keys to Pythium. The College of Estate Management. Whiteknights, Reading, Great Britain
Dohroo NP (2001) Etiology and management of storage rot of ginger in Himachal Pradesh. Indian Phytopathol 54:49–54
Dohroo NP (2005) Diseases of ginger. In: Ravindran PN, Babu KN (eds) Ginger, the genus Zingiber. CRC Press, Boca Raton, pp. 305–340
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13
Gerbore J, Benhamou N, Vallance J, Le Floch G, Grizard D, Regnault-Roger C, Rey P (2014) Biological control of plant pathogens: advantages and limitations seen through the case study of Pythium oligandrum. Environ Sci Poll Res 21:4847–4860
Hogarth J (2000) Buderim ginger: an export success story : a history of the ginger industry of Queensland. Hogarth & Buderim Ginger Ltd, Yandina, Qld
Kavitha PG, Thomas G (2008) Expression analysis of defense-related genes in Zingiber (Zingiberaceae) species with different levels of compatibility to the soft rot pathogen Pythium aphanidermatum. Plant Cell Rep 27:1767–1776
Kim CH, Yang SS, Park KS (1997) Pathogencity and mycological characteristics of Pythium myriotylum causing rhizome rot of ginger. Korean J Plant Pathol 13(3):152–157
Kroon LPNM, Bakker FT, van den Bosch GBM, Bonants PJM, Flier WG (2004) Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet Biol 41:766–782
Kumar A, Reeja ST, Bhai RS, Shiva KN (2008) Distribution of Pythium myriotylum Drechsler causing soft rot of ginger. JOSAC 17(1):5–10
Le PD, Smith MK, Aitken EAB (2010) Pythium spp. on ginger (Zingiber officinale Roscoe) in Australia. In: Sterling GR (ed) 6th ASDS Proceedings, Twin Waters, Queensland, p. 62
Le DP, Smith M, Hudler GW, Aitken E (2014) Pythium soft rot of ginger: detection and identification of the causal pathogens, and their control. Crop Prot 65:153–167
Le DP, Smith MK, Aitken EAB (2015) Pythiogeton ramosum, a new pathogen of soft rot disease of ginger (Zingiber officinale) at high temperatures in Australia. Crop Prot 77:9–17
Levesque CA, De Cock AWAM (2004) Molecular phylogeny and taxonomy of the genus Pythium. Mycol Res 108:1363–1383
Li Y, Mao LG, Yan DD, Liu XM, Ma TT, Shen J, Liu PF, Li Z, Wang QX, Ouyang CB, Guo MX, Cao AC (2014a) First report in China of soft rot of ginger caused by Pythium aphanidermatum. Plant Dis 98:1011
Li YP, You MP, Barbetti MJ (2014b) Species of Pythium associated with seedling root and hypocotyl disease on common bean (Phaseolus vulgaris) in Western Australia. Plant Dis 98:1241–1247
Lumsden RD, Ayers WA, Dow RL (1975) Differential isolation of Pythium species from soil by means of selective media, temperature and pH. Can J Microbiol 21:606–612
Martin FN (2000) Phylogenetic relationships among some Pythium species inferred from sequence analysis of the mitochondrially encoded cytochrome oxidase II gene. Mycologia 92:711–727
McLeod A, Botha WJ, Meitz JC, Spies CFJ, Tewoldemedhin YT, Mostert L (2009) Morphological and phylogenetic analyses of Pythium species in South Africa. Mycol Res 113:933–951
Mu JH, Bollon AP, Sidhu RS (1999) Analysis of β-tubulin cDNAs from taxol-resistant Pestalotiopsis microspora and taxol-sensitive Pythium ultimum and comparison of the taxol-binding properties of their products. Mol Gen Genet 262:857–868
Perneel M, Tambong JT, Adiobo A, Floren C, Saborío F, Lévesque A, Höfte M (2006) Intraspecific variability of Pythium myriotylum isolated from cocoyam and other host crops. Mycol Res 110:583–593
van der Plaats-Niterink AJ (1981) Monograph of the genus Pythium. Studies of Mycology 21:1–244
Robideau GP, Gachon CMM, Hu C-H, Küpper FC, Rintoul TL, Sarhan E, Verstappen ECP, Zhang Y, Bonants PJM, Ristaino JB, Lévesque CA, De Cock AWAM, Coffey MD, Voglmayr H, Brouwer H, Bala K, Chitty DW, Désaulniers N, Eggertson QA (2011) DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Mol Ecol Resour 11:1002–1011
Stanghellini ME, Kronland WC (1985) Bioassay for quantification of Pythium aphanidermatum in soil. Phytopathology 75:1242–1245
Stirling GR, Turaganivalu U, Stirling AM, Lomavatu MF, Smith MK (2009) Rhizome rot of ginger (Zingiber officinale) caused by Pythium myriotylum in Fiji and Australia. Australas Plant Pathol 38:453–460
Teakle DS (1962) Investigation on the genus Pythium in Queensland. The University of Queensland, Master theis of Agricultural Science
Tsai YP (1991) List of plant disease in Taiwan. The plant protection society of the republic of China and the Phytopathological society of the republic of China. Taichung, Taiwan
Wang PH, Chang CW (2003) Detection of the low-germination-rate resting oospores of Pythium myriotylum from soil by PCR. Lett Appl Microbiol 36:157–161
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a Guide to Methods and Applications. Academic Press, Inc., California, pp. 315–322
Zhang BQ, Yang XB (2000) Pathogenicity of Pythium populations from corn-soybean rotation fields. Plant Dis 84:94–99
Zitnick-Anderson KK, Nelson BD (2014) Identification and pathogenicity of Pythium on soybean in North Dakota. Plant Dis 99:31–38
Yuan JD, Zhang YL, Qi JS, Zhang B, Xu ZT, Li L, Li CS (2013) Pathogen identification of ginger stalk rot from Shandong province. Acta Phytopath Sin 43:S525
Acknowledgments
This work is a part of ‘Pythium spp. on ginger in Australia’ project funded by Rural Industries Research and Development Corporation (PRJ-008410) in conjunction with the Australian Ginger Growers Association. We greatly thank Mr. Rob Abbas from Rob Abbas Consulting Pty. Ltd. for sampling diseased ginger; Dr. David Teakle (retired) for critical reading the manuscript. The first author also wants to thank Australian Endeavour Awards for rewarding a PhD scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Le, D.P., Smith, M.K. & Aitken, E.A.B. An assessment of Pythium spp. associated with soft rot disease of ginger (Zingiber officinale) in Queensland, Australia. Australasian Plant Pathol. 45, 377–387 (2016). https://doi.org/10.1007/s13313-016-0424-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-016-0424-5