Abstract
Myasthenia gravis can be efficiently treated with rituximab but there is no consensus regarding administration and dose schedules in this indication. No marker has yet been described to predict the clinical relapse of patients. Our objective was to identify the B cell subpopulations predicting clinical relapse in patients suffering from generalized myasthenia gravis and treated with rituximab. Clinical and biological data of 34 patients followed between 2016 and 2019 were prospectively collected every 3 months. Using multiparameter flow cytometry, we assessed the percentage in leucocytes of lymphocytes and several B cell subpopulations measured in residual disease conditions. CD19+ were also measured in non-residual disease conditions. Clinical examinations were performed by neurologists using the Osserman score. Clinical relapse occurred in 14 patients (41%). No patients required ICU or ventilatory assistance. The mean improvement of the Osserman score was 17.18 (3–45) after the first rituximab treatment (p < 0.0001). The mean delay between the first rituximab maintenance cycle and clinical relapse was 386.8 days. At the time of relapse, CD27+ increased (p = 0.0006) with AUC = 0.7654, while CD19+ did not. At a threshold of 0.01%, the sensitivity and specificity of CD19+CD27+ were 75.8% and 72.8%, respectively, and the positive and negative predictive values were 28.0% and 95.6%, respectively. The percentage of memory B cells in whole blood cells can accurately predict clinical relapse in myasthenia gravis patients treated with rituximab. This monitoring allows physicians to tailor rituximab administration and to decrease the number of infusions over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myasthenia gravis is a B cell–mediated autoimmune disorder of neuromuscular transmission. It is characterized by fluctuating weakness involving variable combinations of ocular, bulbar, limb, and respiratory muscles and marked serologically by the presence of autoantibodies against acetylcholine receptors (AChRs), muscle-specific kinase (MuSK), or lipoprotein-related protein 4 (LPR4). Once uniformly disabling and sometimes fatal, myasthenia gravis can now be managed effectively with anticholinesterase agents, rapid immunomodulatory treatments, chronic immunosuppressive agents, and thymectomy [1, 2]. But despite these advances in therapeutic management, some patients still suffer from clinical relapse, which can be threatening. Anti-CD20 antibody rituximab (RTX) appears to be beneficial in the treatment of refractory myasthenia gravis [1, 3,4,5]. But even if RTX tends to eliminate B cells, auto-reactive precursor B cells that do not express CD20 remain abnormal after regenerating and contribute to the relapse [6, 7]. B cell depletion with RTX has proven efficacious in patients with refractory myasthenia gravis [3, 8,9,10]. The most used administration schedule protocol is based on systematic infusions every 6 months. However, repeated infusions of RTX have a noxious immunosuppressive effect [4, 9, 11, 12]. Clinicians lack efficient biomarkers to predict clinical relapses and tailor the frequency of infusions. A recent study described potential immunologic B cell markers that may guide treatment decisions in future clinical settings [13]. The effects on B cell homeostasis after repeated treatments and the relationship of certain B cell subsets to the clinical response or relapse are currently unknown. Our primary objective was to evaluate the monitoring of B cell subsets for the prediction of clinical relapse after RTX treatment in patients with myasthenia gravis. Memory B cells, characterized as CD27+ expressing B cells, are useful in predicting clinical relapse in neuromyelitis optica spectrum disorders (NMOSD) when their percentage is higher than 0.05% in whole blood [14,15,16]. We hypothesized that the levels of B cells and specifically CD27+ B cells could also be a predictive biomarker for clinical relapse in patients suffering from myasthenia gravis. Our secondary objective was to study the quantitative and phenotypic reconstitution of peripheral blood B cells in patients with myasthenia gravis following B cell depletion with RTX.
Materials and Methods
Data Collection
We conducted a prospective study on 34 myasthenia gravis patients followed between 2016 and 2019 in the neurological unit at the Nice University Hospital. All patients were followed clinically and biologically according to a standardized protocol after having signed an informed consent form. The study protocol was validated by the Nice University ethical committee.
Clinical evaluation was performed every 3 months using the Osserman myasthenia gravis score (OS) [17,18,19]. Each patient was classified as having either a myasthenia gravis relapse (defined by a decreased OS of more than ten points in comparison to the last known score) or a stable myasthenia gravis (defined by a stable or improved OS), and blood samples were classified accordingly as relapse myasthenia gravis positive (MGR+) or negative (MGR−).
RTX Treatment
Patients were treated with a conventional induction RTX treatment (1 g, D1-D15) with clinical and biological monitoring every 3 months. An additional RTX infusion (1 g) was administered either in the case of clinical relapse or when memory B cell levels were above 0.05% in the peripheral blood mononuclear cell (PBMC) population, based on publications in NMOSD [16] and our previous work [20]. However, the first ten patients who had memory B cells just above this threshold showed a 40% clinical relapse [20]. As our main objective was to avoid clinical relapse, we decided to decrease the decisional threshold to 0.01% for all subsequent patients.
Peripheral B Cell Monitoring Using by Flow Cytometry
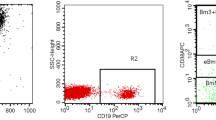
B cell subpopulations were monitored prospectively every 3 months and when clinical symptoms worsened. Peripheral B cells were measured using two different approaches: First, the percentage and absolute values of B cells were measured in routine conditions using the automated method, the BD Multitest™ (BD Biosciences), where only 2500 lymphocytes at most were acquired and analyzed. Peripheral B cells and their different subpopulations including CD27+ memory B cells were also measured by multiparameter flow cytometry (Canto II, BD Biosciences) where one million leucocytes were acquired. Briefly, 1 ml of blood was lyzed (Pharmlyse, Becton Dickinson), washed (cellWASH, BD Biosciences), and then labeled with an eight-color mixture of antibodies, i.e., -V500-CD45; FITC-CD27; PE-anti-IgD; APC-anti-IgM, APC-H7-CD3, -CD14, V450-CD38, and PerCP-Cy5.5-CD24, (all purchased from BD Biosciences); and PE-Cy7-CD19 (Beckman-Coulter), before being resuspended in 500 μl of cell-WASH. The limit of detection for CD27+ B cells in leucocytes was 0.0025%. At least six different B cell populations were identified in each sample: naïve B cells, switched memory B cells, marginal zone-like memory B cells, CD27-negative memory B cells, transitional B cells, and plasmablasts. Figure 1 shows the characterization of B cells using an eight-color panel.
Characterization of B cells using an 8-color panel. (A) Identification of lymphocytes was done using a combination of SSC/FSC properties and CD45 expression. (B) CD19+ B cells (which here represent 4.3% of total lymphocytes and 0.91% of PBMC) are subdivided into CD27 negative and positive B cells using CD27+ T cells (CD3+) as a control; then expression of IgM and IgD among these subpopulations allows the identification of naïve, double-negative memory B cells (lower panel) and switch memory and marginal zone memory B cells (upper panel). The percentage of the different subpopulations among B cells is shown in the upper left corner of the corresponding dot blot. In the example shown here, memory B cells represent 0.013% of PBMC, just above the threshold of 0.01% described in this paper. (C) Plasmablasts and transitional B cells are identified among peripheral B cells using the expression of CD24 and CD38
Patterns of B Cell Repopulation
The pattern of B cell repopulation identified by multiparameter flow cytometry was classified as follows. Type 1 was defined by a predominantly B naïve repopulation (CD27−, IgD+, IgM+). Type 2 a memory B cell repopulation that was further subdivided into Type 2A (a predominantly switched memory population, CD27+, IgD−, IgM−) and 2B (a predominantly marginal zone-like memory B cell repopulation, CD27+, IgD+, IgM+) and type 2C (a predominantly double-negative B cell repopulation; CD27−, IgD−, IgM−). Type 3 was a uniform repopulation of all memory B cells, and type 4 was the absence of repopulation.
Statistical Analyses
Differences between groups were assessed by the Student’s t test, or the Kruskal-Wallis test using Dunn’s post hoc test, when appropriate. Receiver operating characteristic (ROC) curves were established to compare the prognostic values of the different biological markers using the area under the curve (AUC). We also determined the sensitivity and specificity of the different B subset populations at the 0.05% threshold established by Kim et al. [15] in NMO and at lower thresholds down to 0.01% with 0.01% decrements (data not shown). Normality was checked with the Shapiro-Wilk normality test. The alpha risk was set to 5% (α = 0.05). Statistical analyses were performed with Prism (version 6, GraphPad software, San Diego, CA, USA) and EasyMedStat (www.easymedstat.com, Neuilly-sur-Seine, France).
Results
Population Characteristics
Thirty-four patients were included from January 2016 to December 2019. The mean age at diagnosis was 45.8 years (SD: 20.2, range 10–87). There were 14 men (41%) and 20 women (59%). Thirty patients (88%) had AChR, one patient (3%) had MuSK, and five patients (15%) had titin antibodies. Before RTX, 19 patients (56%) received at least one immunosuppressive treatment: azathioprine (14 patients, 41%), IV methylprednisolone (nine patients, 26%), and mycophenolate mofetil (two patients, 6%). A thymoma was diagnosed in eight patients (24%) and thymus hyperplasia in seven patients (21%) (Table 1). The initial mean OS was 66 (SD: 17.5) and increased to 83.1 (SD: 19.3) after the first maintenance RTX cycle (p < 0.001).
Clinical Relapse Characteristics
There were 33 clinical relapses (41%) occurring in 14 patients with 2.36 clinical relapses per patient (SD: 1.39, range 1–5). No patients required ICU or ventilatory assistance. The mean improvement of OS was 17.18 (3–45) after the first RTX treatment (p < 0.0001). The mean delay between the first RTX maintenance cycle and clinical relapse was 386.8 days (SD: 220.26, range 259–514). Most patients increased their OS after the first maintenance RTX, while 6 out of 14 relapsed after the first year of maintenance RTX, and 8 out of 14 in the second year. No correlation between treatment history and clinical relapses was found.
B Cells as a Biomarker of Clinical Relapses
We collected 368 blood samples (10.8 samples per patient on average) of which 99 were excluded since the OS was missing for a period of 3 months after blood collection. Overall, 33 blood samples were followed by a relapse of the disease (MGR+) and 236 were not associated with clinical relapse (MGR−) (Fig. 2). First, we compared the predictivity of CD19+ measured in routine conditions (non-residual conditions) and in residual disease conditions (on average 186,393 lymphocytes were analyzed). According to the ROC curves, CD19+ B cells measured in residual conditions showed a better AUC (0.711) than in routine conditions (0.591) (Fig. 3(A, B), p = 0.046).
CD19+ B cells measured in residual and non-residual disease conditions. We measured CD19+ by multiparameter flow cytometry in residual disease and non-residual disease (routine conditions) to compare the efficacy of CD19+ measurement in each method to predict MGR+. (A) Receiver operating curve (ROC) of CD19+ in residual disease (RD) and non-residual disease (NRD) conditions for the prediction of clinical relapse: CD19+ B cells were measured either in residual disease condition (red line) or in routine conditions (percentage in dark green, absolute value in light green) in 34 patients. CD19+ B cells measured in routine conditions have lower area under curve (AUC) than CD19+ measured in residual disease conditions. (B) CD19+ B cells measured in RD significantly increased in MGR+ compared to CD19+ measured in routine conditions (percentage or absolute value). No significant difference was observed between percentage and absolute values of B cells measured in NRD conditions
The Youden Index enables the selection of an optimal threshold value. For the CD27+ marker, we determined the Youden Index which is higher than 0.0112 for myasthenia gravis patients (Fig. 4(A)). When patients are treated above this 0.01% threshold, clinical relapse is estimated at 21.3% whereas there is 40% chance of clinical relapse at a 0.05% threshold (Fig. 4(B)).
Identification of an optimal memory B cell threshold value to guide RTX infusion and avoid clinical relapse. (A) Determination of the Youden index for myasthenia gravis treated with RTX and monitor using memory B cells. (B) Comparison of the percentage of clinical relapse with different memory B cell threshold ranging from 0.01 to 0.05%
We compared MGR− samples and MGR+ samples which were not associated with higher proportions of lymphocytes measured in residual disease conditions (19.39% vs 22.4% respectively, p = 0.14). On the other hand, higher proportions of CD19+, naïve (N), transitional (T), and plasmablast (P) were observed in the case of MGR+ (Fig. 5(A)). Mean memory B cells were 0.024% in MGR+. The memory B cell ratio was significantly higher in MGR+ than in MGR− (0.024 vs 0.01 respectively p = 0.0004%). In MGR+, CD27+, switch memory (SM), double-negative (DN), and MZ B cells considerably increased p < 0.05 (Fig. 5(B)). Despite a slight difference, CD27+, marginal zone B cells and plasmablasts had the best AUC (AUC = 0.7578, 0.7654 and 0.7258 respectively) to predict MGR+ (Fig. 5(C, D)). For CD27+ cells, the sensitivity and specificity to predict clinical relapse were 75.8% [57.7–88.9] and 72.7% [66.6–78.3%], respectively, at the 0.01% threshold. With the prevalence of clinical relapse of 12% (33 of 269 samples), the positive and negative predictive values of CD27+ were 28.0% and 95.6%, respectively, at 0.01% (Table 2).
Proportion of B cells subsets in MGR+ and in MGR− patients. We compared the percentage among leucocytes of the different B cells subsets in MGR+ and MGR− patients (A). Total B cells (CD19+), naïve B cells (N), transitionals (T), and plasmablasts (P). In panel B, the total population of CD27+ memory B cells and their different subpopulations, SM (switched memory B cell population), DN (double-negative B cell population), and MZ (marginal zone B cell population). In panel C, receiver operating curve for B cells for the prediction of clinical relapse. CD27+ and CD19+ have the highest AUC: 0.7578 and 0.7170, respectively. (D) Among B memory cells, DN, MZ, and SM have similar AUC 0.722, 0.765, and 0.756 respectively
Subgroup Analyses
We compared naïve patients (n = 14) who received RTX as a first-line therapy without other treatments and patients who were thymectomized (n = 15) or received an immunosuppressive treatment (n = 17). Due to the small group number (n = 4), patients who received a double immunosuppressive treatment (RTX in association with another immunosuppressive treatment) were not analyzed.
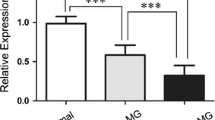
In naïve patients, there were four clinical relapses (4 of 14, 28.5%). The mean OS in naïve patients was 69.75 (range: 54–89) and reached 82.6 (range: 80–100) after RTX p < 0.0003%. Higher proportions of CD19+ were observed in MGR+ (Fig. 6(A)). Proportions of CD27+ and MZ were significantly higher in MGR+, p = 0.01 and p = 0.002, respectively (Fig. 6(B)), and the AUC of CD27+ and MZ were the highest at 0.82 and 0.88, respectively (Fig. 6(C, D)).
Proportion of B cell subsets in the subgroup of naïve patients. We compared the proportion of B cell subsets and AUC in MGR+ and MGR− in naïve patients. (A) Only the percentage of total B cells (CD19+) among leucocytes is statistically significant in MGR+ as compared to MGR−. (B) The percentage of total B cells CD27 and MZ among leucocytes is higher in MGR+ (C). CD27+ have the highest AUC (D). MZ and CD27+ have the highest AUC. AUC: area under the curve; DN: double-negative; MZ: marginal zone; ns, non-significant; MGR: myasthenia gravis relapse; SM: switch memory
We also observed four clinical relapses in the group of thymectomized patients (26.7%). The mean OS was 67 (range: 40–90) and reached 84.5 (range: 51–100) after RTX (p < 0.0001%). The proportions of transitional and DN were significantly higher in MGR+ than in MGR− (p = 0.0387). In this group of patients, the AUC of CD19+ (0.7745) and DN (0.8046) were highest.
In the group of patients treated with IS other than RTX, there were six clinical relapses (6 of 17, 35.3%) and the mean OS was 65.7 (range: 22–90) reaching 83.87 (range: 51–100) after RTX (p < 0.0001%). Proportions of naïve B cells (AUC = 0.81), CD27+ (0.79), SM (0.84), DN (0.80), and MZ (0.70) were significantly higher in MGR+ than in MGR− (p < 0.02).
The proportion of plasmablasts was not significantly higher in MGR+ in any of these three subgroups (p > 0.05).
Pattern of B Cell Repopulation
Next, we analyzed the pattern of B cell repopulation after each infusion of RTX (n = 139). We observed five different patterns of biological repopulation. The most frequent profile was type 3 (67%), defined by a repopulation of all memory B cells, followed by type 2A (16.5%) and type 1 (13%). Type 4 (2%) and type 2C (1.5%) were quite rare (Fig. 7(A)). While the time of repopulation appeared different between different patterns of biological relapses (type 1: 11.5 months; type 2A: 12.1 months; type 3: 10.1 months; and type 4: 13 months), it did not reach statistical significance, likely due to small group sizes (Fig. 7(B)).
Different types of repopulation patterns and time to relapse (A). Frequency of the different type of B cell repopulation: Type 3 was the most frequently observed (67%) followed by type 2A (16.5%) and type 1. The other profiles were quite anecdotal. (B) Analysis of the mean delay between two RTX infusions (expressed in months) according to the repopulation profile. The mean delay between different types was not statistically different. Three patients were excluded from this analysis because they had fewer than two RTX infusions
Mean Delay Between RTX Infusions
The mean delay between two RTX infusions was 10.3 months (5.75–18), corresponding to 1.2 infusions per year. Only one patient (1of 34) required intra-venous Ig, and none needed either ventilation assistance or ICU hospitalization. The mean delay between two RTX injections was not statistically significant between the subgroups.
Discussion
We show here that monitoring B cells in minimal residual disease conditions in myasthenia gravis patients predicts, with high sensitivity clinical relapse in patients treated by RTX.
Several studies have indicated that different schedules of rituximab administration are effective in treating refractory myasthenia gravis with a minimum of two cycles to prevent potential relapses [21, 22]. A systematic review recommended that a repeated rituximab infusion should be considered 4 to 6 months after a cycle [23]. However, many patients do not require such a frequency of infusions which underscores the interest in having predictive markers of disease activity, defined as the occurrence of a clinical relapse. To date, a few studies have reported the usefulness of monitoring of B cells for myasthenia gravis treated by RTX [24,25,26]. In a case report of two patients presenting with myasthenia gravis treated with RTX, Muto et al. [24] found that clinical relapse was associated with a major augmentation of CD20+ CD3− cells and was preceded by an increase of switch memory and double-negative memory B cells a few months prior. Based on the results found in rheumatoid arthritis and NMOSD, Choi et al. [25] used circulating CD19+ and clinical relapse to guide decision about treatment with RTX. Interestingly, up to 57% of patients with clinical symptoms had an increase of CD19+ over 1%. In the study just mentioned, B cells were not a good predictor of clinical relapse at the individual level, probably because the decisional threshold was at 1%. Lebrun et al. [20] used the monitoring of CD27+ cells to guide the administration of RTX, thereby decreasing the number of yearly cycles of RTX from 2 to 1.2 per year with an improvement in clinical status. Lu et al. [22] described the way in which low-dose RTX (600 mg every 6 months) is effective in treating refractory myasthenia gravis patients and how CD27+ memory B cells remain low during the absence of relapse. Recently, Novi et al. [26] showed that a memory B cell–based RTX reinfusion protocol reduced the mean number of RTX reinfusions with a persistent reduction of disease activity. The literature has also shown that plasmablasts may be a good marker to predict clinical relapse [27]. We found that plasmablasts could be a powerful biomarker in predicting clinical relapse in myasthenia gravis but not in all subgroups of the population. In naïve or thymectomy cases or in patients who have undergone immunosuppressive treatment, the proportion of plasmablasts is not significantly higher in MGR+.
These nascent studies about the use of B cell markers in myasthenia gravis are supported by numerous results in other immune-mediated pathologies such as rheumatoid arthritis (RA), systemic lupus erythematosus, Sjogren’s syndrome, and NMO [28,29,30]. Kim et al. [16] used CD27+ memory B cells to guide the treatment of NMO with RTX, achieving remarkable control of the disease. In NMOSD patients with the aquaporin 4 antibody, Durozard et al. [31] showed that memory B cells had reemerged in 12 out of 13 patients treated by RTX (92.5%) who presented clinical relapse.
In untreated early RA and established RA, double-negative (DN) IgD−CD27− memory B cells were significantly increased when compared to controls [32]. All these studies suggest that repopulation subtypes are autoimmune disease-dependent.
Our study confirmed that CD19+ measured in residual disease is a good marker for predicting clinical relapse in myasthenia patients treated with RTX, compared to routine conditions (non-residual diseases). It also showed that memory B cells measured in residual disease conditions by flow cytometry have the highest AUC to predict MGR+ and it suggests that CD27+ could be an alternate marker for tailoring RTX infusion for myasthenia gravis. However, our treatment decision was based on the occurrence of clinical symptoms or the increase in CD27+ in peripheral blood mononuclear cells as measured, using flow cytometry when exceeding 0.01%. Our experience showed at the beginning of the study that 40% of patients (4 of 10) could be MGR+ before reaching this 0.05% threshold, so we planned to treat our patients based on a 0.01% threshold in order to avoid clinical relapse. The sensitivity of CD27+ for the prediction of clinical relapse of RTX increased from 9.1% at a threshold of 0.05% and to 75.8% at a threshold of 0.01%. This necessary threshold modification may have biased the analysis of the CD27+ cells because RTX was introduced shortly after the increase in peripheral blood samples thus preventing them from growing in a significant way in subsequent blood samples. Whether our results can be extrapolated to other immune diseases needs to be further confirmed.
We performed these analyses in residual disease conditions to increase the sensitivity of our techniques and the performance of our results. It is more challenging to assess the monitoring of memory B cells by residual disease mainly because this monitoring requires far more cells to be analyzed than in routine conditions. The monitoring of B memory cells is appropriate in the context of myasthenia, but it requires a low detection threshold, reached in residual disease conditions. Indeed, the sensitivity of CD27+ in predicting clinical relapse of RTX increased from 9.1% at a threshold of 0.05% to 75.8% at a threshold of 0.01% while the specificity was not greatly impacted by this lower threshold. Subgroup analyses confirmed the consistency of our results across patients with different treatments.
To further characterize our samples at the time of RTX infusion, we identified patterns of B cell repopulation. We found that all B cell subtypes were repopulated, without the predominance of a single subpopulation and without a difference, between naïve and non-naïve RTX patients. The patients who required several RTX infusions repeated identical patterns of repopulation without any change between the two repopulations. We did not identify a type of repopulation with a more severe prognosis regarding the evolution of myasthenia gravis. The mean time between the first RTX maintenance cycle and clinical relapse was 386.8 days (55.25 weeks). A recent publication showed similar times of repopulation in NMOSD patients treated with RTX (50.09 weeks) [30].
The strengths of this study include its large collection of matched clinical and biological data and the systematized evaluation of our patients. To our knowledge, it is the first prospective study to analyze the prognostic performances of B cell populations with two different techniques that predict clinical relapse in patients with an immune-mediated pathology.
We believe that the proposed 0.01% threshold should be studied in further prospective clinical studies.
Conclusion
Memory B cell monitoring can accurately predict the clinical relapse of myasthenia gravis patients treated with RTX. An individualized approach to retreatment with RTX based on the assessment of memory B cells could optimize its efficacy while limiting costs and long-term adverse effects.
Abbreviations
- AChRs:
-
Acetylcholine receptors
- AUC:
-
Area under curve
- DN:
-
Double-negative
- MuSK:
-
Muscle-specific kinase
- MFC:
-
Multiparameter flow cytometry
- MZ:
-
Marginal Zone B cells
- NMOSD:
-
Neuromyelitis optica spectrum disorder
- LPR4:
-
Lipoprotein-related protein 4
- MG:
-
Myasthenia gravis
- MGR:
-
Myasthenia gravis relapse
- ROC:
-
Receiver operating characteristic
- RTX:
-
Rituximab
- SM:
-
Switched memory B cells
References
Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM. Myasthenia gravis. Nat Rev Dis Primers. 2019;5:30.
Dalakas MC. Immunotherapy in myasthenia gravis in the era of biologics. Nat Rev Neurol. 2019;15:113-24.
Jing S, Song Y, Song J, Pang S, Quan C, Zhou L, et al. Responsiveness to low-dose rituximab in refractory generalized myasthenia gravis. J Neuroimmunol. 2017;311:14-21.
Afanasiev V, Demeret S, Bolgert F, Eymard B, Laforêt P, Benveniste O. Resistant myasthenia gravis and rituximab: A monocentric retrospective study of 28 patients. Neuromuscul Disord. 2017;27:251-8.
Jing S, Lu J, Song J, Luo S, Zhou L, Quan C, et al. Effect of low-dose rituximab treatment on T- and B-cell lymphocyte imbalance in refractory myasthenia gravis. J Neuroimmunol. 2019;332:216-23.
Jiang R, Fichtner ML, Hoehn KB, Stathopoulos P, Nowak RJ, Kleinstein SH, et al. Single-cell repertoire tracing identifies rituximab refractory B cells during myasthenia gravis relapses. bioRxiv. Cold Spring Harbor Laboratory; 2020;840389.
Crickx E, Weill J-C, Reynaud C-A, Mahévas M. Anti-CD20–mediated B-cell depletion in autoimmune diseases: successes, failures and future perspectives. Kidney International. Elsevier; 2020;97:885-93.
Lebrun C, Bourg V, Tieulie N, Thomas P. Successful treatment of refractory generalized myasthenia gravis with rituximab. Eur J Neurol. 2009;16:246-50.
Landon-Cardinal O, Friedman D, Guiguet M, Laforêt P, Heming N, Salort-Campana E, et al. Efficacy of Rituximab in Refractory Generalized anti-AChR Myasthenia Gravis. J Neuromuscul Dis. 2018;5:241-9.
Chan F, Swayne A, Gillis D, Walsh M, Henderson RD, McCombe PA, et al. Long-term follow-up of patients with myasthenia gravis treated with low-dose rituximab. J Neurol Neurosurg Psychiatry. 2019;90:955-6.
Tony H-P, Burmester G, Schulze-Koops H, Grunke M, Henes J, Kötter I, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID). Arthritis Res Ther. BioMed Central; 2011;13:1-14.
Blum S, Gillis D, Brown H, Boyle R, Henderson R, Heyworth-Smith D, et al. Use and monitoring of low dose rituximab in myasthenia gravis. Journal of Neurology, Neurosurgery and Psychiatry. British Medical Association; 2011;659.
Min YG, Park C, Kwon Y-N, Shin J-Y, Sung J-J, Hong Y-H. B Cell Immunophenotyping and Transcriptional Profiles of Memory B Cells in Patients with Myasthenia Gravis. Exp Neurobiol. 2019;28:720-6.
Kim S-H, Kim W, Li XF, Jung I-J, Kim HJ. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412-20.
Kim S-H, Huh S-Y, Lee SJ, Joung A, Kim HJ. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70:1110-7.
Kim S-H, Jeong IH, Hyun J-W, Joung A, Jo H-J, Hwang S-H, et al. Treatment Outcomes With Rituximab in 100 Patients With Neuromyelitis Optica: Influence of FCGR3A Polymorphisms on the Therapeutic Response to Rituximab. JAMA Neurol. 2015;72:989-95.
Osserman KE, Genkins G. Studies in myasthenia gravis: review of a twenty-year experience in over 1200 patients. Mt Sinai J Med. 1971;38:497-537.
Barnett C, Herbelin L, Dimachkie MM, Barohn RJ. Measuring Clinical Treatment Response in Myasthenia Gravis. Neurol Clin. 2018;36:339-53.
Sharshar T, Chevret S, Mazighi M, Chillet P, Huberfeld G, Berreotta C, et al. Validity and reliability of two muscle strength scores commonly used as endpoints in assessing treatment of myasthenia gravis. Journal of Neurology. 2000;
Lebrun C, Bourg V, Bresch S, Cohen M, Rosenthal-Allieri MA, Desnuelle C, et al. Therapeutic target of memory B cells depletion helps to tailor administration frequency of rituximab in myasthenia gravis. J Neuroimmunol. 2016;298:79-81.
Robeson KR, Kumar A, Keung B, DiCapua DB, Grodinsky E, Patwa HS, et al. Durability of the Rituximab Response in Acetylcholine Receptor Autoantibody-Positive Myasthenia Gravis. JAMA Neurol. 2017;74:60-6.
Lu J, Zhong H, Jing S, Wang L, Xi J, Lu J, et al. Low-dose rituximab every 6 months for the treatment of acetylcholine receptor-positive refractory generalized myasthenia gravis. Muscle Nerve. 2020;61:311-5.
Tandan R, Hehir MK, Waheed W, Howard DB. Rituximab treatment of myasthenia gravis: A systematic review. Muscle Nerve. 2017;56:185-96.
Muto K, Matsui N, Unai Y, Sakai W, Haji S, Udaka K, et al. Memory B cell resurgence requires repeated rituximab in myasthenia gravis. Neuromuscul Disord. 2017;27:918-22.
Choi K, Hong Y-H, Ahn S-H, Baek S-H, Kim J-S, Shin J-Y, et al. Repeated low-dose rituximab treatment based on the assessment of circulating B cells in patients with refractory myasthenia gravis. Ther Adv Neurol Disord. 2019;12:1756286419871187.
Novi G, Bovis F, Fabbri S, Tazza F, Gazzola P, Maietta I, et al. Tailoring B cell depletion therapy in MS according to memory B cell monitoring. Neurol Neuroimmunol Neuroinflamm. 2020;7:e845.
Stathopoulos P, Kumar A, Nowak RJ, O’Connor KC. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight. 2017;2.
Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JCW. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613-20.
Roll P, Mahmood Z, Muhammad K, Feuchtenberger M, Dörner T, Tony H-P. Long-term repopulation of peripheral B-cell subsets after single and repeated rituximab infusions in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2015;33:347-53.
Trewin BP, Adelstein S, Spies JM, Beadnall HN, Barton J, Ho N, et al. Precision therapy for neuromyelitis optica spectrum disorder: A retrospective analysis of the use of class-switched memory B-cells for individualised rituximab dosing schedules. Multiple Sclerosis and Related Disorders. 2020;43:102175.
Durozard P, Rico A, Boutiere C, Maarouf A, Lacroix R, Cointe S, et al. Comparison of the Response to Rituximab between Myelin Oligodendrocyte Glycoprotein and Aquaporin-4 Antibody Diseases. Ann Neurol. 2020;87:256-66.
Moura RA, Quaresma C, Vieira AR, Gonçalves MJ, Polido-Pereira J, Romão VC, et al. B-cell phenotype and IgD-CD27- memory B cells are affected by TNF-inhibitors and tocilizumab treatment in rheumatoid arthritis. PLoS ONE. 2017;12:e0182927.
Acknowledgments
We thank all the patients involved in this study, as well as the laboratory technicians for their help in the laboratory. We had no source of financial support for the work.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Contributions
CRC, SB, MC, MT, and CLF contributed to the conception and design of the study, and the data acquisition and data analysis, and they drafted and critically reviewed the manuscript.
CD and AR contributed substantially to the acquisition of data, and commented on, revised, and approved the final version with respect to data acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Michel Ticchioni and Christine Lebrun-Frenay are co-last authors.
Supplementary Information
ESM 1
(PDF 459 kb)
Rights and permissions
About this article
Cite this article
Ruetsch-Chelli, C., Bresch, S., Seitz-Polski, B. et al. Memory B Cells Predict Relapse in Rituximab-Treated Myasthenia Gravis. Neurotherapeutics 18, 938–948 (2021). https://doi.org/10.1007/s13311-021-01006-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-021-01006-9