Abstract
Amblyopia is a neurodevelopmental disorder of vision caused by abnormal visual experience during early childhood that is often considered to be untreatable in adulthood. Recently, it has been shown that a novel dichoptic videogame-based treatment for amblyopia can improve visual function in adult patients, at least in part, by reducing inhibition of inputs from the amblyopic eye to the visual cortex. Non-invasive anodal transcranial direct current stimulation has been shown to reduce the activity of inhibitory cortical interneurons when applied to the primary motor or visual cortex. In this double-blind, sham-controlled cross-over study we tested the hypothesis that anodal transcranial direct current stimulation of the visual cortex would enhance the therapeutic effects of dichoptic videogame-based treatment. A homogeneous group of 16 young adults (mean age 22.1 ± 1.1 years) with amblyopia were studied to compare the effect of dichoptic treatment alone and dichoptic treatment combined with visual cortex direct current stimulation on measures of binocular (stereopsis) and monocular (visual acuity) visual function. The combined treatment led to greater improvements in stereoacuity than dichoptic treatment alone, indicating that direct current stimulation of the visual cortex boosts the efficacy of dichoptic videogame-based treatment. This intervention warrants further evaluation as a novel therapeutic approach for adults with amblyopia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amblyopia, sometimes referred to as “lazy eye”, is a neurodevelopmental disorder of vision arising from decorrelated binocular input during early visual development (prevalence, approximately 3 %) [1]. Amblyopia results in decreased visual acuity in an otherwise healthy eye and is often associated with suppression of inputs from the amblyopic eye to the visual cortex [2, 3]. This causes an impairment of binocular visual functions such as stereoscopic depth perception. Conventional amblyopia treatment emphasizes patching or penalization of the fellow-fixing (non-amblyopic) eye to force the use of the amblyopic eye [4–6]. While effective at improving visual acuity [6, 7], current treatment is often associated with residual monocular [8] and binocular [9] deficits, and a high rate of recurrence [10]. It has been argued that the monocular treatment approach may not be maximally effective, as it does not directly address suppression [11, 12]. In fact, participants with deeper suppression may have less successful monocular treatment outcomes [13]. Moreover, the standard monocular approach to amblyopia treatment is often considered to be ineffective for adult patients who are past the critical period of visual cortex development [14].
Recent evidence from animal models has shown that reduced cortical inhibition allows for recovery of vision in adult amblyopic eyes [15–19]. This has led to the development of amblyopia treatment interventions designed to reduce suppressive mechanisms within the human visual cortex [11, 20–26]. One approach, based on psychophysical models of binocular vision and supported by initial clinical studies in adults [11, 23, 27] and children [22] involves dichoptic (separate images to each eye) presentation of high contrast images to the amblyopic eye and lower contrast images to the fellow eye. This dichoptic treatment approach has recently been implemented in the form of a videogame that can be played using video goggles [22] or on an iPod touch equipped with a lenticular overlay screen [23].The contrast imbalance overcomes suppression and allows patients to see with both eyes simultaneously. Repeated exposure to such stimuli results in a lasting reduction in suppression, and improvements in both binocular and monocular visual function [11, 22, 23, 27].
In addition to behavioral interventions, non-invasive brain stimulation techniques, such as repetitive transcranial magnetic stimulation and transcranial direct current stimulation (tDCS), are capable of modulating inhibitory networks within targeted areas of the human brain [25, 28–31]. The use of non-invasive brain stimulation techniques for the treatment of amblyopia is developing rapidly [25, 26, 32], and tDCS is particularly attractive owing to its low cost and the possibility that it can be used in the patient’s own home [33]. Anodal tDCS (a-tDCS) tends to increase and cathodal tDCS (c-tDCS) tends to decrease excitability in the stimulated region. Several mechanisms have been proposed for these effects, including alteration of the resting membrane potential [34] and N-methyl-D-aspartate receptor-dependent long term potentiation (LTP)- or long term depression (LTD)-like mechanisms [35]. Of particular relevance for amblyopia, a-tDCS has been associated with a reduction in gamma-aminobutyric acid-mediated inhibition. These effects have been observed when a-tDCS is applied to either the motor [29, 30] or visual [28] cortices. Furthermore, there is evidence that a-tDCS of the visual cortex can enhance the effects of visual rehabilitation for visual field loss following stroke [36, 37]. This is consistent with studies reporting that a-tDCS can augment physiotherapeutic interventions for motor impairments following stroke [38, 39] and enhance learning/skill acquisition [29].
The aim of this study was to determine whether a-tDCS can enhance the effects of dichoptic treatment in adults with amblyopia. To explore the potential of this multimodal treatment approach we designed a double-blind sham-controlled cross-over study in which adults with amblyopia received daily sessions of dichoptic treatment combined with a-tDCS of the visual cortex. Our hypothesis was that dichoptic treatment and a-tDCS would result in greater improvements in visual function than dichoptic treatment alone.
Methods
Participants
Sixteen adults with amblyopia (mean age 22.07 ± 1.1 years SEM) were recruited from the ophthalmology clinics at Zhongshan Ophthalmic Center, Guangzhou, China. Inclusion criteria were a visual acuity of 0 LogMar or better in the fellow fixing eye, at least 0.2 LogMar difference in visual acuity between the eyes, and no contraindications for tDCS. Five participants had previously undergone patching therapy, and 11 patents had received no previous treatment. Clinical details of all participants are summarized in Table 1. Best refractive correction was worn during all testing sessions and prismatic correction was provided when necessary. The study protocols were approved by the Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University, and complied with the Declaration of Helsinki. All participants provided informed consent.
Study Design
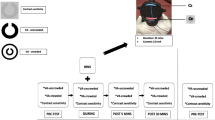
A sham-controlled, cross-over, double-blind study design was adopted (Fig. 1). Participants were randomized into 2 groups. Both groups received an identical dichoptic treatment regimen of 10 sessions lasting 65 mins. Both groups also received tDCS during the first 15 mins of each treatment session; however, group 1 received a-tDCS of the visual cortex during the first 5 training sessions and motor cortex tDCS [sham-tDCS (s-tDCS)] during the second 5 sessions. In group 2, the order of tDCS conditions was reversed.
Experimental design. A sham-controlled, cross-over, double-blind study design was adopted. Group 1 (solid line) received anodal-transcranial direct current stimulation (a-tDCS) of the visual cortex during the first 5 treatment sessions and sham-tDCS (s-tDCS) during the second 5 sessions. The order of tDCS conditions was reversed for group 2 (dashed line). Visual functions were assessed before treatment (baseline), after 5 treatment sessions, and after 10 treatment sessions. tDCS was administered during the first 15 mins of dichoptic training. This was followed by two 25-min training blocks. A 5-min break was provided between each block
Measurements of best-corrected visual acuity and stereopsis were made prior to the first training session (pre), after 5 days of treatment (post-5d) and after 10 days of treatment (post- 10d). In 8 available patients, visual acuity and stereopsis were also measured at 2 weeks (post- 2w) and 3 months (post-3 m) after the final treatment session.
Clinical Assessment
Visual acuity was measured using a tumbling E chart with decimal progression presented using a Topcon ACP-8 projector and viewed from a distance of 3 m. A forced-choice testing method was employed. Lines 1.0–0.2 contained 5 optotypes, lines 0.18–0.05 contained 3 optotypes, and visual acuity was scored using the standard technique of subtracting 0.02 or 0.03 logMAR units, respectively, for each correctly identified optotype. Stereopsis was assessed using the Randot Stereo Test at a 40-cm viewing distance. This test relies on dichoptic separation of 2 disparity-shifted images using polarized glasses and requires patients to detect shapes presented in depth. Measurements of stereopsis can be made in the range of 800 to 40 seconds of arc. Clinical data were collected by an investigator masked to the grouping of the participants.
Dichoptic Treatment
Dichoptic treatment was administered in a clinical assessment room using an iPod touch equipped with a lenticular overlay screen. The videogame-based treatment is described in detail elsewhere [23]. In brief, it consists of a modified version of Tetris, which requires the player to tessellate falling blocks together [23]. Some blocks are presented to the amblyopic eye at high contrast, some to the fellow eye at low contrast, and some to both eyes to aid fusion. Successful game play can only occur if suppression of the amblyopic eye is overcome, thereby allowing all bocks to be perceived simultaneously.
Participants were seated in a chair with their head placed in a chinrest to ensure exact alignment of the lenticular screen. The iPod touch was controlled using a Bluetooth keyboard. Interocular suppression was assessed at the start of each treatment session using an established psychophysical technique that provides a measurement of the interocular contrast difference required to overcome suppression of the amblyopic eye [13, 40, 41]. This technique has recently been modified to allow for measurements to be made in the context of high anisometropia [42]. The suppression measurement was used to set the contrast of the Tetris blocks presented to the fellow eye; amblyopic eye blocks were always presented at 100 % contrast.
Each treatment session was divided into 3 blocks of Tetris play. The first block lasted 15 mins and the second 2 blocks lasted 25 mins. Each block was separated by a 5-min break (Fig. 1). tDCS was delivered during the first block.
tDCS
tDCS was administered using a direct current stimulator (Chattanooga Ionto DJO International, Guildford, Surrey, UK) according to established safety guidelines [43]. This device complies with international electrotechnical commission (IEC) 60601 and is CE-approved. The device is not Food and Drug Administration-approved for tDCS. The stimulating current was delivered via 2 rubber electrodes housed in saline-soaked sponge pockets (Chattanooga Intelect). The sizes of the stimulating and reference electrode were 4 × 6 cm and 8 × 12 cm, respectively. The large size of the reference electrode was chosen to render the electrode inert owing to a low current density [44].
For the a-tDCS condition the stimulating and reference electrode were positioned over Oz and Cz, respectively [28, 45–50], as defined by the 10–20 electroencephalography coordinate system [51].The direct current was ramped up to 2 mA, kept constant for 15 mins, and then ramped down.
Sham stimulation differed from real stimulation in 2 ways. First, sham tDCS was only delivered for 30 s, whereby the current was ramped up to 2 mA and then turned off out of view of the patient [51]. Second, the tDCS electrodes were placed over the motor cortex to ensure that the 30-s period of stimulation could not influence visual cortex function. The stimulating electrode was placed over the nondominant primary motor cortex and the reference electrode was placed over the dominant primary motor cortex (corresponding to 10–20 electroencephalography positions C3 and C4).
Statistical Analysis
Prior to statistical analysis, visual acuity measurements were converted to logMAR units and stereoacuity values were converted to stereosensitivity (stereosensitivity = stereoacuity-1), as many patients had no measureable stereopsis prior to treatment (sensitivity of 0). Visual acuity and stereosensitivity measures made after 5 treatment sessions were normalized to baseline measurements (pretreatment) by subtraction. Measurements made after 10 treatment sessions were normalized to the measurements made after 5 treatment sessions by subtraction. In order to assess any a-tDCS-specific effects on the results of dichoptic treatment, mixed analyses of variance were conducted on normalized data with a between-subject factor “group” (group 1 vs group 2) and within-subject factor “time” (post-5d and post-10d). Post hoc analyses were conducted using paired sample t tests. Means and standard error are reported in the text.
Results
No participants reported any adverse effects apart from a slight tingling sensation under the electrodes.
In agreement with previous studies [11, 23, 24], dichoptic training resulted in improved visual acuity and stereopsis. After all 10 treatment sessions 14 of the 16 participants (78.8 %) exhibited improved stereopsis, including 12 participants who had no measurable stereopsis prior to treatment. The mean improvement was 0.003 ± 0.0004 arc s-1 (t15 = −3.382, p = 0.004) (Fig. 2a). The improvement in amblyopic eye visual acuity ranged from 0.16 to 0.53 logMAR and the mean improvement was 0.34 logMAR ± 0.04 SEM (t15 = 8.725, p < 0.0001) (Fig. 2b). There was a strong positive correlation (Pearson’s r = 0.955, p < 0.0001) between each patient’s baseline visual acuity (pre) and their visual acuity improvement, indicating that patients with deeper amblyopia exhibited greater acuity improvements (Fig. 2c). This correlation remained significant when each patient’s average acuity before and after treatment (mean of pre and post-10d measurements) was compared with their improvement in visual acuity (r = 0.866, p < 0.0001).
Overall improvements in visual function. (a, b) Stereopsis and amblyopic eye visual acuity measurements averaged across all 16 participants at baseline (Pre) and after 5 (post-5d) and 10 (post-10d) treatment sessions. *Significant change from baseline (p < 0.05, 2-sample paired t test). (c) Suppression measured as the Weber contrast that could be tolerated in the fellow eye when the amblyopic eye was presented with a random dot kinematogram (RDK) stimulus at 100 % contrast. Larger values indicate weaker suppression (less contrast difference between the eyes was required to overcome suppression). Error bars represent ± within-subject SEM. (d) Correlation between baseline visual acuity and change in visual acuity after 10 sessions. Each data point represents an individual participant. The positive correlation indicates that the treatment effect was smallest for participants with mild amblyopia and largest for those with more severe amblyopia
A mixed analyis of variance conducted on the normalized stereopsis data revealed an interaction between time and group (F1, 14 = 10.5, p = 0.006), indicating that the effect of a-tDCS differed significantly from the effect of s-tDCS. As shown in Fig. 3a, a-tDCS enhanced the improvement in stereoacuity (group 1 pre to post-5d t7 = −2.553, p = 0.038; group 2 post-5d to post-10d t7 = −3.55, p = 0.009), whereas s-tDCS did not (group 1 post-5d to post-10d t7 = −1.256, p = 0.250; group 2 pre to post-5d t7 = −1.323, p = 0.227). It is notable that across both groups 12/16 patients experienced improved stereopsis when dichoptic treatment was combined with tDCS compared with 4/16 when dichoptic treatment was delivered alone.
Mean stereosensitivity and visual acuity. Mean stereosensitivity (a) and visual acuity (b) for group 1 [open circles; anodal-transcranial direct current stimulation (tDCS) followed by sham-tDCS] and group 2 (filled circles; s-tDCS followed by a-tDCS) at baseline (pre) and after 5 (post-5d) and 10 (post-10d) days of dichoptic treatment. Dashed lines represent dichoptic treatment combined with a-tDCS and solid lines represent dichoptic treatment combined with s-tDCS. *Improvement in visual acuity from baseline (p < 0.05, 2-sample paired t test). # Improvement in stereopsis from pre to post-5d in group 1 and post-5d to post-10d in group 2 (p < 0.05). Error bars represent ± within-subject SEM
Unlike the stereosensitivity measurements, there was no interaction between time and group for the amblyopic eye visual acuity data (Fig. 3b), indicating no difference between a-tDCS and s-tDCS for this measure.
Clinical measurements made at post-2w and post-3 m after the combined treatment in a subset of 8 available patients indicated that the improvements remained stable for both stereopsis (pre to post-2w t5 = −2.611, p = 0.048; pre to post-3 m t5 = −2.445, p = 0.058) and visual acuity (pre to post-2w t7 = 5.36, p = 0.001; pre to post-3 m t7 = 5.841, p = 0.001) (Table 2). Although the post-3 m results for stereopsis were statistically marginal, none of the patients that improved returned to their baseline levels of stereopsis (Table 2).
Discussion
This study has produced confirmatory and novel results. First, in agreement with previous work [11, 22–24, 52], we found that a short period (2 weeks) of dichoptic treatment resulted in pronounced and lasting gains in visual acuity and stereopsis in adult patients with amblyopia, and, second, the novel finding that a-tDCS enhances the effect of dichoptic treatment on stereopsis. These results are consistent with the idea that the adult visual cortex has sufficient plasticity to recover function in adulthood [53–55] and provide the first evidence that brain stimulation techniques can augment treatment interventions for amblyopia.
The dichoptic treatment approach used in this study is based on psychophysical studies demonstrating that information from the amblyopic eye is subject to a strong inhibitory drive from the fellow eye prior to binocular combination [56]. The treatment is designed to reduce suppression of the amblyopic eye and therefore improve binocular functions, such as stereopsis [11, 23]. The fact that monocular function also improves, even though the fellow eye is never occluded during treatment, emphasizes the importance of binocular dysfunction in the visual deficits associated with amblyopia [57–59].
Significant gains in visual function achieved by both groups of patients in this study further support the argument that dichoptic treatment is an effective approach to amblyopia therapy [11, 23, 24]. The magnitude of the stereopsis improvements we report after 2 weeks of dichoptic treatment are consistent with previous studies using this technique in adult patients for comparable periods of time [20, 21, 23, 52]. Interestingly, the improvements in visual acuity were 0.15 log units larger than those reported in previous studies using dichoptic treatment and those using patching of the fellow combined with extended periods (40 h) of videogame play [60]. The protocol in the current study allowed for training sessions to be precisely timed and closely monitored. In addition, the interocular contrast offset used within the videogame was calibrated at the start of every session. These factors may have optimized the treatment effects on visual acuity. The fact that 11/16 (69 %) of the patients in the current study had not previously received treatment may also have resulted in large acuity gains as greater improvements may occur in older patients who have not previously been treated [61].
A novel finding of this study was that a-tDCS enhanced the effects of dichoptic treatment for stereopsis. The rationale for the application of a-tDCS to amblyopia is based on previous work showing that a-tDCS reduces gamma-aminobutyric acid-mediated inhibition [28–30], a key mechanism underlying suppression of the amblyopic eye [3, 57]. A reduction in inhibition may also enhance the potential for experience dependant plasticity [15, 29, 62, 63]. It is possible that a-tDCS further reduced suppression of the amblyopic eye, therefore enhancing the effects of dichoptic treatment on stereopsis, which requires precise binocular integration. a-tDCS did not appear to enhance the effects of dichoptic treatment on amblyopic eye visual acuity in this study; however, it is likely that any effect of a-tDCS was masked by the pronounced improvements in visual acuity that occurred with the dichoptic treatment alone.
a-tDCS of the visual cortex has been reported to enhance the rehabilitation of visual field deficits following stroke [36, 37] and non-invasive transorbital alternating current stimulation has been applied to patients with chronic optic neuropathy [64]. Here we have shown for the first time that a-tDCS can enhance treatment effects in adults with amblyopia, raising the possibility that a-tDCS can improve outcomes for adult patients with amblyopia who are typically left untreated.
References
Vinding T, Gregersen E, Jensen A, Rindziunski E. Prevalence of amblyopia in old people without previous screening and treatment. Acta Ophthalmol (Copenh) 1991;69:796–798.
Holmes JM, Clarke MP. Amblyopia. Lancet 2006;367:1343–1351.
Sengpiel F, Jirmann KU, Vorobyov V, Eysel UT. Strabismic suppression is mediated by inhibitory interactions in the primary visual cortex. Cereb Cortex 2006;16:1750–1758.
Pediatric Eye Disease Investigator Group. A randomized trial of atropine vs. patching for treatment of moderate amblyopia in children. Arch Ophthalmol 2002;120:268–278.
Pediatric Eye Disease Investigator Group. A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology 2004;111:2076–2085.
Pediatric Eye Disease Investigator Group. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol 2003;121:603–611.
Pediatric Eye Disease Investigator Group. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology 2003;110:2075–2087.
Pediatric Eye Disease Investigator Group. A randomized trial of atropine vs patching for treatment of moderate amblyopia: follow-up at age 10 years. Arch Ophthalmol 2008;126:1039–1044.
Wallace DK, Lazar EL, Melia M, et al. Stereoacuity in children with anisometropic amblyopia. JAAPOS 2011;15:455–461.
Bhola R, Keech RV, Kutschke P, Pfeifer W, Scott WE. Recurrence of amblyopia after occlusion therapy. Ophthalmology 2006;113:2097–2100.
Hess RF, Mansouri B, Thompson B. Restoration of binocular vision in amblyopia. Strabismus 2011;19:110–118.
Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res 2013;33:67–84.
Narasimhan S, Harrison ER, Giaschi DE. Quantitative measurement of interocular suppression in children with amblyopia. Vision Res 2012;66:1–10.
Epelbaum M, Milleret C, Buisseret P, Dufier JL. The sensitive period for strabismic amblyopia in humans. Ophthalmology 1993;100:323–327.
Maya-Vetencourt JF, Baroncelli L, Viegi A, et al. IGF-1 restores visual cortex plasticity in adult life by reducing local GABA levels. Neural Plas 2012;250421.
He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci 2006;26: 2951–2955.
Harauzov A, Spolidoro M, DiCristo G, et al. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci 2010;30:361–371.
Sale A, Maya Vetencourt JF, Medini P, et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci 2007;10:679–681.
Sale A, Berardi N, Spolidoro M, Baroncelli L, Maffei L. GABAergic inhibition in visual cortical plasticity. Front Cell Neurosci 2010;4:10.
Hess RF, Thompson B, Black JM, et al. An iPod treatment of amblyopia: An updated binocular approach. Optometry 2012;83:87–94.
Black JM, Hess RF, Cooperstock JR, To L, Thompson B. The measurement and treatment of suppression in amblyopia. J Vis Exp 2012;70:e3927.
Knox PJ, Simmers AJ, Gray LS, Cleary M. An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci 2012;53:817–824.
To L, Thompson B, Blum J, Hess R, Maehara G, Cooperstock J. A game platform for treatment of amblyopia. IEEE Trans Neural Syst Rehabil Eng 2011;PP:1–1.
Hess RF, Mansouri B, Thompson B. A new binocular approach to the treatment of amblyopia in adults well beyond the critical period of visual development. Restor Neurol Neurosci 2010;28:793–802.
Thompson B, Mansouri B, Koski L, Hess RF. Brain plasticity in the adult: modulation of function in amblyopia with rTMS. Curr Biol 2008;18:1067–1071.
Spiegel DP, Byblow WD, Hess RF, Thompson B. Anodal transcranial direct current stimulation transiently improves contrast sensitivity and normalises visual cortex activation in individuals with amblyopia. Neurorehab Neural Repair 2013 Jun 17 [Epub ahead of print].
Hess RF, Mansouri B, Thompson B. A binocular approach to treating amblyopia: antisuppression therapy. Optom Vis Sci 2010;87:697–704.
Spiegel DP, Hansen BC, Byblow WD, Thompson B. Anodal transcranial direct current stimulation reduces psychophysically measured surround suppression in the human visual cortex. PLoS One 2012;7:e36220.
Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol 2011;21:480–484.
Stagg CJ, Best JG, Stephenson MC, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 2009;29:5202–5206.
Thompson B, Mansouri B, Koski L, Hess RF. From motor cortex to visual cortex: the application of noninvasive brain stimulation to amblyopia. Dev Psychobiol 2010;54: 263–273.
Clavagnier S, Thompson B, Hess RF. Long lasting effects of daily theta burst rTMS sessions in the human amblyopic cortex. Brain Stimul 2013 Apr 28 [Epub ahead of print].
Benninger DH, Lomarev M, Lopez G, Pal N, Luckenbaugh DA, Hallett M. Transcranial direct current stimulation for the treatment of focal hand dystonia. Mov Disord 2011;26:1698–1702.
Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 1965;28:166–185.
Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC stimulation induced after effects of human motor cortex excitability. Brain 2002;125:2238–2247.
Plow EB, Obretenova SN, Fregni F, Pascual-Leone A, Merabet LB. Comparison of visual field training for hemianopia with active versus sham transcranial direct cortical stimulation. Neurorehab Neural Repair 2012;26:616–626.
Plow EB, Obretenova SN, Jackson ML, Merabet LB. Temporal profile of functional visual rehabilitative outcomes modulated by transcranial direct current stimulation. Neuromodulation 2012;15:367–373.
Butler AJ, Shuster M, O'Hara E, Hurley K, Middlebrooks D, Guilkey K. A meta-analysis of the efficacy of anodal transcranial direct current stimulation for upper limb motor recovery in stroke survivors. J Hand Ther 2013;26:162–170.
Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci 2007;25:123–129.
Black JM, Thompson B, Maehara G, Hess RF. A compact clinical instrument for quantifying suppression. Optom Vis Sci 2011;88:334–343.
Mansouri B, Thompson B, Hess RF. Measurement of suprathreshold binocular interactions in amblyopia. Vision Res 2008;48:2775–2784.
Li J, Hess RF, Chan LYL, et al. How best to assess suppression in patients with high anisometropia. Optom Vis Sci 2013;90:e47-e52.
Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 2003;114:2220–2222.
Nitsche MA, Doemkes S, Karakose T, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 2007;97:3109–3117.
Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci 2004;45:702–707.
Antal A, Kincses TZ, Nitsche MA, Paulus W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp Brain Res 2003;150: 375–378.
Antal A, Nitsche MA, Paulus W. External modulation of visual perception in humans. Neuroreport 2001;12:3553–3555.
Antal A, Kincses TZ, Nitsche MA, Paulus W. Modulation of moving phosphene thresholds by transcranial direct current stimulation of V1 in human. Neuropsychologia 2003;41:1802–1807.
Antal A, Nitsche MA, Paulus W. Transcranial direct current stimulation and the visual cortex. Brain Res Bull 2006;68:459–463.
Antal A, Paulus W. Transcranial direct current stimulation and visual perception. Perception 2008;37:367–374.
Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activities. Am J EEG Technol 1985;25:83–92.
Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF. Dichoptic training enebles the adult amblyopic brain to learn. Curr Biol 2013;23:R308-309.
Li RW, Klein SA, Levi DM. Prolonged perceptual learning of positional acuity in adult amblyopia: perceptual template retuning dynamics. J Neurosci 2008;28:14223–14229.
Li RW, Provost A, Levi DM. Extended perceptual learning results in substantial recovery of positional acuity and visual acuity in juvenile amblyopia. Invest Ophthalmol Vis Sci 2007;48:5046–5051.
Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci USA 2004;101:6692–6697.
Baker DH, Meese TS, Mansouri B, Hess RF. Binocular summation of contrast remains intact in strabismic amblyopia. Invest Ophthalmol Vis Sci 2007;48:5332–5338.
Mower GD, Christen WG. Evidence for an enhanced role of GABA inhibition in visual cortical ocular dominance of cats reared with abnormal monocular experience. Brain Res Dev Brain Res 1989;45:211–218.
Bi H, Zhang B, Tao X, Harwerth RS, Smith EL, Chino YM. Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cereb Cortex 2011;21:2033–2045.
Li J, Thompson B, Lam CS, et al. The role of suppression in amblyopia. Invest Ophthalmol Vis Sci 2011;52:4169–4176.
Li RW, Ngo C, Nguyen J, Levi DM. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biology 2011;9: e1001135.
Pediatric Eye Disease Investigator Group. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol 2005;123:437–447.
Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Maffei L, Sale A. Brain plasticity and disease: a matter of inhibition. Neural Plas 2011;286073.
Scali M, Baroncelli L, Cenni MC, Sale A, Maffei L. A rich environmental experience reactivates visual cortex plasticity in aged rats. Exp Gerontol 2012;47:337–341.
Sabel BA, Fedorov AB, Naue N, Borrmann A, Herrmann C, Gall C. Non-invasive alternating current stimulation improves vision in optic neuropathy. Restor Neurol Neurosci 2011;29:493–505.
Acknowledgments
This work was supported by a Faculty of Science Research Development Fund and Early Career Research Excellence Award, University of Auckland; an Auckland Medical Research Foundation Project Grant; and a Health Research Council Grant to BT; a National Natural Science Foundation of China Grant (81200715); a Thrasher Research Fund for Early Career Award to JL; and a Canadian Institutes of Health Research Grant (53346) to RFH. We thank Dr Long To and Dr Jeremy Cooperstock for their collaboration, and Dr Avinesh Pillai for valuable assistance with the statistical analysis.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Daniel P. Spiegel and Jinrong Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Spiegel, D.P., Li, J., Hess, R.F. et al. Transcranial Direct Current Stimulation Enhances Recovery of Stereopsis in Adults With Amblyopia. Neurotherapeutics 10, 831–839 (2013). https://doi.org/10.1007/s13311-013-0200-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-013-0200-y