Abstract
Biological control, especially with Bacillus-based biocontrol agents, offers an attractive alternative to synthetic pesticides for sustainable management of white mold disease caused by Sclerotinia sclerotiorum. In this study, eight effective Bacillus isolates were isolated from rhizospheric soil samples as potential bacterial biocontrol agents. Cultural, biochemical, and molecular analyses of 16S rDNA and gyrase subunit A (gyrA) confirmed that all isolates were identified as Bacillus amyloliquefaciens subsp. plantarum. The production of hydrolytic enzymes and the plant growth-promotional attributes of these isolates confirmed their multifaceted potential. Molecular analysis of the eight biosynthetic genes, which are related to antibiotic properties of bacilli, revealed that all of the isolates possess five genes: bacA for bacilysin, dfnM for difficidin, fenA for fengycin, ituA for iturin, and sfp for surfactin. The Bacillus isolates inhibited mycelial growth and suppressed formation of sclerotia during an in vitro test against S. sclerotiorum. Deformities and cell-wall lysis of mycelia, abnormalities of apothecia, and germination failure of ascospores through interaction with the Bacillus isolates were observed with light and scanning electron microscopes, suggesting that they have high antagonistic effects against S. sclerotiorum. Seed bacterization with the Bacillus isolates protected mustard seedlings in vitro up to 98 % against S. sclerotiorum. In a pot experiment, damages of mustard plants against the pathogen decreased up to 90 % after foliar spray of the Bacillus isolates. In addition, the isolates increased seed germination and accelerated seedling vigor of mustard, suggesting that they have plant growth-promoting functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sclerotinia sclerotiorum (Lib.) de Bary is a soil-borne, necrotrophic, and cosmopolitan plant pathogenic fungus (Purdy, 1979) that attacks more than 400 plant species in the world at all stages of growth and harvested products (Bolton et al., 2006). The fungus forms sclerotia, which serve as infectious propagules in soil and survival structures that can remain viable for several years in fields even in adverse conditions such as heat, drought, and the presence of fungicides. Hyphae that are produced through the myceliogenic germination of sclerotia under optimum moisture conditions can infect plant tissues directly or after producing ascospores through carpogenic development of apothecia (Bolton et al., 2006).
Crop management practices such as use of clean seeds, adjustment of sowing time, tillage of soil, and maintaining the density of the plant population can be used to help reduce white mold disease caused by S. sclerotiorum, although there are some limitations to these effective means to control disease (Steadman, 1979; Mueller et al., 2002). Cultural means like crop rotation are practically impossible because of the persistence of sclerotia in the soil for a long time and the wide host range of S. sclerotiorum (Bolton et al., 2006). Resistant cultivars remain the most economic and long-term approach for controlling any disease, but resistance to S. sclerotiorum is largely controlled by many genes and some agronomic traits (Steadman, 1979). The effectiveness of fungicides to control S. sclerotiorum has been inconsistent (Mueller et al., 2002), because it is difficult to achieve appropriate coverage with fungicides and appropriately time the application in relation to ascospore release (Steadman, 1979). Moreover, fungicides have adverse effects on non-target organisms, and continuous use of chemicals may increase the resistance of the pathogen to fungicides (Mueller et al., 2002). Therefore, biological control is gaining interest as a suitable alternative to chemicals to control phytopathogens like S. sclerotiorum because of unreliable control with cultural or traditional methods and the deleterious effects of fungicides on the environment (Pérez-García et al., 2011).
Over the last two decades, the biological control of plant pathogens has emerged as a viable disease control strategy that is an alternative to chemical pesticides (Choudhary and Johri, 2009). A number of biocontrol agents from different genera such as Bacillus, Pseudomonas, and Trichoderma were reported against S. sclerotiorum (e.g., Hou et al., 2006; Abdullah et al., 2008). In addition, the highly specific biocontrol agent Coniothyrium minitans was used commercially to destroy sclerotia of S. sclerotiorum (Gerlagh et al., 1999). However, it has the limitation of slow germination of pycnidiospores (Shi et al., 2004).
Bacillus spp. can form endospores that are resilient structures capable of surviving adverse situations (McSpadden Gardener, 2004). The ecological traits of bacilli offer advantages for long-term survival to the easy commercialization of biocontrol products (Pérez-García et al., 2011). Bacillus-based biocontrol agents produce a range of different metabolites and antibiotic compounds to suppress various pests such as fungi, bacteria, nematodes, viruses, and insects (Kloepper et al. 2004, Choudhary and Johri, 2009). In recent years, numerous Bacillus spp. have been reported to suppress various air and soil-borne fungal diseases from different genera such as Rhizoctonia (Yu et al., 2002), Colletotrichum (Ashwini and Srividya, 2013), Fusarium (Zhao et al., 2014), and Sclerotinia (Hou et al., 2006). The antagonistic ability and plant growth-promotional attributes of Bacillus rhizobacteria might be useful for efficient biocontrol strategies of sustainable cropping systems (Choudhary and Johri, 2009; Pérez-García et al., 2011). Bacillus subtilis and other closely related Bacillus spp. can control phytopathogens by producing various antimicrobial metabolites and two dozen antibiotics including surfactin, iturin, and fengycin (Arguelles-Arias et al., 2009; Stein, 2005). Moreover, surfactin lipopeptide acts as a potent biosurfactant to microbial biocontrol by maintaining the densely packed multicellular structure of biofilms (Stein, 2005).
There is a need to develop beneficial microbial resources for Bacillus-based biofungicides from diverse ecological niches around the world (Pérez-García et al., 2011). The multifaceted ability of biocontrol agents is suitable for sustainable agricultural production systems (Choudhary and Johri, 2009). Therefore, a pool of Bacillus isolates was isolated from soil for the biocontrol of the most omnitrophic phytopathogen, S. sclerotiorum. Newly isolated multifarious bacilli were analyzed for their suppressive ability against S. sclerotiorum; additionally, a putative mode of action and plant growth-promotional attributes were studied.
Materials and methods
Isolation of Bacillus spp. from soil
Bacterial isolates were randomly isolated from the rhizospheric soil samples of various plants collected from different places in Nihonmatsu, Fukushima, Japan. Soil suspensions were prepared with gentle shaking for 1 h at 25 °C in sterilized water under aseptic conditions. Suspensions were serially diluted (10−3 to 10−5), followed by a heat treatment at 80 °C for 10 min, and 100 μL was plated on a tryptic soy agar (TSA) plate containing 3 % tryptic soy broth (TSB; BD, Bacto™, Becton Dickinson and Company, Franklin Lakes, NJ, USA) and 1.5 % agar. Bacilli-like colonies were roughly identified on the basis of their colony characteristics such as size, shape, color, margin, elevation and opacity, and Gram staining. In addition, motility, formation of endospores, tolerance at 80 °C, and survival on TSA supplemented with 10 % NaCl were examined. All Bacillus isolates used in this study were routinely maintained on TSA at 30 °C. For broth culture, bacteria were grown using TSB on a rotary shaker (BR-40LF Bio-Shaker; Taitec, Saitama, Japan) for 48 to 72 h at 30 °C and 150 rpm.
Initial screening for anti-pathogenic activity
The anti-pathogenic activity of the Bacillus isolates was screened in a dual culture with S. sclerotiorum MAFF244850 (Rahman et al., 2015) as described by Souto et al. (2004). First, S. sclerotiorum MAFF244850 was grown on potato dextrose agar (PDA) at 22 °C. The bacterial isolates were streaked on one side of an S. sclerotiorum plug on PDA medium. Plates that were inoculated with an S. sclerotiorum plug alone were used as the negative control. After incubation for 5 days at 22 °C, the mycelial growth inhibition was evaluated using a scoring system (Perneel et al., 2007): hyphae growing on bacteria =0, hyphae growing by the edge (<0.5 cm) of the bacterial colony =1, and a distinct inhibition zone (>0.5 cm) is found around the bacterial colony =2. The bacterial colonies that induced a distinct inhibition zone (scored as 2) were selected as being active producers of diffusible antagonistic compounds and were selected for further characterization.
Molecular phylogenetics of the isolates
Molecular characteristics of the isolates that demonstrated high antagonistic effects in the control of S. sclerotiorum MAFF244850 in the primary screen were determined by partial sequencing of 16S rDNA (Weisburg et al., 1991) and gyrase subunit A gene (gyrA) (Chun and Bae, 2000) followed by phylogenetic analysis. Total DNA was extracted from each isolate using an ISOPLANT kit (Nippon Gene, Toyama, Japan). Part of 16S rDNA was amplified in a 25-μL reaction tube with the universal primers 27F and 1492R (Table 1) using a KOD FX Neo PCR mix (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. The reaction mixtures were incubated in a Takara PCR Thermal Cycler Dice® Gradient (Takara, Ohtsu, Japan) according to the following program: initial denaturation at 94 °C for 2 min; 30 cycles of denaturation at 98 °C for 10 s, annealing at 62 °C for 30 s, and polymerization at 68 °C for 1 min; and final elongation at 68 °C for 7 min. Amplification of gyrA was performed using primers p-gyrA-f and p-gyrA-r (Table 1) and the following program: initial denaturation at 94 °C for 2 min, followed by 30 cycles of denaturation at 98 °C for 20 s, annealing at 59 °C for 35 s, polymerization at 68 °C for 35 s, and final elongation at 68 °C for 10 min. The other reaction conditions were same as those for 16S rDNA. Five microliters of each amplification mixture was used to verify the results by agarose (1 % w/v) gel electrophoresis in 0.5× Tris-borate-EDTA buffer.
Subsequently, PCR products were purified with the diluted ExoSAP-IT (USB, Cleveland, OH, USA). The nucleotide sequences were then determined by dideoxy sequencing at the sequencing facility in FASMAC Co. Ltd. (Kanagawa, Japan). Partial 16S rDNA and gyrA sequences were generated using the primers described in Table 1. The sequences were combined with BioEdit Sequence Alignment editor 7.0.9®, checked manually, corrected, and then analyzed by the National Center for Biotechnology Information basic local alignment search tool (http://blast.ncbi.nlm.nih.gov/) to identify isolates. Phylogenetic analysis was carried out with the MEGA 6 program, and a neighbor-joining tree was constructed using the Kimura-2-parameter distance model. The phylogenetic trees for 16S rDNA and gyrA were created with sequences of bacteria in GenBank with the greatest homology, and Brevibacillus agri DSM 6348T and Bacillus lichiniformis KCTC 1918T as the outgroup, respectively. Confidence values were assessed from 1000 bootstrap replicates of the original data.
Production of hydrolytic enzymes and plant growth-promotional compounds
Protease activity was expressed as a clearing zone on a Luria-Bertani (LB) plate amended with 2 % skim milk powder after incubation at 28 °C for 48 h (Perneel et al., 2007). The extracellular chitinase activity was studied on chitin minimal agar medium (0.2 % colloidal chitin) (Singh et al., 1999), and β-1,3-glucanase activity was examined on minimal medium supplemented with laminarin as the sole carbon source (Dunne et al., 1997). The isolates were spot-inoculated at the center of the plates. After incubation at 30 °C for 7 days, the development of clear zones around colonies was examined. The cellulase production was determined by the appearance of a halo around a bacterial colony on a carboxymethyl cellulose-amended Czapek-mineral salt agar plate.
Indoleacetic acid (IAA) production by the isolates was examined according to Minaxi et al. (2012). In brief, test bacterial cultures were inoculated separately in LB broth containing L-tryphtophan (3 mg ml−1) and incubated at 28 ± 2 °C for 5 days. Cultures were centrifuged at 3000 rpm for 30 min, and 2 ml of the supernatant was mixed with two drops of orthophosphoric acid and 4 ml Salkowski’s reagent (50 ml 35 % perchloric acid, 1 ml 0.5 M FeCl3). The appearance of pink color indicated the production of IAA. Bacterial isolates were tested for siderophore production on Chrome-azurol S medium (Alexander and Zuberer, 1991). The bacterial isolates were spot-inoculated and incubated at 30 °C for 48–72 h. The formation of an orange to yellow halo around the colonies indicated positive siderophore production. Phosphate solubilization on solid medium was detected by spotting the isolates separately on Pikovskaya’s agar plates containing tricalcium phosphate (Kumar et al., 2012). The plates were incubated at 30 °C for 4–5 days. The development of a halo zone around the colony indicated the phosphate solubilizing capacity of isolates. Bacterial isolates were screened for the production of ammonia in peptone water broth (Ahmad et al., 2008). Bacterial cultures were inoculated separately in 10 ml peptone water and incubated at 30 °C for 4 days. The accumulation of ammonia was detected with the addition of 0.5 ml Nessler’s reagent. A brown to yellow color indicated positive ammonia production.
Biofilm formation
The biofilm formation of Bacillus isolates was monitored using a microtiter plate assay based on the methods of Jackson et al. (2002). Briefly, LB medium was inoculated with diluted bacterial cells of mid-exponential growth, aliquoted into each well of 96-well cell culture plates, and incubated at 28 °C. After incubation for 24, 48, 72, and 96 h, the medium with bacterial cells was discarded from the plate, and the plate was washed with water and stained with 1 % crystal violet. The plates were then rinsed with water again, and the dye was solubilized with 33 % acetic acid. Biofilm formation was quantified by measuring the optical density at 630 nm of each well using a microplate reader (model: 680; Bio-Rad Laboratories Inc., Hercules, CA, USA).
PCR detection of antibiotic biosynthesis genes
Putative genes related to the biosynthesis of antifungal compounds including surfactin, iturin, fengycin, bacilysin, and difficidin were examined by PCR. Total DNA was extracted as described above and was diluted 100-fold in sterile distilled water. PCR for each gene was performed using the KOD FX Neo (Toyobo, Osaka, Japan) PCR mix following the manufacturer’s instructions. The lipopeptide antibiotic surfactin, dipeptide antibiotic bacilysin, iron-siderophore bacillibactin, polyketide antibiotic bacillaene, and difficidin were reported as antibiotic biosynthetic loci in the genome of Bacillus isolates (Chen et al., 2009a; Stein, 2005). Considering this view, eight pairs of primers (Table 1) were used to screen the putative synthase genes bacA, beaB, dfnM, dhbF, fenA, ituA, sfp, and srf (Arguelles-Arias et al., 2009; Zhao et al., 2014). PCR amplification was employed using the following program: initial denaturation at 94 °C for 2 min; 30 cycles of denaturation at 94 °C for 30 s, annealing at the temperature shown in Table 1 for 30 s, and extension at 72 °C for 1 min; and final extension at 72 °C for 10 min. After verification by agarose gel electrophoresis, PCR products were purified with an ExoSAP-IT kit, and nucleotide sequencing was performed at FASMAC. Sequences of respective biosynthesis genes were compared with the similar genes associated with Bacillaceae group bacteria. Phylogenetic trees using the neighbor-joining method were constructed following the procedure described above.
In vitro antagonisms against S. sclerotiorum
Bacterial isolates were tested for their ability to suppress the growth of S. sclerotiorum by an in vitro dual-culture method (Souto et al., 2004). A plug of 0.5-cm diameter containing mycelia taken from a 3-day-old culture of S. sclerotiorum MAFF244850 was placed on one side of PDA plates, and single bacterial colonies were streaked in a straight line at a distance of about 4 cm from the fungal plug. Plates without inoculation of the bacterial isolate served as the control.
The antifungal activities of cell-free culture filtrate (CFCF) of the isolates were also evaluated. CFCF of each isolate was obtained from its log-phase culture by centrifugation at 8000 rpm and 4 °C for 10 min and subsequent filtration with a 0.20-μm membrane filter (DISMIC®-25CS; Advantec, Toyo Roshi Kaisha Ltd., Tokyo, Japan). A mycelial plug of S. sclerotiorum MAFF244850 was prepared as above and was placed in the center of a PDA plate. The bacterial CFCF was aliquoted into wells (0.5 cm) made on four sides of each plate 3 cm from the fungal plug. Plates without CFCF served as the control.
In addition, growth inhibition of S. sclerotiorum with volatile compounds released by the Bacillus isolates was tested. Two-day-old culture of each bacterial isolate was streaked on a TSA plate and was incubated for 24 h at 28 °C. A PDA plate with actively growing S. sclerotiorum MAFF244850 was prepared as described above. The lids of the two plates were then removed, and the fungal plate was inverted onto the Bacillus plate. The edges of the two plates were taped together with plastic paraffin film. Control plates were also prepared in the same way except that a non-inoculated plate was used instead of the Bacillus plate.
In the three in vitro assays, there were three replicates per treatment and five plates per replication. In all cases, these plates were incubated for 5 days at 22 °C, and the inhibition of fungal growth was measured using the following formula:
where C = colony growth of S. sclerotiorum in the control and T = colony growth of the fungus. In all cases of the dual-culture, CFCF, and volatile assays, the number of sclerotia per plate was also recorded at 15 days of incubation. The percentage reduction of sclerotia was calculated relative to the control plate without bacterial cells during the tests.
Microscopic studies
For light microscopic studies, the inhibition zone on the dual-culture plate of fungal interaction with bacterial antagonists was focused with an inverted compound microscope, and the same microscopic field was observed every day up to 7 days. Samples of similar areas of interaction were used in scanning electron microscopy (SEM) to evaluate the antagonist effects of the bacterial isolates on S. sclerotiorum according to Sharma and Sharma (2008). Around 2–3-mm segments of the mycelium with a thin layer of upper portion of PDA were cut and rapidly placed in glass vials containing 3 % glutaraldehyde prepared with 0.05 M phosphate buffer (pH 7.2). Samples were then fixed in this solution at 4 °C for 48 h, washed with the same buffer, and dehydrated in an ethanol series for 30 min in each dilution and finally in absolute ethanol for 24 h. Then, dried samples were mounted over the stubs with double-stick adhesive carbon tape and coated with gold using a vacuum ion sputter coater (Eiko IB-3; Eiko Engineering Co. Ltd., Tokyo, Japan) for about 3 min. Finally, samples were analyzed by a scanning electron microscope (JSM-6510LA; JEOL Ltd., Tokyo, Japan) operating at 20 kV with different magnification.
Effects of the Bacillus isolates on apothecia of S. sclerotiorum were also examined. Young and small apothecia developed by the method described in Rahman et al. (2015) were used with stalk and sclerotia and were soaked in an actively grown bacterial suspension separately for 24 h and then placed on sterile moist soil in a Petri dish. After incubation for two weeks, apothecia were subjected to electron microscopy. As a control, similar apothecial samples were prepared following the same procedure using sterilized water. Ascospore germination tests were done following the method described by Souto et al. (2004) using diluted (50 %) cell-free supernatant and observed under a light microscope.
Seed bacterization study
Seeds of mustard (Kibana-no-chikara, Takii Co., Kyoto, Japan) after surface sterilization were immersed in diluted bacterial culture (104 cfu ml−1) of the Bacillus isolates for 30 min. With modification of the method of Abdullah et al. (2008), two mycelial plugs (5 mm in diameter) of S. sclerotiorum were placed on both sides of a PDA plate. Five bacterized seeds were then placed in a row between the two mycelial plugs. For each bacterial isolate, 10 plates were maintained, and the experiment was performed in triplicate. There were three replicates per treatment and 10 plates per replication. Untreated seeds were used as a control. After incubation for 3 and 7 days at 25 °C, numbers of healthily germinated seeds and infected seedlings were recorded.
Detached leaf inoculation assay
Detached leaves of the same mustard variety of the same age, size, and position were used for in vitro evaluation of efficacy of the bacterial antagonists. A single leaf was detached from each 3-week-old plant to maintain the uniformity of the leaf. The cells of 8 bacterial isolates were harvested separately from 48-h broth culture by centrifugation at 3000 rpm for 10 min, and the pellet was resuspended in sterile distilled water amended with 0.05 % Tween 20 and adjusted to 5 × 108 cfu ml−1. Detached leaves were soaked in a prepared bacterial suspension and were air dried inside a clean bench. Three treated leaves were placed on a glass slide on moist blotter in a 15-cm plastic Petri plate to avoid direct contact with water. Mycelial discs that were 5 mm in diameter were taken from an actively grown culture of S. sclerotiorum on PDA and inoculated singly on each leaf at the same position. A control treatment was inoculated with culture of S. sclerotiorum with soaking in sterilized distilled water. Plates were incubated in a growth chamber (Biotron, NK System, Osaka, Japan) maintained at 22 °C, 85 % relative humidity, and 12-h alternating photoperiod under 12,000 lx (fluorescent lighting). The experiments were conducted with three replications, and 9 leaves were inoculated in each replication. The area of infection with S. sclerotiorum on each detached leaf was recorded after 1–5 days of incubation based on the average infection area (%), and the area under disease progress curve (AUDPC) was calculated using the following formula (Shaner and Finney, 1977):
where Y i = diseased area (%) at the ith observation, X i = time (days) at the ith observation, and n = total number of observations. Finally, data were tabulated as the percent reduction of AUDPC value relative to the control treatment.
Suppression of white mold disease with foliar spray of lead bacterial isolates
Mustard seedlings (Kibana-no-chikara, Takii Co.) were grown in a greenhouse to assess the efficacy of foliar spray of the Bacillus isolates to suppress white mold disease. Seeds were planted in a pot filled with commercially available potting soil mix after autoclaving. Water was regularly applied to mustard seedlings when needed. Top meristems of V3-stage seedlings were cut off to keep tri-foliar blades during experiments (Zhang and Xue, 2010). Bacillus isolates prepared using the procedure described in the detached leaf inoculation test were applied to V3-stage mustard with a hand-pump sprayer at a rate of 5 ml per plant. After air drying for 1 h, the plants were inoculated with 5 ml homogenized mycelial suspension (optical density at 600 nm [OD600] = 1.8) of S. sclerotiorum MAFF244850. Mustard inoculated with S. sclerotiorum alone was prepared as a control. The pots were randomly arranged in a growth chamber set to cycle at 12 h light (20,000 lx, fluorescent lighting) at 22 °C and 12 h dark at 18 °C. The relative humidity was maintained at 95 %. Fourteen days after the inoculation with S. sclerotiorum, disease severities were evaluated with a 0–5 scale (Zhang and Xue, 2010), where 0 = no disease, 1 = few necrosis or lesions on leaves with a total diseased area < 5 %, 2 = several lesions on leaves with total diseased area of 5–10 %, 3 = many lesions with total diseased area of 11–30 % on leaves and stem rot occurring on stem tops, 4 = large lesions on leaves with total diseased area of 31–50 % and rotted stem top, and 5 = lesions on leaves with total diseased area of more than 50 % and rot on stem top progressing downward or plant dying. The experiment was performed twice, and each treatment within an experiment had three replicates that contained 15 plants. Using the disease rating scale, the percent disease suppression of white mold disease was calculated according to Idris et al. (2007) as follows:
where A is the disease severity exhibited by the white mold disease due to S. sclerotiorum alone, and B is the disease severity exhibited after application of both the pathogen and bacterial antagonists.
Effect on seed germination and seedling vigor
For the seed germination study, 25 ml bacterial inoculum (108 cfu ml−1) and 0.1 g carboxymethyl cellulose (CMC) were added into a 100-ml Erlenmeyer conical flask. Seeds (10 g) were surface sterilized and soaked in a bacterial suspension of the isolate using a rotary shaker for 30 min at 150 rpm. The bacterial suspension was drained, the seeds were dried overnight aseptically in laminar air flow, and 10 seeds per plate were placed on solid agar plates. The experiment was done in triplicate, and seeds soaked in sterile deionized water amended with CMC served as a control. All plates were incubated at 28 ± 2 °C for 7 days. The number of germinated seeds and length of radicles and plumules were recorded, and the vigor index was calculated using the following formula (Abdul-Baki and Anderson, 1973):
Statistical analysis
Experimental data were analyzed using standard analysis of variance. The treatment means were separated at the 5 % significance level using Tukey’s multiple range test.
Nucleotide sequence accession numbers
The sequences for 16S rDNA and gyrA obtained in this study have been deposited in the DNA Data Bank of Japan (DDBJ) under accession numbers AB971198 to AB97120 and LC096068 to LC096075, respectively. Sequences of biosynthetic genes were also assigned in DDBJ under accession numbers AB973460 to AB973467, AB973469 to AB973476, AB973478 to AB973485, AB973487 to AB973494, and AB971633 to AB971640 for bacA, dfnM, fenA, ituA, and sfp, respectively.
Results
Isolation and characterization of antifungal Bacillus isolates
Among the total collection of 122 Bacillus isolates, 16 showed antifungal activity against S. sclerotiorum MAFF244850 (Table S1). All isolates were Gram-positive, endospore-forming, motile, and rod-shaped isolates with folded and irregular-edged colonies and showed heat and salt resistance. On the basis of the antifungal activity in the initial screening (Table S1), eight isolates, RNB-88, 92, 98, 100, 107, 110, 113, and 117, were selected for further investigation. Molecular characterization of the eight effective isolates by 16S rDNA and gyrA sequencing indicated that they belonged to B. amyloliquefaciens subsp. plantarum (Fig. 1a and b).
Phylogenetic trees of the Bacillus isolates (filled diamond) based on the 16S rDNA (a) and gyrA (b) sequences. The trees were constructed using the neighbor-joining method, and genetic distances were generated using the Kimura 2-parameter method. The numbers at the branches are bootstrap confidence percentages from 1000 trial replications. Brevibacillus agri DSM 6348T and Bacillus licheniformis KCTC 1918T were used as an outgroup in respective trees. Numbers in parentheses indicate the accession numbers in GenBank. Bar: 2 % of sequence divergence
Antifungal enzymatic production and plant growth-promotional attributes
It is common to consider different traits of bacteria as biocontrol agents to screen suitable microorganisms for disease control. In this study, we first investigated different modes of action such as direct antibiosis through the antifungal enzymatic production for the eight isolates as shown in Table 2. The eight Bacillus isolates showed protease activity. All isolates showed chitinase activity except RNB-107. All isolates except RNB-100 grew on minimal medium supplemented with laminarin azure, indicating that they had β-1,3-glucanase activity. Cellulase activity was not detected. We then investigated the plant growth-promotional attributes of the eight isolates. Isolates RNB-88, 92, 98, 113, 117, and 110 produced IAA (Table 2). Siderophore production, phosphate solubilization, and NH3 production were common features of the isolates.
Biofilm formation
All of the tested bacterial isolates formed biofilms that yielded varied OD630 values in a microtiter plate assay. High levels of biofilm formation were recorded by RNB-113, RNB-107, and RNB-88 followed by RNB-92. Biofilm formation was reduced after 72 h of incubation except by RNB-98 and RNB-107 (Fig. S2).
Molecular analysis and genetic evidence of antibiotic biosynthesis genes
Five biosynthetic genes, bacA, dfnM, fenA, ituA, and sfp, of eight tested genes had positive bands in the eight Bacillus isolates. According to the genetic diversity of biosynthetic genes, the bacA, dfnM, and sfp showed little diversity among the isolates (Fig. 2a, b, and e). In contrast, the fenA and ituA were more diverse in the isolates (Fig. 2c and d).
Antagonistic activity against S. sclerotiorum
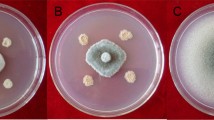
Mycelial growth inhibition and reduced sclerotia formation were observed in in vitro tests like dual culture, CFCF assays, and volatile assays. Bacillus isolates produced distinct “zones of inhibition” on PDA plates in both dual culture and CFCF assays (Fig. 3). S. sclerotiorum was suppressed 65.9–82.5 % in dual-culture interaction with the Bacillus isolates (Fig. 4a). The highest (82.5 %) growth suppression was recorded from isolate RNB-88 followed by RNB-92 (82.0 %), RNB-110 (75.3 %), and RNB-117 (74.9 %). The other four isolates, RNB-98 (71.3 %), RNB-100 (65.9 %), RNB-107 (66.8 %), and RNB-113 (68.6 %), showed statistically similar growth suppression of S. sclerotiorum in dual culture on PDA. CFCF of Bacillus isolates showed potential performance to suppress the growth of S. sclerotiorum from 38.3 to 62.2 % (Fig. 4b). Isolates RNB-92 showed the highest (62.2 %) growth inhibition of S. sclerotiorum followed by RNB-117 (59.4 %), RNB-110 (57.8 %), and RNB-88 (57.5 %). Statistically similar performance of growth suppression using CFCF was recorded from isolates RNB-98 (50.8 %), RNB-100 (46.1 %), RNB-107 (40.6 %), and RNB-113 (38.3 %). In addition, the eight potential isolates also produced a varied range of volatiles that inhibit mycelia of the fungus (Fig. 4c). High volatile suppression was recorded in RNB-92 (54.4 %) and RNB-110 (53.3 %) in an assay against S. sclerotiorum. Isolates RNB-88 (51.0 %), RNB-92, and RNB-110 suppressed the growth of S. sclerotiorum similarly. Comparatively lower growth inhibition through volatile production was recorded with isolates RNB-100 (34.0 %) and RNB-107 (28.3 %). The overall trend of mycelial growth suppression of the fungus was dual culture > CFCF assay > volatile assay in vitro tests.
Growth suppression and reduction of number of sclerotia of Sclerotinia sclerotiorum MAFF244850 with antifungal Bacillus isolate RNB-92 in dual culture and cell-free culture filtrate (CFCF) assay. Mycelial growth inhibition was observed as a distinct zone of inhibition with the bacterial line on a dual-culture plate (a), full-plate growth on the control plate of the dual-culture assay (b), mycelial growth retardation around the hole containing CFCF (c), and full-plate growth of S. sclerotiorum in the control (d)
The development and formation of sclerotia was also reduced during the interaction with the Bacillus isolates in in vitro culture on PDA plates (Table 3). The number of sclerotia reduced over control varied from 70.7 to 82.7 %, 48.7 to 59.5 %, and 48.9 to 66.0 % during dual culture, CFCF assay, and volatile assay, respectively. The reduction of sclerotia was greatest in the dual culture, and the general trend of reduction was dual culture > volatile assay > CFCF assay.
Microscopic studies
During dual culture with the bacterial isolates, while no mycelial abnormality was observed by microscopy after 3-days incubation (Fig. 5a-1), morphological deformities such as lysis of mycelia, exudation of cytoplasmic materials, and blackening of mycelia were found after 5-days incubation (Fig. 5a-2). In all cases of Bacillus spp., the intensity of mycelial deformities increased with incubation period from 4 to 7 days. SEM confirmed the curling of mycelia, along with mycelial abnormalities like the breakdown of the cell wall (Fig. 5b and c). Control plates without bacterial isolates showed normal and actively growing hyphae with no such deformities. In all cases of bacterial treatments, apothecia were abnormal and dead, and they failed to produce ascospores (Fig. 5d); apothecia from the control treatment yielded ascospores as usual. Germination of ascospores was normal in the water treatment (Fig. 5e). Cell-free supernatants from all Bacillus isolates inhibited germination of ascospores in separate tests (Fig. 5f).
Microscopic observation of the antagonistic effect of Bacillus isolate RNB-92 against S. sclerotiorum MAFF244850 on a potato dextrose agar plate during dual culture. Normal mycelia after incubation for 3 days (a-1) and mycelial tip and cell wall lysis with blackening of hyphae after incubation for 5 days (a-2) in the same examination field of interaction observed under an inverted compound microscope. Mycelial tip lysis with curling (b) and cell wall breakdown of the mycelium (c) were also observed with a scanning electron microscope (JEOL, JSM-6510LA) after incubation for 5 days. Abnormal and dead apothecia after treatment with diluted bacterial culture (d), germination of an untreated ascospore (e), and inhibition of ascospore germination due to treatment with cell-free supernatant (f)
Seed bacterization for protection from S. sclerotiorum
Figure 6a indicates that seed bacterization with Bacillus isolates gave protection from seed rot due to infection by S. sclerotiorum; this protection was up to 98.3 % by isolates RNB-92, followed by RNB-110 (97.7 %) and RNB-88 (95.3 %). Through seed bacterization, at least 87.3 % (RNB-107) of seedlings were healthy. Seed rotting was avoided even with a high level of inoculum pressure of S. sclerotiorum from both sides on PDA. In contrast, seedlings in the control treatment without bacterial isolates were infected by the fungus after 7 days of incubation (Fig. 7a and b).
Control of seedling rot, leaf rot, and white mold disease using antagonist Bacillus isolates. Inhibition of mycelia of S. sclerotiorum resulting in protection from seedling rot through seed bacterization with Bacillus RNB-92 (a) and seedling rot with black sclerotia on the control plate without treatment with the antagonist (b). No infection was observed after 72 h of incubation on the detached leaf of mustard treated with Bacillus isolates followed by inoculation with a mycelial block of S. sclerotiorum (c); rotting from the inoculation point was observed on a leaf that was not treated with a Bacillus isolate within 72 h after infection by S. sclerotiorum (d). Healthy plants were obtained after application of Bacillus RNB-92 followed by spray inoculation of S. sclerotiorum (e), and leaf rot and stem rot including leaf-dropping symptoms were observed in the control treatment without application of Bacillus biocontrol agents (f)
Detached leaf inoculation methods
In the detached leaf assay, disease suppression by antagonist bacteria also was recorded by evaluating disease progress after calculating the AUDPC value (Fig. 6b). The AUDPC valued was reduced 71.6 to 91.1 % of that of the control by using bacterial isolates in the in vitro conditions of a detached leaf on moist blotter method. The highest reduction (91.1 %) of AUDPC was recorded from the application of RNB-110, followed by RNB-92 (90.7 %), RNB-88 (90.0 %), and RNB-113 (87.4 %). Bacterial cell suspensions on a detached leaf protected and delayed the leaf infection by S. sclerotiorum up to a certain period of time compared to the untreated control (Fig. 7c and d).
Suppression of white mold disease after foliar spray with bacterial isolates
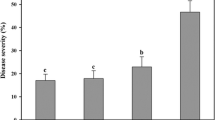
The suppression of white mold disease of mustard with the foliar spray ranged from 80.0 to 90.0 % with the four lead bacterial isolates (Fig. 6c). The highest (90.0 %) reduction of white mold disease was observed after the application of isolate RNB-92. Foliar spray of RNB-88, RNB-110, and RNB-113 reduced mold disease over the pathogen control by 88.5 %, 87.1 %, and 80.1 %, respectively. Leaves and plants remained healthy with the application of bacterial cells. Conversely, leaves and stems rotted and the disease incidence reached the top grade of the disease rating scale (5 in the 0–5 scale) with the sole application of S. sclerotiorum (Fig. 7e and f).
Effect on seed germination and seedling vigor
Evidence of plant growth promotion and an increase germination rate were observed through seed bacterization on agar plates (Table S3). The seed germination (%) in all cases except the control treatment was statistically similar, and the germination increase over the control ranged from 25.3 % to 30.2 %. In seedling vigor, statistically similar growth was recorded from RNB-113 (700), RNB-92 (675), RNB- 98 (672), RNB- 88 (668), and RNB-117 (665); this was followed by treatment with RNB-110 (624). A range from 30.8 % to 87.2 % increase in seedling vigor was recorded from the assay, suggesting that there was significant growth promotion over the untreated control (Table S3).
Discussion
In this study, eight potential isolates that showed antagonistic performance against S. sclerotiorum were isolated. Phenotypic characteristics and 16S rDNA gene sequencing confirmed that all of the isolates belonged to the Bacillus genus (Fig. 1a). Although 16S rDNA sequencing is a powerful tool for identification along with phylogenetic and evolutionary relationship among bacteria (Weisburg et al., 1991), it often shows a limitation in case of closely related taxa. Protein-coding genes exhibiting high genetic variation can be used for more accurate classification and identification. Therefore, we determined partial gyrA sequences of the isolates (Chun and Bae, 2000: Borriss et al., 2011) and confirmed that all of them could be assigned to B. amyloliquefaciens subsp. plantarum (Fig. 1b).
The eight B. amyloliquefaciens isolates were endospore formers and showed an ability to endure stress conditions like high temperature (80 °C) and high NaCl concentration. Bacillus isolates were commonly reported to survive in stress and adverse environmental conditions (Pérez-García et al., 2011). Various diseases caused by fungi, bacteria, systematic viruses, and nematodes on a diversity of hosts were reduced and managed with the application of several isolates of Bacillus including B. amyloliquefaciens, B. subtilis, B. cereus, and B. pumilis (Choudhary and Johri, 2009; Kloepper et al., 2004). Major biocontrol mechanisms used by Bacillus spp. are direct antagonism to phytopathogens (Yu et al., 2002) and stimulation of plant host defenses through the induction of systemic resistance (Kloepper et al., 2004; Whipps, 2001; Choudhary and Johri 2009).
The biocontrol mechanism of the isolates regarding the production of lytic enzymes to hydrolyze the cell wall of spores and hyphae of fungal pathogens was studied (Table 2 and Fig. 5). Chitinase, which seven of the eight isolates produced, hydrolyzes chitin to oligo- and monomeric components through the breakdown of glycoside. Chitinases are important constituents of several bacterial species, including Bacillus spp., in the biological control of fungal pathogens (e.g., Singh et al., 1999). Like chitinase, β-1,3-glucanase is a potent enzyme in the control of fungal pathogens that is also produced by several microorganisms including Bacillus spp. (Leelasuphakul et al., 2006). Purified forms of such metabolites and lytic enzymes inhibit the mycelial growth of certain fungi (Leelasuphakul et al., 2006).
In this study, we did not observe a positive reaction regarding the excretion of cellulase by the tested Bacillus isolates (Table 2). This result regarding the production of cellulase is consistent with the report of Cazorla et al. (2007). Extracellular lytic enzymes like proteases protect fungi like Pythium ultimum, the causal agent of damping disease of sugarbeet (Dunne et al., 1997).
IAA was produced by the Bacillus isolates in an in vitro test that used tryptophan as a precursor for IAA biosynthesis (Table 2). Similar results were obtained for Bacillus isolates reported by Ahmad et al. (2008). According to the findings of Minaxi et al. (2012), the production of IAA was much higher in the presence of higher amounts of tryptophan in the medium. Ammonia production was also reported as a common trait of several plant growth-promoting rhizobacteria (Ahmad et al., 2008).
The eight isolates showed phosphate solubilization and siderophore formation (Table 2). Ahmad et al. (2008) reported that 80 % of tested Bacillus isolates showed phosphate-solubilizing ability. Siderophores produced by rhizospheric bacteria increase the availability of iron near roots that can enhance plant growth (Alexander and Zuberer, 1991). Additionally, rhizobacteria can protect plants from disease by producing a range of iron-chelating compounds or siderophores that make iron unavailable, resulting in the growth retardation of pathogenic fungi (Whipps, 2001).
All of the Bacillus isolates formed biofilms (Fig. S1). A surfactin-producing biofilm is expected to prevent other microbes and is suggested as an important mode for the colonization of Bacillus isolates on root and leaf surfaces (Pérez-García et al., 2011). Stable biofilms and the secretion of surfactin act together for biological protection from pathogenic microbes (Stein, 2005; Ongena and Jacques, 2007).
The PCR detection methods confirmed that the isolates possessed multiple genes for the biosynthesis of several metabolites involved in disease suppression by Bacillus bacteria (Fig. 2). An average of 4–5 % and around 8 % of its genome is devoted to antibiotic biosynthesis in B. subtilis (Stien, 2005) and B. amyloliquefaciens (Arguelles-Arias et al., 2009, Chen et al., 2009a), respectively. Various biologically active molecules synthesized by Bacillus species have been reported to show antagonistic actions, suppressing the growth and development of different phytopathogens through hyphal cell perturbation and/or permeabilization of spores or conidia, resulting in germination failure (Zhao et al., 2014). Three main families of lipopeptides, surfactins, iturins, and fengycins, are mostly reported for their antagonistic activity toward a wide range of phytopathogens (Ongena and Jacques, 2007). In addition, antibacterial polyketides like difficidin and the dipeptide antibiotic bacilysin were responsible for the biocontrol of plant pathogens (Arguelles-Arias et al., 2009). Surfactin, a potent biosurfactant, is reported to demonstrate hemolytic and antimicrobial activities along with biofilm formation (Ongena and Jacques, 2007). Antibiotic lipopeptides like iturin showed their growth-inhibitory effect toward several pathogenic fungi (e.g., Yu et al., 2002). The lipopeptide fengycin can efficiently suppress the growth and development of filamentous fungi like Sclerotinia (Hou et al., 2006) and Fusarium (Zhao et al., 2014). Difficidin shows broad-spectrum antibacterial activity. Bacilysin and difficidin inhibit the growth of Erwinia amylovora (Chen et al., 2009b). Moreover, potential cyclic lipopeptides like surfactins and fengycins were reported as elicitors of induced systemic resistance (Ongena and Jacques, 2007).
All eight screened Bacillus isolates showed high inhibition in dual culture with the target fungus (Fig. 4a). There were clear inhibition zones (>0.5 cm) similar to the initial screening step for isolating effective isolates (Table S1). The percentage of mycelial growth inhibition in vitro by Bacillus isolates was up to 82.5 % in the dual-culture test. These results concur with those of other researchers (Souto et al., 2004) regarding a dual-culture–based approach, which can be an important tool to evaluate fungal growth suppression with bacterial biocontrol agents.
The formation of an inhibition zone through the reduction of radial growth of target phytopathogens (Fig. 3) was due to the excretion of antimicrobial metabolites (Kumar et al., 2012). Changes in mycelial growth color with growth suppression also were observed during the co-culture of S. sclerotiorum with antagonistic bacteria. Similar observations regarding the retardation of in vitro radial extension of S. sclerotiorum and mycelial discoloration with the suppression of germ tube formation were reported by Yang et al. (2009) and Sharma and Sharma (2008).
The zone of inhibition formed in both the dual culture and the cell-free supernatant bioassay plates (Fig. 4a and b) indicated the presence of biologically active metabolites that diffused in agar medium. According to Yoshida et al. (2001), culture filtrate inhibited the in vitro growth of several phytopathogenic fungi and bacteria such as Rosellinia necatrix, Pyricularia oryzae, S. sclerotiorum, Agrobacterium tumefaciens, and Xanthomonas campestris pv. campestris and exhibited preventive effects against disease development.
Antagonistic bacterial volatiles adversely affect fungal growth and development (Fig. 4c). Interactions with volatiles from B. subtilis revealed inhibitory effects like morphological aberration of hyphae and conidia of plant pathogens (Chaurasia et al., 2005). In the present study, the overall growth suppression during dual culture and the cell-free supernatant assay was higher than the volatile inhibition; these results differed from the findings of Chaurasia et al. (2005).
Microscopic observations during the interaction between antagonistic Bacillus spp. and S. sclerotiorum revealed morphological abnormalities in fungal structures (Fig. 5). Lysis of hyphae and cytoplasmic exudation from the fungus were recorded when it was challenged with the eight Bacillus isolates; these observations were supported by reports of Abdullah et al. (2008) and Chaurasia et al. (2005). Morphological changes including twisting and coiling of mycelia and pore formation in the mycelial wall of S. sclerotiorum occurred during interactions with biocontrol agents like B. subtilis (Sharma and Sharma, 2008; Yang et al., 2009). Although there was no report regarding direct antagonism on apothecia, the failure of ascospores to germinate with antifungal metabolites of Bacillus sp. was reported by Souto et al. (2004).
Our observations regarding seed bacterization (Fig. 6a) comply with other reports where seed treatment with Bacillus isolates had a positive effect on seed germination of different crops and suppressed seedling diseases caused by several phytopathogens (Zheng and Sinclair, 2000). The treatment of seeds with Bacillus isolates reduced disease incidence up to 65 % when co-inoculated with Colletotrichum gloeosporioides (Ashwini and Srividya, 2013).
Bacillus isolates substantially reduced the growth of S. sclerotiorum in the detached leaf inoculation method (Fig. 6b). In another report, B. subtilis reduced Alternaria spore germination on pieces of mustard leaf in an in vitro test (Sharma and Sharma, 2008).
The four lead bacterial isolates that we identified effectively suppressed white mold disease of mustard in growth chambers (Fig. 6c). The mechanism involved in foliar disease suppression might involve inhibiting inocula and enhancing induced systemic resistance. Hu et al. (2005) observed a significant reduction of disease incidence caused by S. sclerotiorum on oilseed rape under artificial infection. Foliar application of B. subtilis on soybean plants under control conditions effectively reduced stem rot caused by S. sclerotiorum (Zhang and Xue, 2010).
The ability of bacilli to promote plant growth is well documented. This study also revealed that seed germination and vigor of mustard seedlings were improved by seed bacterization with the Bacillus isolates (Table S3). Bacillus populations increase the bioavailability of mineral nutrients and essential compounds to the host rhizosphere. Several Bacillus species, such as B. amyloliquefaciens, contribute to promote auxin (IAA), which might stimulate root proliferation and nutrient uptake (Pérez-García et al., 2011). Beneduzi et al. (2012) also mentioned that bacteria of diverse genera were effective promoters of plant growth; among this, Bacillus spp. form a predominant group. Plant growth promotion and the elicitation of induced systemic resistance by plant-associated bacilli such as B. amyloliquefaciens, B. subtilis, B. cereus, B. pumilus, and B. mycoides were explained against various phytopathogens (Choudhary and Johri, 2009; Kloepper et al., 2004).
In conclusion, the isolated Bacillus spp. effectively suppressed the growth of S. sclerotiorum and the formation of the sclerotia in in vitro and in vivo assays. The efficacy of biological control agents depends on their reliability in field conditions because of the high variability in efficacy, survival, and production of metabolites. As future work, the Bacillus isolates should be tested in field trials under different environmental conditions.
References
Abdul-Baki AA, Anderson JD (1973) Vigour determination in soybean seed by multiple criteria. Crop Sci 13:630–633
Abdullah MT, Ali NY, Suleman P (2008) Biological control of Sclerotinia sclerotiorum (Lib.) de bary with Trichoderma harzianum and Bacillus amyloliquefaciens. Crop Prot 27:1354–1359
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45
Arguelles-Arias A, Ongena M, Halimi B, Lara Y, Brans A, Joris B, Fickers P (2009) Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb Cell Factories 8(63). doi:10.1186/1475-2859-8-63
Ashwini N, Srividya S (2013) Potentiality of Bacillus subtilis as biocontrol agent for management of anthracnose disease of chilli caused by Colletotrichum gloeosporioides OGC1. 3 Biotech. doi:10.1007/s13205-013-0134-4
Beneduzi A, Ambrosini A, Passaglia LMP (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35:1044–1051
Bolton MD, Thomma BPHJ, Nelson BD (2006) Sclerotinia sclerotiorum (Lib.) de bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol 7:1–16
Borriss R, Chen XH, Rueckert C, Blom J, Becker A, Baumgarth B, Fan B, Pukall R, Schumann P, Spröer C, Junge H, Vater J, Pühler A, Klenk HP (2011) Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7T and FZB42T: a proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. Int J Syst Evol Microbiol 61:1786–1801
Cazorla FM, Romero D, Pérez-García A, Lugtenberg BJJ, de Vicente A, Bloemberg G (2007) Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J Appl Microbiol 103:1950–1959
Chaurasia B, Pandey A, Palni LMS, Trivedi P, Kumar B, Colvin N (2005) Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol Res 160:75–81
Chen XH, Koumoutsi A, Scholz R, Schneider K, Vater J, Süssmuth R, Piel J, Borriss R (2009a) Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol 140:27–37
Chen XH, Scholz R, Borriss M, Junge H, Mögel G, Kunz S, Borriss R (2009b) Difficidin and bacilysin produced by plant-associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. J Biotechnol 140:38–44
Choudhary DK, Johri BN (2009) Interactions of Bacillus spp. and plants with special reference to induced systemic resistance (ISR). Microbiol Res 164:493–513
Chun J, Bae KS (2000) Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie Van Leeuwenhoek 78:123–127
Dunne C, Crowley JJ, Moënne-Loccoz Y, Dowling DN, de Bruijn FJ, O’Gara F (1997) Biological control of Pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiol 143:3921–3931
Gerlagh M, Goossen-van d GHM, Fokkema NJ, Vereijken PFG (1999) Long-term biosanitation by application of Coniothyrium minitans on Sclerotinia sclerotiorum-infected crops. Phytopathology 89:141–147
Hou X, Boyetchko SM, Brkic M, Olson D, Ross A, Hegedus D (2006) Characterization of the anti-fungal activity of a Bacillus spp. associated with sclerotia from Sclerotinia sclerotiorum. Appl Microbiol Biotechnol 72:644–653
Hu X, Roberts DP, Jiang M, Zhang Y (2005) Decreased incidence of disease caused by Sclerotinia sclerotiorum and improved plant vigor of oilseed rape with Bacillus subtilis Tu-100. Appl Microbiol Biotechnol 68:802–807
Idris HA, Labuschagne N, Korsten L (2007) Screening rhizobacteria for biological control of Fusarium root and crown rot of sorghum in Ethiopia. Biol Control 40:97–106
Jackson DW, Suzuki K, Oakford L, Simecka JW, Hart ME, Romeo T (2002) Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J Bacteriol 184:290–301
Kloepper JW, Ryu C-M, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167:493–499
Leelasuphakul W, Sivanunsakul P, Phongpaichit S (2006) Purification, characterization and synergistic activity of β-1,3-glucanase and antibiotic extract from an antagonistic Bacillus subtilis NSRS 89-24 against rice blast and sheath blight. Enzym Microb Technol 38:990–997
McSpadden Gardener BB (2004) Ecology of Bacillus and Paenibacillus spp. in agricultural systems. Phytopathology 94:1252–1258
Minaxi LN, Yadav RC, Saxena J (2012) Characterization of multifaceted Bacillus sp. RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi arid deserts. Appl Soil Ecol 59:124–135
Mueller DS, Dorrance AE, Derksen RC, Ozkan E, Kurle JE, Grau CR, Gaska JM, Hartman GL, Bradley CA, Pedersen WL (2002) Efficacy of fungicides on Sclerotinia sclerotiorum and their potential for control of Sclerotinia stem rot on soybean. Plant Dis 86:26–31
Ongena M, Jacques P (2007) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Pérez-García A, Romero D, de Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of bacilli in agriculture. Curr Opin Biotechnol 22:187–193
Perneel M, Heyrman J, Adiobo A, De Maeyer K, Raaijmakers JM, De Vos P, Höfte M (2007) Characterization of CMR5c and CMR12a, novel fluorescent Pseudomonas strains from the cocoyam rhizosphere with biocontrol activity. J Appl Microbiol 103:1007–1020
Purdy LH (1979) Sclerotinia sclerotiorum: history, diseases and symptomatology, host range, geographic distribution and impact. Phytopathology 69:875–880
Rahman MME, Dey TK, Hossain DM, Nonaka M, Harada N (2015) First report of white mold caused by Sclerotinia sclerotiorum on jackfruit. Australasian Plant Dis Notes 10:10. doi:10.1007/s13314-014-0155-9
Shaner G, Finney RE (1977) The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology 67:1051–1056
Sharma N, Sharma S (2008) Control of foliar diseases of mustard by Bacillus from reclaimed soil. Microbiol Res 163:408–413
Shi J, Li Y, Qian H, Du G, Chen J (2004) Pre-germinated conidia of Coniothyrium minitans enhances the foliar biological control of Sclerotinia sclerotiorum. Biotechnol Lett 26:1649–1652
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 89:92–99
Souto GI, Correa OS, Montecchia MS, Kerber NL, Pucheu NL, Bachur M (2004) Genetic and functional characterization of a Bacillus sp. strain excreting surfactin and antifungal metabolites partially identified as iturin-like compounds. J Appl Microbiol 97:1247–1256
Steadman JR (1979) Control of plant diseases caused by Sclerotinia species. Phytopathology 69:904–907
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–511
Yang D, Wang B, Wang J, Chen Y, Zhou M (2009) Activity and efficacy of Bacillus subtilis strain NJ-18 against rice sheath blight and sclerotinia stem rot of rape. Biol Control 51:61–65
Yoshida S, Hiradate S, Tsukamot T, Hatakeda K, Shirata A (2001) Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology 91:181–187
Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL (2002) Production of iturinA by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963
Zhang JX, Xue AG (2010) Biocontrol of sclerotinia stem rot (Sclerotinia sclerotiorum) of soybean using novel Bacillus subtilis strain SB24 under control conditions. Plant Pathol 59:382–391
Zhao P, Quan C, Wang Y, Wang J, Fan S (2014) Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. spinaciae. J Basic Microbiol 54:448–456
Zheng XY, Sinclair JB (2000) The effects of traits of Bacillus megaterium on seed and root colonization and their correlation with the suppression of Rhizoctonia root rot of soybean. BioControl 45:223–243
Acknowledgments
The authors wish to thank Dr. Guan and Mr. Shoji, Niigata University, Japan, for assisting with the collection of soil samples for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interest regarding the publication of this article.
Rights and permissions
About this article
Cite this article
Rahman, M.M., Hossain, D.M., Suzuki, K. et al. Suppressive effects of Bacillus spp. on mycelia, apothecia and sclerotia formation of Sclerotinia sclerotiorum and potential as biological control of white mold on mustard. Australasian Plant Pathol. 45, 103–117 (2016). https://doi.org/10.1007/s13313-016-0397-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-016-0397-4