Abstract
To evaluate the short- and long-term survival of hyperthermic intraperitoneal chemotherapy (HIPEC) in the patients with advanced gastric cancer (AGC) through randomized controlled trials (RCTs). We analyzed the endpoints of AGC patients including 1-, 2-, 3-, and 5-year overall survival (OS), intestinal anastomotic leakage, myelosuppression, nausea and vomiting from included studies. And we retrieved RCTs from medical literature databases. Risk ratios (RR) was used to calculated the endpoints. Totally, we retrieved 13 articles (14 trial comparisons) which contained 1091 patients. They were randomized to HIPEC group and control group. The results showed that there was no significant differences in survival rates between HIPEC group and control group at 1-, 2- and 3-year follow-up, while a statistical significant overall survival effect was found at the 5-year follow-up [RR: 1.20, 95% CI 1.01 to 1.43, I2 = 0.0%]. And there is no significant difference in the risk of intestinal anastomotic leakage, myelosuppression and nausea and vomiting. Compared with the control group, HIPEC could improve the long-term OS without increasing the risk of adverse effect in AGC patients with/without peritoneal carcinomatosis, but there was no benefit at short-term OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Gastric cancer is one of the most common malignant tumors of digestive tract [1]. According to the global cancer statistics in 2020, the incidence of gastric cancer ranks fifth among malignant tumors, and the fatality rate ranks fourth [2]. The incidence of gastric cancer is hidden, and there are no obvious symptoms in the early stage of gastric cancer. Therefore, when gastric cancer is found, it is mostly advanced gastric cancer (AGC), which has a poor prognosis and a high mortality rate [3]. AGC refers to cancer tissue that has invaded the muscularis or even serosa layer of the gastric wall, regardless of the size of the lesion or the presence or absence of metastasis. Postoperative local recurrence and peritoneal metastasis are important factors affecting the prognosis of patients with AGC, and peritoneal metastasis is the most common outcome and cause of death in AGC [4]. The diagnosis rate of peritoneal metastasis in patients with gastric cancer is 14–30%. Even if there is no peritoneal metastasis in the initial treatment, the incidence of peritoneal recurrence after radical gastric cancer surgery is 34–60% [5].
Hyperthermic intraperitoneal chemotherapy (HIPEC) is an adjuvant therapy technology that infuses mixed lavage solution of chemotherapy drugs into the abdominal cavity and kills tumor cells by the synergistic mechanism of temperature and chemotherapy drugs [6]. In 1980, Spratt et al. [7] first reported the treatment of pseudomyxoma of peritoneum by HIPEC, which officially began the clinical exploration and practice of HIPEC treatment. HIPEC has been widely used in the treatment of various primary and secondary peritoneal tumors and their complicated malignant ascites [8,9,10]. At present, the application of HIPEC in advanced gastric cancer is mainly divided into prophylactic and therapeutic [11]. At present, prophylactic HIPEC is mainly used after R0 resection in patients with advanced gastric cancer who have high risk factors but do not have visible peritoneal metastasis [12, 13]. Therapeutic HIPEC is mainly applied to gastric cancer patients with peritoneal metastasis or accompanied by cancerous ascites, with the main purpose of alleviating the symptoms of cancerous ascites and trying to prolong the survival time to the maximum [14].
In the past 5 years, a number of studies on the role of HIPEC in AGC have been published. The effectiveness of HIPEC to AGC remains hot and controversial. Therefore, the purpose of this meta-analysis is to systematically explore and summarize the efficacy and safety of HIPEC in patients with AGC through randomized controlled trials, and to report the relationship between HIPEC and complications for the first time.
Materials and methods
Search strategy
We searched published studies following the preferred report items of systematic review and meta-analysis (PRISMA) guidelines [15]. We conducted a systematic search for RCTs in databases, such as the Cochrane Library, PubMed, Embase, Pubmed, Google Scholar, Baidu Scholar and other databases. We searched for relevant studies published up to January 20th, 2022 with language restriction to English. Combining the main keywords and free words, the complete search strategy was as follows: (“hyperthermic intraperitoneal chemotherapy” OR “intraperitoneal chemotherapy” OR “hyperthermic perfusion chemotherapy” OR “intraperitoneal hyperthermic perfusion chemotherapy” OR “chemotherapy for peritoneal perfusion” OR “HIPEC”) AND (“advanced gastric cancer” OR “stomach cancer” OR “gastric cancer” OR “AGC”). Besides, we reviewed the reference list of retrieved articles to look for other potential experiments.
Study selection
The studies included in this meta-analysis were RCTs which evaluate the efficacy of HIPEC in the ACG. The main endpoints were 1-, 2-, 3- and 5-year overall survival (OS) of patients with gastric cancer, while the safety endpoints were intestinal anastomotic leakage, myelosuppression, nausea and vomiting. Summary studies, animal, cellular studies, or low-quality studies were excluded.
Data extraction
Two authors (H.D. and B.L.) independently extracted the following data from each included study: study design, author, publication date, study country, participant characteristics, gender, age, HIPEC regimen, interventions, treatment cycle and endpoint indicators. When differences arise, all the authors negotiate together until the differences are resolved.
Quality assessment of study and evidence
The quality assessment is based on the Cochrane bias risk standard and is independently assessed by two reviewers (H.D. and B.L.) [16]. Five items were used to estimate bias in each study, including bias due to deviations from intended interventions, bias arising from the randomization process, bias in selection of the reported result, bias in measurement of the outcome and bias due to missing outcome data.
Statistical analysis
The aggregate risk ratio (RR) and 95% confidence interval (CI) of hyperthermic intraperitoneal chemotherapy and unexposed intraperitoneal chemotherapy are the criteria for measuring the efficacy of hyperthermic intraperitoneal chemotherapy. Q test (p < 0.05) was used to assessed the heterogeneity among included studies. The Higgins I2 statistic was also examined, I2 value > 50 and 75%, respectively, means substantial heterogeneity and high heterogeneity existed in the trials. A random-effects model was used when significant heterogeneity was detected; otherwise, a fixed-effects model was preferred. If there were more than ten studies assessed one endpoint, we examined the publication bias and explored sources of heterogeneity by funnel plot. We conducted a subgroup analysis to evaluate the sources of heterogeneity. And sensitivity analysis was used to determine the reliability and stability of the pooled results. All statistical analyses were performed with the STATA 12.0 (Stata Corporation, College Station, Texas, USA). A threshold of p < 0.05 was considered significant without anything special.
Results
Literature retrieval process and baseline characteristics of included studies
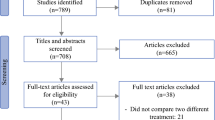
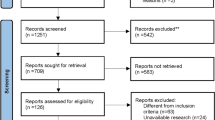
According to PRISMA guidelines, 678 studies were enrolled. We then eliminated a portion of the articles by screening the abstracts, and identified the final articles for inclusion after reading the full text. Finally, 13 studies [17,18,19,20,21,22,23,24,25,26,27,28,29] (14 trial comparisons) were included which contained 1091 patients as shown in (Fig. 1). 556 patients (51.0%) were randomized to HIPEC group whereas 535 patients (49.0%) were randomized to control group. All of included studies used HIPEC as a preemptive strategy. All included studies were RCTs. The basic characteristics of the included studies are described in Table 1.
Assessment of quality of the studies
Two authors evaluated the quality of the retrieved studies by The Cochrane Risk of Bias criteria [30]. 13 studies [17,18,19,20,21,22,23,24,25,26,27,28,29] described random sequence generation and allocation concealment. None of the studies described other biases. The included studies were all RCTs. The literature quality score is shown in Table 2.
Endpoints
Overall survival (OS)
The overall survival analysis in AGC showed no significant differences in survival rates between HIPEC group and control group at 1-year [RR: 1.23, 95% CI 0.89 to 1.70, I2 = 82.2%, Fig. 2], 2-year [RR: 1.14, 95% CI 0.59 to 2.17, I2 = 78.6%, Fig. 3] and 3-year [RR: 1.21, 95% CI 0.86 to 1.70, I2 = 75.7%, Fig. 4] follow-up, while a statistical significant overall effect was found at the 5-year follow-up [RR: 1.20, 95% CI 1.01 to 1.43, I2 = 0.0%, Fig. 5] favoring the HIPEC procedure.
And we performed a subgroup analysis by country, peritoneal carcinomatosis and year of publication. The results of the subsequent subgroup analysis showed that there was no significant difference between HIPEC group and control group at 1, 2, 3-year OS, regardless of country and peritoneal carcinomatosis as show in (Table 3). And included studies published before 2010 demonstrated that HIPEC could improve 1- and 3-year OS as show in (Table 3). According to the country subgroup analysis, the heterogeneity of China subgroup decreased at 2-year OS (I2 = 0.0%), 3-year OS (I2 = 64.7%). And the heterogeneity of Japan subgroup decreased at 3-year OS (I2 = 46.9%). According to peritoneal carcinomatosis subgroup analysis, the heterogeneity of peritoneal carcinomatosis subgroup decreased at 3-year OS (I2 = 0.0%). According to year of publication subgroup analysis, the heterogeneity of studies published after 2010 subgroup decreased at 1-year OS (I2 = 79.5%) 2-year OS (I2 = 0.0%), 3-year OS (I2 = 64.7%).
Safety endpoints
The safety endpoints mainly including the risk of intestinal anastomotic leakage, myelosuppression, nausea and vomiting. There was no significant difference between HIPEC group and control group in the risk of intestinal anastomotic leakage (RR: 0.89, 95% CI 0.38 to 2.13, I2 = 0.0%, Supplementary 1), myelosuppression (RR: 1.09, 95% CI 0.90 to 1.32, I2 = 0.0%, Supplementary 2), nausea and vomiting (RR: 1.22, 95% CI 0.98 to 1.52, I2 = 12.5%, Supplementary 3). The random effect model was applied.
Sensitivity analysis and publication bias
The funnel plots show a low probability of publication bias (all the p > 0.05) for the included studies, as shown in Supplementary 4–5. The results of the sensitivity analyses show the heterogeneity mainly comes from the studies of Cui et al. [25] and Huang et al. [26] as shown in Supplementary 6-8.
Discussion
HIPEC could selectively kill tumor cells by inhibiting DNA replication, transcription and repair. Under high temperature, the fluidity of cancer cell membrane is enhanced, and the permeability of cell membrane and tumor blood vessels is increased, which is conducive to the penetration and absorption of chemotherapeutic drugs [31]. It refers to the precise constant temperature, circulating perfusion, filling the abdominal cavity and maintaining it for a certain time, to prevent and treat the implantation and metastasis of the peritoneal cavity [32]. HIPEC is an adjuvant therapy for abdominal malignant tumors. It has unique therapeutic effects in the prevention and treatment of peritoneal carcinomas, colorectal cancer, ovarian cancer, gastric cancer, and so on [33, 34]. The advantage of HIPEC is that drug directly acts on cancer cells, affecting the peritoneal microenvironment and inhibiting the implantation of cancer cells. Another advantage is that the adverse reaction is small [35].
Advanced gastric cancer is often accompanied by peritoneal metastasis [36]. Even with D2 radical surgery, peritoneal metastasis and recurrence may occur [37]. How to treat peritoneal metastasis of ACG is the key to prolong the survival of patients and improve the quality of life of patients.
Since Koga et al. [17] first applied HIPEC to gastric cancer patients in 1988, domestic and foreign scholars have conducted in-depth research on this method. HIPEC can effectively remove peritoneal free cancer cells and micro metastases, and prevent and treat peritoneal metastasis of gastric cancer.
Nowadays, there are a few meta-analyses to study the efficacy and safety of HIPEC in the AGC patients with/without peritoneal carcinomas. In 2017, Desiderio et al. [38] conducted a meta-analysis to evaluate the role of HIPEC in gastric cancer and clarify its effectiveness at different stages of peritoneal disease progression. They found that preventive HIPEC could bring survival benefits. In particular, patients whose disease burden is limited to positive cytology and limited nodal involvement may benefit the most from HIPEC. The authors included both RCTs and nRCTs, while we included only RCTs, In addition, a number of other studies on the role of HIPEC in AGC have been published in the last 5 years. Liu et al. [39] comprehensively analyzed the effect of HIPEC for gastric cancer patients by including twenty-one trials. They concluded that HIPEC had a beneficial effect on 3-year survival rate and complete response in patients with AGC and peritoneal metastases. But they did not report the relationship between the HIPEC and complications. Besides, all of included studies in their meta-analysis were from China, which is unrepresentative and limited.
Our meta-analysis evaluated the efficacy and safety of HIPEC in patients with AGC. The results showed that no significant differences in survival rates between HIPEC group and control group at 1, 2 and 3-year follow-up, while a statistical significant overall effect was found at the 5-year follow-up. And there is no significant difference in the risk of intestinal anastomotic leakage, myelosuppression and nausea and vomiting.
There is a large heterogeneity in the endpoint of 1, 2 and 3-year OS (I2 = 82.2, 78.6 and 75.7%). Through sensitivity analysis, we found that the heterogeneity mainly comes from the study of Cui et al. [25] and Huang et al. [26] We consider that this may be because the sample size of Huang’s study is small (n = 42), which may affect the reliability of the pooled effect size. And Cui et al. and Huang et al. are from China, while most of the other studies are from Japan, which may have ethnic and geographical differences, leading to correlation bias. Besides, the HIPEC regimens in Cui’s and Huang’s studies were different from the other included studies which may lead to methodological heterogeneity.
The potential clinical implications of this meta-analysis are as follows: (1) this is an updated meta-analysis to evaluate the efficacy and safety of HIPEC in AGC patients with/without peritoneal carcinomatosis. Compared to previous studies, we included 13 RCTs that contained a large sample size of 1091 participants. (2) Sensitivity analyses and subgroup analyses were conducted to explore heterogeneity. We found the source of heterogeneity (Cui’s and Huang’s studies). And we successfully decreased the heterogeneity of OS by performing a subgroup analysis according country and peritoneal carcinomatosis. (3) All the included studies were RCTs and the literature was of high quality. (4) Only 2 studies [26, 27] had a sample size of less than 45. (5) There was a low probability of publication bias for the included studies. (6) Compared to control group, HIPEC had no benefit in short-term OS, but in long-term OS, which is different from the conclusion of previous studies. And the pooled effect of long-term OS (5-year OS) was derived from studies conducted prior to 2001, and there was doubt whether the findings were still relevant, which needs to be further confirmed by large sample size and higher quality RCTs.
The limitations of our study are as follows: (1) several baseline characteristics (HIPEC regimes, preoperative nutritional status, and related underlying diseases) were not considered which may lead to mixed bias. (2) Most of included RCTs did not describe the blinding method used, which may lead to selection bias. (3) The data of 5-year OS were prior to 2001, which was too far away from now. (4) Included studies were mainly from Japan and China, lacking studies from other regions, which was not representative. (5) The heterogeneity of this meta-analysis was high at 1-, 2-, and 3-year OS and the conclusions were not reliable enough. (6)The role and timing of adjuvant chemotherapy and its impact on multimodal treatment approaches have not been clearly described.
In summary, our meta-analysis has demonstrated that compared with the control group, HIPEC could improve the long-term OS without increasing the risk of adverse effect in AGC patients with/without peritoneal carcinomatosis, but there was no benefit at short-term OS.
For AGC patients, peritoneal metastasis still occurs frequently, chemotherapy including HIPEC has not been widely adopted, and peritoneal metastasis still exist. In this context, the next steps in developing treatment regiments should consider combining with neoadjuvant chemotherapy, immunotherapy, targeted therapy and surgical treatment. And large sample size, multicenter and long-term follow-up RCTs are necessary to conducted to further evaluate the efficacy of HIPEC.
Data availability
The author could be contacted for data requests.
References
Correa P (2013) Gastric cancer: overview. Gastroenterol Clin N Am 42:211–217
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 71(3):209–249
Chen WQ, Li H, Sun KX et al (2018) Report of cancer incidence and mortality in China, 2014 Zhonghua zhong liu za zhi. Chin J Oncol 40(1):5–13
Fujitani K (2013) Overview of adjuvant and neoadjuvant therapy for resectable gastric cancer in the East. Dig Surg 30(2):119–129
Yarema R, Mielko J, Fetsych T et al (2019) Hyperthermic intraperitoneal chemotherapy (HIPEC) in combined treatment of locally advanced and intraperitoneally disseminated gastric cancer: a retrospective cooperative central-Eastern European study. Cancer Med 45:144
Zheng GZ, Rui T, Min Y et al (2006) Efficacy and safety of intraoperative peritoneal hyperthermic chemotherapy for advanced gastric cancer patients with serosal invasion. A long-term follow-up study Dig Surg 23(1–2):93–102
Spratt JS, Adcock RA, Muskovin M et al (1980) Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Can Res 40(2):256–260
Mar A, Jpm B, Rb C et al (2020) A retrospective study comparing the efficacy of dose-dense chemotherapy, intraperitoneal chemotherapy and dose-dense chemotherapy with hyperthermic intraperitoneal chemotherapy in the treatment of advanced stage ovarian carcinoma. Eur J Obstet Gynecol Reprod Biol 244:101–105
Klaver C, Wisselink DD, Punt C et al (2019) Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol 4(10):761–770
Cortés-Guiral D, Mohamed F, Glehen O et al (2020) "Prehabilitation of patients undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal malignancy. Eur J Surg Oncol 47(1):60–64
Dahdaleh FS, Turaga KK (2018) Evolving treatment strategies and outcomes in advanced gastric cancer with peritoneal metastasis. Surg Oncol Clin N Am 27(3):519–537
Beeharry MK, Zhu ZL, Liu WT et al (2019) Correction to: Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: personal experience from a randomized case control study. BMC Cancer 19(1):1–3
Golse N, Bakrin N, Passot G et al (2012) Iterative procedures combining cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal recurrence: postoperative and long-term results. J Surg Oncol 106(2):197–203
Sugarbaker PH (2016) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev 48:42–49
Page MJ, Mckenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10(1):1–11
Higgins JPT, Altman DG, Gtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Koga S, Hamazoe R, Maeta M et al (1988) Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer 61:232–237
Kaibara N, Hamazoe R, Iitsuka Y et al (1989) Hyperthermic peritoneal perfusion combined with anticancer chemotherapy as prophylactic treatment of peritoneal recurrence of gastric cancer. Hepatogastroenterology 36(2):75–78
Hamazoe R, Maeta M, Kaibara N (2015) Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 73(8):2048–2052
Fujimura T, Yonemura Y, Muraoka K et al (1994) Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: randomized controlled study. World J Surg 18(1):150
Ikeguchi M, Kondou A, Oka A et al (1995) Effect of continuous hyperthermic peritoneal perfusion on prognosis of gastric cancer with serosal invasion. Eur J Surg 161(8):581–586
Fujimoto S, Takahashi M, Mutou T et al (2015) Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 85(3):529–534
Yonemura Y, Aretxabala XD, Fujimura T et al (2001) Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology 48(42):1776–1782
Yang XJ, Huang CQ, Suo T et al (2011) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 18(6):1575–1581
Cui H, Ge H, Bai X et al (2014) Effect of neoadjuvant chemotherapy combined with hyperthermic intraperitoneal perfusion chemotherapy on advanced gastric cancer. Exp Ther Med 7(5):1083–1088
Huang O, Lu XH, Xu XD et al (2015) Fibrin-sealant-delivered cisplatin chemotherapy versus cisplatin hyperthermic intraperitoneal perfusion chemotherapy for locally advanced gastric cancer without peritoneal metastases: a randomized phase-ii clinical trial with a 40-month follow-up. Cell Biochem Biophys 71(2):1171–1180
Rudloff U, Langan RC, Mullinax JE et al (2014) Impact of maximal cytoreductive surgery plus regional heated intra-peritoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 110(3):275–284
Lu C, Li L, Luo Z et al (2016) Clinical efficacy of type-B ultrasound-guided intraperitoneal hyperthermic chemoperfusion combined with systemic chemotherapy in advanced gastric cancer patients with malignant ascites. Neoplasma 63:299–303
Fan B, Bu Z, Zhang J et al (2021) Phase II trial of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer after curative surgery. BMC Cancer 21(1):216
Sterne J, Savovi J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ Clin Res 366:l4898
Afsar B, Kanbay M (2018) Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 378(14):1362
Nissan A, Garofalo A, Esquivel J (2010) Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy (HIPEC) for gastric adenocarcinoma: why haven’t we reached the promised land? J Surg Oncol 102(5):359–360
Reutovich MY, Krasko OV, Sukonko OG (2021) Hyperthermic intraperitoneal chemotherapy in prevention of gastric cancer metachronous peritoneal metastases: a systematic review. J Gastrointest Oncol 12(Suppl 1):S5–S17
Ji Z, Zhang Y, Li Y (2021) Intra-operative hyperthermic intraperitoneal chemotherapy for prevention and treatment of peritoneal metastases from gastric cancer: a narrative review. J Gastrointest Oncol 12(1):S70–S78
Pallas N, Karamveri C, Kyziridis D et al (2017) Cytoreductive surgery and hyperthermic intraperitenoal chemotherapy (HIPEC) for colorectal and appendiceal carcinomas with peritoneal carcinomatosis. J BUON: Off J Balk Union Oncol 22(6):1547–1553
Di N, Liu F et al (2009) Destruction of gastric cancer cells to mesothelial cells by apoptosis in the early peritoneal metastasis. J Huazhong Univ Sci Technol Med Sci 29:163–168
Liu G, Huang JP (2011) The clinical analysis of laparoscopic assisted D_2 radical gastrectomy for progressed gastric carcinoma. Chin J Mod Op Surg 15(5):355-357
Desiderio J, Chao J, Melstrom L et al (2017) The thirty-year experience - a meta-analysis of randomized and high-quality non-randomized studies of hyperthermic intraperitoneal chemotherapy(HIPEC) in the treatment of gastric cancer. Eur J Cancer 79:1–14
Liu Y-W, Ying Du et al (2019) Effect of hyperthermic intraperitoneal chemotherapy for gastric cancer patients: a meta-analysis of the randomized controlled trials. J Int Med Res 47(12):5926–5936
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13304_2022_1376_MOESM1_ESM.tif
Supplementary file1 Forest plot of the risk of intestinal anastomotic leakage in HIPEC group and the Control group. HIPEC hyperthermic intraperitoneal chemotherapy (TIF 1446 KB)
13304_2022_1376_MOESM2_ESM.tif
Supplementary file2 Forest plot of the risk of myelosuppression in HIPEC group and the Control group. HIPEC hyperthermic intraperitoneal chemotherapy (TIF 1462 KB)
13304_2022_1376_MOESM3_ESM.tif
Supplementary file3 Forest plot of the risk of nausea and vomiting in HIPEC group and the Control group. HIPEC hyperthermic intraperitoneal chemotherapy (TIF 1512 KB)
13304_2022_1376_MOESM4_ESM.tif
Supplementary file4 Funnel plot of OS at 1 year follow-up. RR= Risk Ratio, HIPEC hyperthermic intraperitoneal chemotherapy, OS overall survival (TIF 475 KB)
13304_2022_1376_MOESM5_ESM.tif
Supplementary file5 Funnel plot of OS at 3 years follow-up. RR Risk Ratio, HIPEC hyperthermic intraperitoneal chemotherapy, OS overall survival (TIF 475 KB)
13304_2022_1376_MOESM6_ESM.tif
Supplementary file6 Sensitivity analysis of OS at 1 year follow-up. HIPEC hyperthermic intraperitoneal chemotherapy, OS overall survival (TIF 442 KB)
13304_2022_1376_MOESM7_ESM.tif
Supplementary file7 Sensitivity analysis of OS at 2 years follow-up. HIPEC hyperthermic intraperitoneal chemotherapy, OS overall survival(TIF 442 KB)
13304_2022_1376_MOESM8_ESM.tif
Supplementary file8 Sensitivity analysis of OS at 3 years follow-up. HIPEC hyperthermic intraperitoneal chemotherapy, OS overall survival (TIF 442 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, H., Li, B. & Qin, X. The short- and long-term survival of hyperthermic intraperitoneal chemotherapy (HIPEC) in the advanced gastric cancer with/without peritoneal carcinomatosis: a systematic review and meta-analysis of randomized controlled trials. Updates Surg 74, 1805–1816 (2022). https://doi.org/10.1007/s13304-022-01376-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-022-01376-5