Abstract
Hypoglossal nerve stimulation (HGNS) therapy was approved in 2014 for the treatment of obstructive sleep apnea in patients who are intolerant to continuous positive airway pressure (CPAP) therapy, which is reported in up to 40–60% of patients. This therapy works via direct neurostimulation of the hypoglossal nerve in synchrony with respiration, to open the airway via tongue stiffening and protrusion. Studies have demonstrated significant reductions in both respiratory parameters such as disordered breathing indices, as well as subjective sleep complaints, such as daytime sleepiness, with the use of this therapy. This has increased the repertoire of treatment options for sleep providers to recommend to those patients that are intolerant to CPAP therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) refers to repetitive partial or complete airway obstruction that occurs during sleep, often leading to interrupted sleep and consequent daytime sleepiness. Outside of these effects to quality of life, impaired cognition and mood changes can also occur. Vascular risk is increased, most notably in those with moderate to severe disease, including hypertension, atrial fibrillation, coronary artery disease, congestive heart failure, and stroke, as well as increased all-cause mortality [1,2,3]. The lifetime prevalence of the disease is approximately 5% [4], and this has increased over the past 20 years, depending upon age and gender [5]. Factors that predispose to this condition include age, male gender, elevated body mass index (BMI), increased neck circumference, African American ethnicity, and pregnancy.
The mechanics of upper airway obstruction during sleep are important to understand, specifically in relation to modalities used to manage the obstruction. The pathophysiology is generally thought to encompass two etiologies: (1) decreased muscle activity and (2) decreased airway size and compliance. The changes to the airway as a consequence of these physiologic changes in the respiratory system contribute to a spectrum of sleep disordered breathing severity. Patients without sleep disordered breathing exhibit a physiologic increase in PaCO2 of 4–5 mmHg as a result of decreased ventilatory motor output and relaxation of the upper airway, leading to increased upper airway resistance [6]. Individuals with increasing susceptibility to airway collapse develop snoring via vibration of soft tissues—usually palatal tissue—but are able to maintain oxygenation through a combination of favorable anatomy, airway tone, and adequate muscle activity. Finally, in those individuals with obstructive sleep apnea, factors identified above, often in combination with each other, lead to a lack of respiratory homeostasis, causing a varying degree of obstruction and related hypoxemia, which contribute to the long-term health consequences of sleep apnea.
OSA is a chronic disease with protean manifestations that requires a multifaceted approach for optimal treatment. Key components of therapy are (1) patient education about the disease, (2) self-management via lifestyle modifications, (3) interventions to improve airway patency, (4) addressing sleep hygiene and duration issues, and (5) referral for or management of diseases influenced by OSA, such as hypertension, diabetes mellitus type II, and cardiac diseases. Lifestyle modifications/self-management strategies that are commonly recommended include moderation of alcohol or sedative intake (if applicable), exercise, and achievement of ideal body weight. Interventions to improve airway patency include avoidance of supine sleep (if disease is noted to occur predominantly in the supine position), mandibular advancement devices, continuous positive airway pressure (CPAP), or surgical procedures. Effectiveness and compliance amongst treatment options do vary.
Alternative Therapy(S) for Obstructive Sleep Apnea
Lifestyle modifications/self-management strategies are indicated for all severities of obstructive sleep apnea and include avoidance of alcohol and other sedatives (if applicable) and weight loss in those who are overweight or obese. Weight loss in particular should initially be attempted with dietary modifications and increased physical activity. Sometimes, formal medication therapy or surgical treatment options may need to be considered [7, 8]. Alcohol avoidance can be helpful for multiple reasons, including reduction of snoring, sleep disordered breathing, and weight loss [9].
Avoidance of supine sleep can be helpful when airway obstruction is noted to occur predominantly in the supine position and can be achieved with the use of formal position restriction therapy via either mechanical or commercially available devices utilizing a vibratory feedback around the chest or neck [10, 11]. Position restriction therapy should be used cautiously, as there are no long-term adherence and efficacy data with such devices.
The “gold standard” treatment for obstructive sleep apnea is continuous positive airway pressure (CPAP) delivered via various nasal or oronasal interfaces, achieving increased pressure within the intraluminal space and exceeding the surrounding pressure, thereby maintaining upper airway patency and also stabilizing the upper airway [12]. There are several randomized trials and meta-analyses outlining the benefits of PAP therapy in improving sleep disordered breathing [13, 14]. However, PAP compliance is not optimal in all individuals. Various intolerances can contribute to decreased long-term adherence, including a sense of claustrophobia or suffocation, inappropriate mask style or size, mask discomfort or facial pain, inadequate humidity with subsequent nasal and/or oral dryness, nasal obstruction, and others. Customized mandibular advancement devices, also known as oral appliances, can be beneficial, most notably in those with mild or even moderate disease, and should be considered when PAP therapy fails, or in those who have a preference for such treatment [15].
Ultimately, in those who fail PAP and/or customized oral appliance therapy, surgical alternative treatments may need to be considered. In broad categories, these may include soft tissue surgery, orthognathic surgery, or hypoglossal nerve stimulation therapy.
Description of Therapy
Hypoglossal nerve stimulation therapy (HGNS) works through stimulation of specific branches of the hypoglossal nerve to induce tongue stiffening and protrusion. The protrusion of a stiffened tongue is beneficial to the patient’s airway by increasing the cross-sectional sizes of the airway, as well as via the prevention of airway collapse. An important understanding of how this device works comes from an awareness of the anatomic tongue musculature, which consists of both extrinsic and intrinsic muscles. The intrinsic tongue muscles are responsible for alterations in tongue shape, while the extrinsic muscles are responsible for the positioning of the tongue within and outside of the mouth. These muscles are the genioglossus, hyoglossus, styloglossus, and palatoglossus muscles. The two muscles of greatest interest in hypoglossal nerve stimulation are the genioglossus muscle, which provides the protrusion of the tongue, and the hyoglossus/styloglossus muscles, which lead to retraction when activated (Fig. 1).
Tongue musculature and hypoglossal nerve branching overview. T/V = transverse and vertical muscles (intrinsic), IL = inferior longitudinal muscle (intrinsic), SG = styloglossus muscle (extrinsic), HG = hyoglossus muscle (extrinsic), GG = genioglossus muscle with the oblique and horizontal components, GH = geniohyoid (extrinsic), XII = hypoglossal nerve with the lateral and medial branches, C1 = first cervical nerve branch (courtesy of Inspire Medical Systems, used with permission)
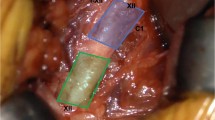
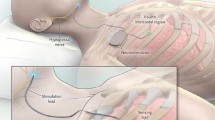
Inspire Medical Systems, located in Minneapolis, Minnesota, is the only Food and Drug Administration (FDA)–approved system for stimulation of the hypoglossal nerve for the treatment of obstructive sleep apnea. The device consists of three components: the implant pulse generator (IPG), a pressure sensor to detect respiration, and a stimulation lead attached to the hypoglossal nerve. The IPG is the pacemaker-like component that houses the battery as well as the processor which analyzes the inputs from the pressure sensor to deliver the stimulation to the hypoglossal nerve (Fig. 2). The IPG is typically placed approximately three finger breadths below the inferior border of the clavicle on the right chest, above the fascia of the pectoralis major muscle. The pressure sensor, placed between the external and internal intercostal muscles along the lateral chest wall, allows for analysis of chest wall motion, which in turn, allows the processor to signal the end of expiration and the start of inspiration, the pivotal time at which airway obstruction can be reversed (Fig. 3). The last component, the stimulation lead, is attached to the specific branches of the hypoglossal nerve through a 2-cm incision below the inferior border of the mandible, allowing for the direct stimulation of the specific branches of the hypoglossal nerve which potentiates tongue stiffening and protrusion (Fig. 3). The stimulation lead is comprised of three contacts, made of platinum/iridium, which can be programmed to stimulate in a positive or negative configuration (Fig. 4). The typical configuration is programmed with the central electrode serving as the negative with the two outer electrodes as positives, but this can be altered to improve patient tolerance if needed. In addition, the IPG also has a built in electrode which can be used to change stimulation electrode configuration as well. Voltage amplitudes across the electrodes can range from 0.1 to 5.0 V, with a stimulation range of 20–40 Hz (generally 33 Hz) and pulse width of 60–210 μs (generally 90 μs).
Additional device considerations include magnetic resonance image (MRI) limitations. The device is compatible in patients who are undergoing MRI scans of the head and extremities, with certain limitations regarding magnet size and types of magnetic coils utilized. Scans of the cervical, thoracic, and lumbar spine as well as the chest and abdomen are not permissible under any circumstance. Other than these MRI limitations, there are no additional limitations in terms of lifestyle modifications needed, such as avoidance of contact sports.
Clinical Studies
Pre-market Trials
A variety of feasibility studies were performed many decades ago to study the effects of hypoglossal nerve stimulation on airway patency [16, 17]. In 2003, Oliven and colleagues demonstrated that the protrusor branches that control the genioglossus muscle could be selectively stimulated to improve airway obstruction [18]. This was a pivotal proof of concept that determined that selective branches of the hypoglossal nerve could be isolated and stimulated. After these initial concept studies were completed, there were three different medical device companies which started human trials on their respective hypoglossal nerve stimulators. These included Apnex Medical Inc. (St. Paul, MN), ImThera Medical Inc. (San Diego, CA), and finally Inspire Medical Systems (Minneapolis, MN). Currently, only one, from Inspire Medical Systems, has traversed the pre-market, FDA approval trial, and has had significant post-market analyses. Hence, most studies on HGNS therapy are focused on the outcomes of this device.
Stimulation Therapy for Apnea Reduction Trial
The Stimulation Therapy for Apnea Reduction Trial (STAR) was the landmark trial assessing treatment outcomes of unilateral hypoglossal nerve stimulation in the treatment of obstructive sleep apnea. The study, completed in 2014, performed across multiple sites, implanted 126 patients who met specific criteria. Patients implanted during the trial were 22 years of age or older, and had previous CPAP trial(s) with intolerance, body mass index (BMI) of 32 kg/m2 or less, apnea/hypopnea index (AHI) between 20 and 50 events per hour (of which less than 25% of the total AHI could include central or mixed sleep apnea), lack of significant tonsillar hypertrophy (+ 3 to + 4 tonsil size), and no concentric collapse of the palatal tissues seen on pre-operative drug-induced sleep endoscopy (DISE) [19].
Primary endpoints for the study included the AHI and oxygen desaturation index (ODI) measured 12 months after implantation. Secondary outcomes included Epworth Sleepiness Scale (ESS) and Functional Outcomes of Sleep Questionnaire (FOSQ) scores, in addition to recording of severe postoperative events. At the 12-month post-implant polysomnography (PSG), 124 out of the initial 126 implanted patients were analyzed, and the median AHI reduction was 68%, with events reduced from a mean of 29.3 to 9.0 events per hour (p < 0.001). The ODI was similarly reduced by 70% from baseline (p < 0.001). Similar statistically significant reductions were seen in the secondary outcome measurements of the ESS and FOSQ. Less than 2% of patients had a serious adverse event. These events included device repositioning to alleviate patient discomfort. No patient had permanent tongue weakness, and 21% of patients had tongue soreness, which most often resolved with acclimatization of the device over time. After the 12-month PSG was performed in all patients, the trial included a randomized therapy withdrawal segment within the trial in 23 patients. In this group, after a week of cessation of therapy, a repeat PSG demonstrated a return of the OSA with a median AHI of 25.8 events per hour while off therapy versus 7.9 events per hour noted in the PSG the week before (p < 0.001).
Post-market Trials
In 2014, based on the previous feasibility trials and the pivotal STAR trial, the FDA approved the Inspire Medical Systems hypoglossal nerve stimulator for the treatment of moderate to severe OSA in adult patients with CPAP intolerance. After this approval, a variety of studies performed by different institutions, in addition to long-term follow-up of the STAR trial participants, have been completed. To date, the initial STAR trial participants have been followed for 60 months, and in the 71 who consented to repeat evaluation, 75% where noted to have a durable response (AHI <20/h or 50% reduction in AHI) [20]. Serious device-related adverse events were minimal at 6% and were all related to lead/device challenges. Secondary outcomes such as ESS, FOSQ, and other respiratory outcomes were persistently improved at the 5-year mark.
A meta-analysis done in 2015 looked at 6 prospective studies in approximately 200 patients and demonstrated a similar significant reduction in respiratory parameters, subjective sleepiness complaints, and low adverse effects in these patients [21]. This further solidified that the outcomes were reproducible across institutions and in a variety of patients who qualified for the device. International studies, including a 60 patient study in Germany, demonstrated similar positive results [22]. Of note, in this study, the inclusion criteria included an elevated BMI cutoff of 35 kg/m2, which demonstrated that consistent outcomes could be obtained in patients with higher body weights.
Finally, the ADHERE registry, the Inspire Medical Systems post-FDA approval longitudinal outcomes study cohort, has continually evaluated the results of the implants in greater than 1000 patients. A study by Thaler et al., looking at 382 of these patients who had completed the 12-month follow-up sleep study, demonstrated AHI reductions from 32.8 to 9.5 events per hour; in addition, ESS was significantly improved (11 to 7), and importantly, nightly usage was noted to be 5.6 h ± 2.1 h in those patients who were studied at the 12-month mark [23].
Clinical Indications
In the initial FDA approval trial for HGNS therapy, patient inclusion criteria included the following for implantation: patient age 22 years or older, previous CPAP trial(s) and intolerance, body mass index (BMI) of 32 kg/m2 or less, apnea/hypopnea index (AHI) between 20 and 50 events per hour (less than 25% of the total AHI could include central or mixed sleep apnea), lack of significant tonsillar hypertrophy (+3 to +4 tonsil size), and no concentric collapse of the palatal tissues seen on pre-operative drug-induced sleep endoscopy (DISE) [19]. After the initial trial was completed, further alterations to the indications have been made. The AHI range has been modified with current limits spanning between 15 and 65 events per hour. In addition, in February of 2020, the Centers for Medicare and Medicaid Services (CMS) published their local coverage determination with slight alterations to HGNS therapy indications. The BMI criterion for Medicare and Medicaid patients is now less than 35 kg/m2 instead of less than 32 kg/m2. CMS also requires that an in-lab polysomnography or home sleep apnea test be completed within 24 months of the first consultation for HGNS implantation.
An additional patient population that has been shown to benefit from HGNS therapy is that of pediatric patients with Down syndrome [24]. Implantation criteria for these patients are different than those in the general population and are as follows: age between 10 and 21 years, prior adenotonsillectomy, body mass index (BMI) < 95th percentile for age, obstructive sleep apnea with AHI between 10 and 50 events per hour, persistent AHI above 10 events per hour during CPAP usage, and unwillingness or intolerability to use of CPAP.
Finally, a particularly interesting question is whether patients who fall outside of the above mentioned criteria would benefit from HGNS therapy. A study published in 2020 by Sarber et al. demonstrated in a cohort of 18 patients implanted outside of the FDA criteria that similar reductions in post-operative outcome measures were seen in comparison to those in the initial STAR trial [25]. Patients in the study were implanted with AHI numbers above and below the typical criteria of 15 to 65 events per hour (8 patients), with elevated BMI above 32 kg/m2 (12 patients), or in patients with central or mixed indices above 25% of the total AHI (2 patients). We suspect that given this study, and the previous modifications to implantation criteria seen in the past, further changes to HGNS criteria may continue to evolve as other studies demonstrate efficacy outside of traditional HGNS inclusion criteria.
Care Pathway
Those who are interested in hypoglossal nerve stimulation therapy are initially seen for Sleep Medicine consultation. During the course of the consultation, indications and contraindications are carefully reviewed. If these are met, formal referral to Sleep Surgery is then pursued. Thereafter, a thorough anatomic evaluation and discussion of all surgical options are outlined by the sleep surgeon. If HGNS is offered, and if drug-induced sleep endoscopy (DISE) does not demonstrate concentric collapse at the level of the palate, implantation of the hypoglossal nerve stimulator is completed. One month following implantation, individuals return to the Sleep Medicine provider to have their device activated and are given the device remote (Figs. 5, 6). Thereafter, over a 3-month period, they are instructed to gradually increase the voltage levels as tolerated. Within the first month after activation, they are contacted by the Sleep Medicine provider to monitor their progress. At the conclusion of this 3-month period, individuals return to undergo an in-laboratory polysomnography study to “fine-tune” their voltage settings. The titration is generally begun at 0.2 V below the maximum level of voltage the patient had achieved in their prior 3-month at-home advancement and is taken to the level required to optimally maintain upper airway patency during all stages and positions of sleep. The goal of the titration is to lower the patient’s disordered breathing indices to the lowest levels achievable with good tolerability. Thereafter, they are sent home with newer settings to continue to utilize on a nightly basis. There is generally an initial follow-up visit within the first 3 months. If they are doing well, follow-up visits are generally every 6–12 months thereafter.
For those with persistently elevated disordered breathing indices in the moderate or severe category after device optimization, additional therapeutic options may need to be considered and include weight loss, supine position restriction therapy, or further discussions with the sleep surgeon to consider additional surgical procedures.
Operative Technique
Once a patient is deemed to be an appropriate candidate for the procedure via Sleep Medicine consultation, and Sleep Surgery consultation, and after the required drug-induced sleep endoscopy (DISE) is performed, they are brought to the operating room and are placed under general anesthesia without the use of long acting paralytics. Next, the protrusor (genioglossus) and retractor (hyoglossus and styloglossus) tongue muscles are monitored via continuous neuromonitoring (NIM response 3.0 system, Medtronic, Jacksonville, FL, USA) utilizing trans-oral electrodes and intraoperative stimulation.
In an effort to prevent infection, meticulous surgical preparation is completed and perioperative antibiotics are given. A clear surgical drape is placed over the patient’s mouth to allow for visualization of the tongue protrusion at the end of the case, and the rest of the patient is sterilely draped. The three surgical sights which correspond to the three components of the device are marked on the patient, typically on the right side, but reports of left-sided placement have been made [26]. There is one incision approximately 2 cm below the mandibular border just anterior to the edge of the submandibular gland (stimulation lead), one incision approximately 3 finger breadths below the clavicle (implant pulse generator), and the most inferior incision is located on the inferior lateral chest (respiratory sensor lead) (Fig. 5).
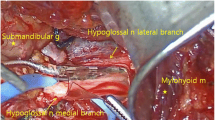
The first part of the operation includes identification of the hypoglossal nerve. The submental incision is created and dissection is carried down to the first landmark, the tendon of the digastric muscle, which is attached to the hyoid bone. Next, after identifying the anterior belly of the digastric muscle, the lateral border of the mylohyoid muscle is retracted medially, and the hypoglossal nerve and its accompanying venae comitans, otherwise known as the ranine vein, is identified. Once the ranine vein is ligated, dissection of the hypoglossal nerve to identify the functional breakpoint between the protrusors and retracting branches is undertaken. A bi-polar stimulator is utilized to identify and assist in separation of these branches. In addition, it is typical that the first cervical nerve branch to the geniohyoid muscle is included in the dissection and ultimately within the stimulation electrode; however, recent data suggest that this may not assist in any additional reductions in sleep apnea postoperatively [27]. Once the nerve branches to the protrusors are identified, the stimulation electrode is wrapped around the branches, and the electrode cuff is held in place via friction and an anchoring site sutured to the digastric tendon.
The second stage of the operation is dissection through the upper chest incision to the pectoralis major muscle fascia, where the implant pulse generator (IPG) is secured in the chest. Once the fascia is identified, an appropriately sized pocket is developed to accept the device. Two securing stitches are placed through the pectoralis muscle fascia to prevent IPG movement.
The third stage includes placement of the respiratory sensor. A 4- to 5-cm incision is made on the lateral chest wall, and the dissection is carried out through the serratus anterior muscle until the external intercostal muscle is identified. A careful dissection is undertaken through the external intercostal muscle until the internal intercostal muscle is identified. The change in the muscle fiber direction is the key finding which alerts the surgeon to prevent an inadvertently deep dissection that could increase the risk for pneumothorax. A malleable ribbon retractor is then placed between the external and internal intercostal muscles to separate the flimsy fibrous tissue that separates the two muscles. Next, the retractor is removed, and the respiratory sensor lead is carefully inserted between the muscles and secured to the chest wall via the anchoring tab on the lead. Using a tunneling catheter, the stimulation lead is passed under the platysma muscle towards the IPG pocket. A similar tunneling is performed with the respiratory sensor lead as well. The two leads are then attached to the IPG, and the device is tested with visualization of the tongue during stimulation, ensuring unopposed protrusion is seen. The tracing from the respiratory lead is analyzed next to ensure the proper rise and fall of respiration is being analyzed appropriately by the IPG.
Finally, the patient’s wounds are meticulously closed in multiple layers and bandaged. The patient is placed in a simple arm sling for 1 week to prevent excessive arm motion. After a short post-operative stay in the anesthesia recovery unit, the patient is discharged to home and outpatient for recovery before being seen in 1 month for a wound check and device activation. The operation typically takes 2–3 h to complete.
Complications
Complications associated with hypoglossal stimulation therapy are rare. In the initial STAR trial, the rate of serious complications was less than 2% [19]. These complications included device repositioning secondary to patient discomfort. Other less serious events included tongue soreness, tongue abrasion, or tongue weakness, most of which were transient. Of those patients with tongue complaints, which occurred in 40% of patients, most of the complaints resolved with continued use of the device. In a recent study from 2019, a cohort of 600 patients that had undergone HGNS placement from October 2016 to April 2018, and were followed for 1 year after implantation, no long-term complications were noted in this group [28].
More serious complications such as device exposure, pneumothorax, wound infection requiring explantation, hematoma requiring evacuation, and device malfunction after cardiac cardioversion have all be described, but are rare in frequency [29, 30]. In general, when compared to alternative surgical therapies for the treatment of CPAP-resistant OSA, such as soft tissue surgery and orthognathic procedures, HGNS therapy complication rates are equally as low, if not lower, in these patients.
Finally, in the rare circumstance that the patient cannot tolerate therapy or needs the device removed, all parts of the device should be removed, typically performed in the operating room under general anesthesia.
Future Directions
Hypoglossal nerve stimulation therapy for obstructive sleep apnea has been a remarkable advance for patients intolerant to CPAP. In the past, soft tissue surgery and orthognathic surgery were our only options for patients and had significant challenges with regard to outcomes and surgical morbidity. Furthermore, elderly patients, who would not otherwise qualify for orthognathic surgery or be willing to undergo a painful soft tissue surgery, are able to undergo this outpatient procedure to help improve their obstructive sleep apnea.
Regardless of this advance, given that surgery for obstructive sleep apnea, even in its most successful form (orthognathic surgery), is considered salvage therapy, we need to continually advance the science in measures that improve CPAP compliance and also improve the outcomes for those who undergo surgery. Currently, the Inspire device is the only FDA-approved HGNS to treat obstructive sleep apnea, but other devices are currently in development, such as bilateral hypoglossal nerve stimulation devices, the Nyxoah (Gilde Healthcare, Mon-Saint-Guibert, Belgium), which has been investigated [31]. Investigations into other peripheral nervous system targets, such as branches of the vagus nerve (ansa cervicalis), are ongoing, in an effort to improve the outcomes of hypoglossal nerve stimulation, and are showing promise [32].
Summary
Hypoglossal nerve stimulation therapy is a novel neurotherapeutic device that has positively impacted the way we treat CPAP-intolerant patients with obstructive sleep apnea. It has brought forth new treatment options for a wider range of these patients and has been demonstrated to be both effective and tolerable. Future advances in neurostimulation will no doubt be pursued given the success of this device and will hopefully provide patients with further options to treat this important disease.
References
Somers VK, White DP, Amin R et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol, 52(8), 686-717 (2008).
Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep, 31(8), 1079-1085 (2008).
Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine, 10(4), 355-362 (2014).
Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. American journal of respiratory and critical care medicine, 165(9), 1217-1239 (2002).
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol, 177(9), 1006-1014 (2013).
Skatrud JB, Dempsey JA, Badr S, Begle RL. Effect of airway impedance on CO2 retention and respiratory muscle activity during NREM sleep. Journal of applied physiology (Bethesda, Md. : 1985), 65(4), 1676-1685 (1988).
Qaseem A, Holty JE, Owens DK et al. Management of obstructive sleep apnea in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med, 159(7), 471-483 (2013).
Randerath WJ, Verbraecken J, Andreas S et al. Non-CPAP therapies in obstructive sleep apnoea. The European respiratory journal, 37(5), 1000-1028 (2011).
Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry, 45(4), 353-359 (1982).
van Maanen JP, de Vries N. Long-term effectiveness and compliance of positional therapy with the sleep position trainer in the treatment of positional obstructive sleep apnea syndrome. Sleep, 37(7), 1209-1215 (2014).
Eijsvogel MM, Ubbink R, Dekker J et al. Sleep position trainer versus tennis ball technique in positional obstructive sleep apnea syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine, 11(2), 139-147 (2015).
Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet, 383(9918), 736-747 (2014).
Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev, (3), CD001106 (2006).
McDaid C, Duree KH, Griffin SC et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep medicine reviews, 13(6), 427-436 (2009).
Iftikhar IH, Bittencourt L, Youngstedt SD et al. Comparative efficacy of CPAP, MADs, exercise-training, and dietary weight loss for sleep apnea: a network meta-analysis. Sleep Med, 30, 7-14 (2017).
Eisele DW, Smith PL, Alam DS, Schwartz AR. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Archives of otolaryngology--head & neck surgery, 123(1), 57-61 (1997).
Hida W, Kurosawa H, Okabe S et al. Hypoglossal nerve stimulation affects the pressure-volume behavior of the upper airway. American journal of respiratory and critical care medicine, 151(2 Pt 1), 455-460 (1995).
Oliven A, O'Hearn DJ, Boudewyns A et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. Journal of applied physiology (Bethesda, Md. : 1985), 95(5), 2023-2029 (2003).
Strollo PJ, Jr., Soose RJ, Maurer JT et al. Upper-airway stimulation for obstructive sleep apnea. The New England journal of medicine, 370(2), 139-149 (2014).
Woodson BT, Strohl KP, Soose RJ et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery, 159(1), 194-202 (2018).
Certal VF, Zaghi S, Riaz M et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: A systematic review and meta-analysis. The Laryngoscope, 125(5), 1254-1264 (2015).
Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of Upper Airway Stimulation for Obstructive Sleep Apnea in a Multicenter German Postmarket Study. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery, 156(2), 378-384 (2017).
Thaler E, Schwab R, Maurer J et al. Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy. The Laryngoscope, 130(5), 1333-1338 (2020).
Caloway CL, Diercks GR, Keamy D et al. Update on hypoglossal nerve stimulation in children with down syndrome and obstructive sleep apnea. The Laryngoscope, 130(4), E263-e267 (2020).
Sarber KM, Chang KW, Ishman SL, Epperson MV, Dhanda Patil R. Hypoglossal Nerve Stimulator Outcomes for Patients Outside the U.S. FDA Recommendations. The Laryngoscope, 130(4), 866-872 (2020).
Deep NL, Hines JP, Parish JM, Hinni ML, Bansberg SF. Subpectoral implantation of the hypoglossal nerve stimulator: An effective technical modification. The Laryngoscope, 129(10), 2420-2423 (2019).
Kumar AT, Vasconcellos A, Boon M, Huntley C. Inclusion of the first cervical nerve does not influence outcomes in upper airway stimulation for treatment of obstructive sleep apnea. The Laryngoscope, 130(5), E382-e385 (2020).
Withrow K, Evans S, Harwick J, Kezirian E, Strollo P. Upper Airway Stimulation Response in Older Adults with Moderate to Severe Obstructive Sleep Apnea. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery, 161(4), 714-719 (2019).
Vasconcellos AP, Huntley CT, Schell AE, Soose RJ, Boon MS. Dysfunctional hypoglossal nerve stimulator after electrical cardioversion: A case series. The Laryngoscope, 129(8), 1949-1953 (2019).
Arteaga AA, Pitts KD, Lewis AF. Iatrogenic pneumothorax during hypoglossal nerve stimulator implantation. American journal of otolaryngology, 39(5), 636-638 (2018).
Eastwood PR, Barnes M, MacKay SG et al. Bilateral hypoglossal nerve stimulation for treatment of adult obstructive sleep apnoea. The European respiratory journal, 55(1) (2020).
Kent DT, Zealear D, Schwartz AR. Ansa Cervicalis Stimulation: A New Direction in Neurostimulation for Obstructive Sleep Apnea. Chest, (2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 571 kb)
Rights and permissions
About this article
Cite this article
Olson, M.D., Junna, M.R. Hypoglossal Nerve Stimulation Therapy for the Treatment of Obstructive Sleep Apnea. Neurotherapeutics 18, 91–99 (2021). https://doi.org/10.1007/s13311-021-01012-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-021-01012-x