Abstract
Background
Microinvasion (MI), defined as infiltration of the portal or hepatic vein or bile duct and intrahepatic metastasis are accurate indicators of a poor prognosis for mall hepatocellular carcinomas (HCC). A previous study showed that intraoperative ultrasound (IOUS) definition of MI-HCC had a high concordance with histological findings. Aim of this study is to evaluate overall survival and recurrence patterns of patients with MI-HCC submitted to hepatic resection (HR) or laparoscopic ablation therapies (LAT).
Methods
A total of 171 consecutive patients (78 h; 93 LAT) with single, small HCC (< 3 cm) with a MI pattern at IOUS examination were compared analyzing overall survival and recurrence patterns using univariate and multivariate analysis and weighting by propensity score.
Results
Overall recurrences were similar in the 2 groups (HR: 51 patients (65%); LAT: 66 patients (71%)). The rate of local tumor progression in the HR group was very low (5 pts; 6%) in comparison to LAT group (22 pts; 24%; p = 0.002). The overall survival curves of HR are significantly better than that of the LAT group (p = 0.0039). On the propensity score Cox model, overall mortality was predicted by the surgical treatment with a Hazard ratio 1.68 (1.08–2.623) (p = 0.022).
Conclusions
If technically feasible and in patients fit for surgery, HR with an adequate tumor margin should be preferred to LAT in patients with MI-HCC at IOUS evaluation, to eradicate MI features near the main nodule, which are relatively frequent even in small HCC (< 3 cm).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several studies showed that some pathological features, such as differentiation grade, presence of vascular microinfiltration and satellites, are critical determinants of early recurrence and survival after successful hepatic resections (HR) for hepatocellular carcinoma (HCC) [1, 2] and these features could be useful to a rapid allocation in the waiting list for transplantation [3, 4]. A previous study by Yamashita et al. [5] showed that the presence of microinvasion (MI), represented by infiltration of the portal or hepatic veins, of bile ducts and/or intrahepatic metastases (satellite nodules), impacted prognosis after HR and transplantation: in fact, the recurrence-free survival of the MI-HCC after HR was significantly lower than that of the MI-negative group. Furthermore, the histological degree of HCC influences prognosis after surgery and high-grade HCC was a prognostic factor of vascular micro-infiltration [5,6,7,8,9].
Therefore, the capacity to detect both microinvasive hepatocellular carcinoma (MI-HCC) and histological grade of HCC could be important features for a treatment strategy [10, 11]. However, the histological grade of HCC and MI patterns can only be determined based on surgical specimens, limiting their clinical applicability: in fact, they are difficult to detect preoperatively with the current advanced imaging modality. Recent studies have shown that contrast-enhanced CT [12], diffusion-weighted magnetic resonance imaging (MRI) [13] and contrast-enhanced ultrasound [14] could be useful to predict the histological grade and/or the presence of MI, but these techniques using radiomics score are difficult to apply in the clinical practice. On the other hand, our recent study [15] suggested that patterns of aggressive behavior (MI-HCC) could be identified by the intraoperative ultrasound (IOUS) evaluation influencing the intraoperative treatment plan with respect to laparoscopic and open surgical intervention. In this prospective double-blind study, we showed that IOUS patterns of aggressive behavior correlate with both the presence of the same histological patterns and differentiation grade of HCC on the histologic specimen of the primary resected tumor.
Aim of this study is to evaluate if the selected treatment according to the actual guidelines, HR or laparoscopic ablation therapies (LAT), has a different prognostic outcome (in terms of HCC recurrences and survival) in a group of patients with a HCC tumor with an aggressive behavior (MI-HCC) defined by the IOUS evaluation.
Methods
Until 2000, a disease staging with a pre-established protocol was accomplished in all patients [16]; assessment of Barcelona Clinic Liver Cancer (BCLC) staging was retrospectively applied in all patients treated before its accessibility [17].
The recommended treatment was given by a multidisciplinary team, which includes surgeons, radiologists, and hepatologists. Particularly, LAT has been proposed according to specific indications already described [16]. Non-invasive imaging diagnosis of HCC including triple-phase computed tomography (CT) and/or MRI was accepted according to the Barcelona-2000 European Association for the Study of the Liver (EASL) Conference, while biopsy for a histological assessment should be performed when the imaging-based diagnosis remains inconclusive [17]. In all patients, Ct or MRI scan have been performed for the diagnosis of HCC: in the LAT group only 8 patients were submitted to histological confirmation (5 grade I and 3 grade II according to Edmondson classification), while in HR group only 5 patients (1 grade I, 3 grade II and 1 grade III according to Edmondson classification). Eligibility for liver transplantation (according to age, etiology, Child–Pugh and MELD score (Model for end-stage liver disease)) or HR was evaluated. HR was proposed for patients who had a single lesion if they had cirrhosis with preserved liver function: we did not consider nodule number and size as absolute exclusion criteria from surgical treatment. The presence of portal hypertension was not considered a contraindication for HR [18].
In this series, the function hepatic reserve was evaluated using the Child–Pugh classification [19] and MELD (model for end-stage liver disease) score [20]. Before surgery, all patients were submitted to serum laboratory tests, routine physical examination and radiographic studies.
This study was approved by Institutional Review Board at Milan University. The patient records were anonymized and de-identified prior to analysis, and informed consent to storage the clinical data of patients with HCC was obtained from each participant.
IOUS evaluation
All patients underwent IOUS evaluation (Aloka Alfa 10; Aloka Co, Tokyo and from 2018 Arietta V70; Hitachi Europe S.r.l. Italy) during the surgery. For laparotomic IOUS, T-shaped linear-type US probe was used at frequencies of both 5 MHz and 7.5 MHz, while for laparoscopic IOUS we used a laparoscopic ultrasound probe with a flexible tip, 10 mm in diameter and 50 cm in length and a 5–7.5 MHz linear-array transducer side-mounted near the tip of the shaft. Two surgeons (R.S., M.B.) with more than 10 years of experience in ultrasonography performed the IOUS exploration in all patients, reviewing (if it is necessary) the sonographic images recorded on videotape and magneto-optical disks. From the IOUS features of the primary HCC nodule [21], the surgeons classified the IOUS patterns according to diameter (registering also cut-off ≤ 20 mm), presence of satellites (identification of small nodules within 2 cm away from HCC lesion) and vascular/biliary micro-infiltration (either presence of vascular/biliary encasement or strict contact between vascular/biliary wall and nodule margins). Number of HCC nodules was also registered: in this case, IOUS pattern has been determined for the largest nodule. As described in our previous article [15], MI-HCC was defined as a tumor with vascular/biliary infiltration and/or the presence of satellites (Fig. 1).
Surgical procedures
Laparotomy for HR was carried out following a standardized technique while the technique of laparoscopic HR has been described elsewhere [22]. The indications for the HR and the type of operation were usually based on the HCC location, remnant liver volume and the hepatic functional reserve. Anatomical resection (AHR) was defined as any type of complete excision of at least one segment, included segmentectomy, subsegmentectomy, sectoriectomy and hemihepatectomy. Subsegmentectomy was defined as the complete removal of territories of the third-order portal vein branch smaller than one segment. US-guided atypical resection (UGAR) was defined as local resection or enucleation without regard to the segmental structure.
For the radiofrequency ablation procedure, a 200-W, 480 kHz monopolar radiofrequency generator (AMICA-GEN, HS Hospital Service SpA, Aprilia, Italy (®)) was used. Usually, the used delivered power was 150–170 W on average, for a total period of 10–12 min. Since February 2009, for the microwave ablation procedure, a 2.45 MHz microwave generator (AMICA-GEN, HS Hospital Service SpA, Aprilia, Italy (®)) providing energy through a 14- or 16-gauge internally cooled coaxial antenna and since April 2017, a 2.45 MHz microwave generator (EMPRINT Microwave Generator, Medtronic (®)) providing energy through a 14 internally cooled coaxial antenna was also used. According to the tumor size, a single microwave energy application is delivered to the patient, ranging from 70 to 100 W net power at the applicator end, for a total period of 3–10 min. The laparoscopic procedure was performed as described elsewhere [16, 22]. Absolute (99%) ethanol was injected at a dose of 5–10 ml for new small lesions (satellites < 1 cm) detected by IOUS.
Postoperative complications
Severity of postoperative morbidity was defined according to the Dindo-Clavien classification of surgical complications [23]: considering the impact of major complications on postoperative outcome in patients with HCC in high-risk locations, only complications of Grade III or higher were described.
Post-treatment imaging evaluation
Ultrasound and CT scan (or MRI) were repeated within one month and 3 months after the procedure. Experienced radiologists reviewed all CT/MRI exams.
As described elsewhere [22, 24], local tumor progression (LTP) was defined as the reappearance of enhancing tissue within and around the ablation zone, the latter case secondary to the presence of residual unablated tumor in a patient previously considered as completely treated. Intrasegmental recurrence (ISR) (including local tumor progression) was defined as the occurrence of HCC nodule in the same segment submitted to the surgical treatment, while intrahepatic recurrence (IHR) was defined as the recognition of new HCC tumors in other liver segments. HCC recurrence was also classified as early or late, using a cutoff of 12-months.
Statistical analysis
Initial evaluation and subsequent follow-up data were collected in a dedicated database (FileMaker Pro, FileMaker Inc., Santa Clara, California, USA) for personal computer input (Apple Computer Inc., Cupertino, California, USA). This retrospective study protocol was approved by our Institutional Review Board and waived the requirement for informed consent.
Statistical analysis was performed with R version 4.0.0 (2020–04-24) – "Arbor Day" Copyright (C) 2020 The R Foundation for Statistical Computing Platform: x86_64-w64-mingw32/ × 64 (64-bit)—Vienna, Austria. URL https://www.R-project.org/.
Categorical variables are reported as number (percentage) and compared with the Pearson’s Chi-squared test with Yates' continuity correction. Continuous variables are reported as mean (SD) or [median (IQR)] if not normally distributed and compared with the Wilcoxon rank Sum test.
Two Survival Analysis models (overall mortality and HCC recurrence) were built. Follow-up was calculated from the day of surgery. Censoring was applied to the last day of follow-up for patients not known to be dead or, respectively, to have had HCC recurrence.
Univariate analysis was performed on the Kaplan–Meier curves with the log-rank test for dichotomous variables and with a Cox proportional hazard model for continuous variables. Prognostic factors with p values < 0.2 on univariate analysis were subject to multivariate analysis using a Cox proportional-hazards regression model. The following dichotomous variables were studied in variable selection: gender, age, cirrhosis etiology, BCLC score, Child–Pugh’s score, MELD score, Charlson’s index, previous HCC, diabetes, esophageal varices, HCC maximum diameter, platelet count, prothrombin time, alfa-fetoprotein, new HCC nodule at IOUS, type of treatment.
A p < 0.05 was considered statistically significant in the final model. Proportionality of hazards was verified for each variable by visual inspection of log–log plots and with the proportional-hazards assumption test based on Schoenfeld residuals. Plotting the Martingale residuals against continuous covariates was used to verify their linearity. Interactions between significant variables in the final model were searched. Influential observations were checked by “dfbeta” values.
A sensitivity analysis was performed for the two survival models with Cox Proportional Hazard models weighted by propensity score with the inverse probability of treatment weighting (IPTW). Propensity scores were estimated using a simple logistic regression. Variance estimation takes into account the propensity score estimation step with the method proposed by Hajage et al. [25]. Average treatment effect on the treated (ATT) population is estimated. Ties are handled through the Breslow approximation. The following confounders are accounted for in the propensity model: age, Child, BCLC, and Meld scores, presence of diabetes mellitus, platelet and bilirubin levels.
Results
Subjects
Data included in this study came from patients who were treated with either HR or LAT starting in 1998 to April 2020. Table 1 shows the preoperative characteristics of the patients. They were divided into 2 groups: 78 patients submitted to HR and 93 to LAT. Differences in the surgical indications reflected some differences in the 2 groups: LAT group shows higher rates of signs of liver dysfunction (MELD ≥ 9, BCLC stage > 1, esophageal varices, lower platelet count and higher INR values) and lower HCC maximum diameter.
Surgical procedures and findings
AHR was performed in 54 cases (69%), while UGAR was chosen in 24 cases (31%). A laparoscopic approach was chosen in 23 (29%) patients. The Pringle maneuver was used in 41 patients (53%) (total duration with interval maneuver: 35 ± 20 min). In the LAT group, a single electrode technique (radiofrequency ablation: RFA) was used in 61 patients (66%) and a cluster-electrode system in 1 case (exposed tip 2.5 cm), while a microwave antenna was used in 31 patients (33%): in 36 patients (39%) a single needle insertion was sufficient while in 43 patients (46%) two-needle insertions and in 14 (15%) patients three or more needle insertions were necessary to obtain adequate tissue coagulation. Mean procedure time for the RFA portion of LAT treatments was 17.2 ± 5.6 min (median: 16; IR: 12–22) and for MWA was 9.7 ± 4.3 min (median: 9; IR: 7–12), while the procedure duration was significantly longer for HR (205 ± 57 min; median: 206; IR: 160–245) compared to LAT (81 ± 30 min, median: 74; IR: 60–90) (p < 0.0001).
During IOUS evaluation, 16/78 (20.5%) patients who underwent HR were found to have additional lesions (10 in a different segment, 6 within 1 cm of the primary HCC location); of these, six were submitted to enlarged resection, 5 to thermoablation and 5 to ethanol injection. In the LAT group, IOUS identified 37/93 (40%) cases (p = 0.007) with previously undetected lesions (25 in a different segment, 12 within 1 cm of the primary HCC); of these, 32 cases were treated with additional thermoablation and 5 with ethanol injection.
Postoperative results
Table 2 illustrates the postoperative outcomes at both short- and long-term following LHR and LAT. As expected, HR was characterized by a significantly longer postoperative hospital stay and higher complication rates compared to LAT in the short term (but the 2 groups had similar rates for severe complications), while morbidity rates became similar during the follow-up period. There were 1 post-operative death in the HR group at 30 days due to sepsis while other four patients died within 90 days: 1 patient for liver failure, 1 for liver failure with diffuse HCC, 1 for hepatic abscess (in the LAT group) and the other for cerebro-vascular accident.
The margins of resection specimens in HR group were negative in all patients with a distance between lesion and margin of 7.1 ± 7.4 mm (median 5; IR: 3–9). In LAT group, a complete necrosis was obtained at 1 month in 88 patients (95%) patients: these patients required an additional trans-arterial chemoembolization in 3 cases and another thermoablation session in the other 2 to achieve complete HCC necrosis.
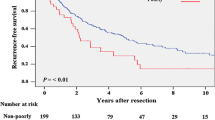
During the follow-up period (47.2 ± 37 months; median 40; IR 18–68.5), 53 (68%) patients in the HR group and 65 (70%) of the LAT group died (Table 2). Overall recurrences were similar in the 2 groups, as well as for the type of HR (61% in AHR and 75% in UGAR), the surgical approach (65% for laparoscopic or open HR) and the type of LAT (77% for RFA and 58% for MWA). Recurrence rates near the surgical margins in the HR group were lower than LTP in LAT group (p = 0.002), with no statistical differences between the type of HR (4% after AHR and 12.5% after UGAR), the surgical approach (4% after laparoscopic and 7% after open) and the type of LAT (29% after RFA and 21% after MWA). The actuarial recurrence rate calculated by Kaplan–Meier product-limit method in patients treated with LAT was significantly different compared to cases treated with HR (p = 0.012) (Fig. 2a). Furthermore, the actuarial survival rates at 1, 3 and 5 years of follow-up were respectively 91%, 76%, and 50% in the HR group and 89%, 60%, and 32% in the LAT group (p = 0.0039) (Fig. 2b).
Table 3 summarize the results of univariate analysis of the influence of preoperative and intraoperative factors on the recurrence rates and survival among the 171 patients with HCC included in the present study, regardless of the surgical approach. Of note, the choice of HR or LAT was significantly associated both with HCC recurrence and with patient survival.
Probability of hepatocellular carcinoma (HCC) recurrences (a) and overall survival (b) actuarial curves comparing surgical resection (HR) and laparoscopic radiofrequency (LAT). The differences between these groups were statistically significant for recurrence (p = 0.012) and overall survival (p = 0.0039)
On multivariate analysis, overall mortality was predicted by the MELD score (Hazard ratio = 1.32 (1.16–1.51), p < 0.001) and serum bilirubin (Hazard ratio = 0.49 (0,27–0.88), p = 0.017), with the surgical treatment (Hazard ratio = 1.55 (0.98–2.44), p = 0.059) barely entering the model. On the propensity score Cox model, overall mortality was predicted by the surgical treatment with Hazard ratio 1.68 (1.08–2.623) and p = 0.022.
On multivariate analysis HCC recurrence was predicted only by the surgical treatment (Hazard ratio = 1.8 (1.2–2.7). p = 0.003). On the propensity score Cox model, HCC recurrence was predicted by the surgical treatment with Hazard ratio 1.82 (1.13- 2.94) and p = 0.014 (Table 4).
Discussion
HCC remains a great challenge because of the high recurrence rates after radical treatments, mainly due to new HCC tumors in the remaining liver. This situation significantly impaired long-term overall survival. This study of 171 patients with small HCC nodule (≤ 3 cm) with a microinvasive ultrasound feature showed that this aggressive biological behavior has an important impact on recurrences and survival which the type of treatment could modify.
Many scoring systems for preoperative non-invasive procedures have been evaluated to identify the MI-HCC, but they have only a potential value to discriminate MI-HCC patients. Dong et al. [26] used a radiomic algorithm base on grayscale ultrasound features but the results showed some biases as limited resolution and relatively low accuracy, highly depending by operator experience. Also, the use of contrast-enhanced ultrasound [14] did not improve the results and the Authors outlined that radiomics technique needs to be developed simpler to promote its application in clinical practice. The use of CT images [12] to investigate prognostic aspects of computational-assisted models, especially the CT tumor radiomic features, could predict the microvascular infiltration in the preoperative period, but it showed several technical and methodological challenges. Similar problems [27] have occurred with combined models based on MRI and clinical features for the prediction in MI-HCC.
Several studies have confirmed that the presence of microvascular infiltration and satellitosis were risk factors of HCC recurrence after curative treatments [1,2,3] and therefore the importance to have a technique for their identification before the histological examination [4,5,6]. In fact, if it is possible to know the biological aggressiveness of the primary tumor, the patient with a MI-HCC could be treated differently. A recent study [28] of 496 patients with single, small (< 2 cm) HCC submitted to hepatectomy confirmed that the presence of microvascular infiltration correlated with a poor prognosis: the Authors concluded that the identification of this pattern should help in avoiding local ablation as first-line treatment and in enlarging the surgical margins during the resection. Another study confirmed this suggestion [29]: narrow resection margins (< 1 cm) in patients with MI-HCC determined the worst oncological outcomes after HR. These results have not been completely confirmed by another study [30] comparing anatomical versus non-anatomical resections. The group submitted to anatomical resections did not suffer of any local recurrences, but this advantage did not produce a significant effect on overall recurrence and survival rates. On the other hand, a comparison between percutaneous radiofrequency ablation and HR [31] showed that the rate of early HCC recurrences was lower after HR than percutaneous ablation for patients with small HCC (< 3 cm) at high risk of microvascular infiltration detected by a prediction model.
In a previous study [15], we have demonstrated that IOUS is able to detect the features of MI-HCC and these findings strongly correlated with histopathologic criteria. These IOUS findings are important to knowledge the biological behavior of HCC and to predict the risk of long-term recurrence and overall survival rates. The capacity of IOUS to detect the MI-IOUS pattern permit to identify these high-risk patients also in the case of LAT, a treatment without a pathological specimen to evaluate.
This study showed some differences in comparison with previously cited studies, both for the methods and for the results. Particularly:
-
Neither radiomics methods nor predictive scores have been used to identify patients with MI-HCC, but IOUS has been used to identify MI-HCC. In our previous studies [15, 21], we demonstrated that high-frequency probes of IOUS is able to identify the presence of microvascular infiltration and satellitosis. These ultrasound features strongly correlated with histological findings producing a beneficial impact on the management choices.
-
Different classification of MI-HCC has been proposed as summarized in the article of Erstad et al. [2]. Our study, according to the definition of Yamashita [5], did not include only the microvascular infiltration. We defined as MI-HCC [15] all nodule with the presence of vascular/biliary encasement or strict contact between vascular/biliary wall and nodule margins and the identification of small nodules within 2 cm away from the primary lesion.
-
HR guarantees lower rates of LTP and intra-segmental recurrences than LAT, while the overall intra-hepatic recurrence rate is significantly different only analyzing the actuarial curves. The intraoperative definition of MI-HCC defined a group of patients at high-risk to develop a new nodule, also away distant from the primary tumor.
-
Definitely, in patients with MI-HCC, LAT showed a higher risk of LTP, probably due to the microvascular infiltration and/or satellitosis: these results are similar to those obtained for lesions adjacent the major vessels as described elsewhere [32]. On the other hand, the specific indications for the laparoscopic approach (difficult, deep or dangerous position) is a justification for overlapping needle insertions to obtain a larger necrosis area and to include the microvascular infiltration and/or satellitosis. In the future, an extensive use of laparoscopic contrast-enhanced IOUS could permit to increase the efficacy of LAT evaluating intraoperatively the necrosis area and the presence of residual disease;
-
On multivariate analysis, HCC recurrence was predicted only by the surgical treatment (Hazard ratio = 1.8 (1.2–2.7) (p = 0.003), confirmed also by the propensity score Cox model. Post-treatment HCC recurrence is a common problem in cirrhotic patients: several studies confirmed that patients with LTP or recurrence after local treatments had a higher risk to develop new HCC during the follow-up [33,34,35,36].
-
On multivariate analysis, overall mortality was predicted by the MELD score and serum bilirubin with the surgical treatment barely entering the model. As regards the two first variables, it is well known that hepatic function scores and bilirubin levels, also in Child A patients, were important prognostic factors for overall survival not only for HR [18, 37] or LAT [38] but also for liver transplantation [39]. However, on the propensity score Cox model, overall mortality was predicted only by the surgical treatment with a Hazard ratio 1.68 (1.08–2.623) (p = 0.022).
-
Recent meta-analysis confirmed that HR offers better long-term oncologic outcomes than percutaneous RFA [40], also for patients with very-early HCC. Furthermore, our previous study comparing LAT and HR [22] showed some advantages for surgery in terms of recurrences and overall survival, even if LAT is a valid alternative for tumors would require complex HR or for patients with liver disfunction. In this group of patients including small HCC with aggressive behavior (MI-HCC), HR seems guarantee both a better overall survival and the recurrences rates.
The present study, despite the proper methodology used to balance the baseline characteristics of patients included in the analysis, is limited by the clinical tendency to select each treatment according to specific technical variables: a lesion sited in the periphery versus centrally located, its proximity to major bile ducts, a patient’s body habitus with or without comorbidities, or the provider’s experience could introduce an inherent selection bias.
In conclusion, IOUS is able to detect patients with MI-HCC. If the nodule position and/or the liver function permits a surgical resection, HR is the best option and, if it is possible, through a laparoscopic approach guarantying wide margins. At the contrary, LAT is a valid alternative even if ablation through a surgical approach seems to be not able to improve LTP rates: probably IOUS evaluation cannot completely detect the exact extension of microvascular infiltration and satellitosis resulting in a not-completely ablation effect. Further studies are necessary to elucidate if MWA or other technologies (laparoscopic contrast-enhanced IOUS) could improve oncological outcomes of patients with MI-HCC in comparison to RFA.
Data availability
Data are collected in database which could be checked.
References
Chen ZH, Zhang XP, Wang H, Chai ZT, Sun JX, Guo WX, Shi J, Cheng SQ (2019) Effect of microvascular invasion on the postoperative long-term prognosis of solitary small HCC: a systematic review and meta-analysis. HPB 21:935–944
Erstad DJ, Tanabe KK (2019) Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol 26:1474–1493
Ferrer-Fabrega J, Forner A, Liccioni A, Miquel R, Molina V, Navasa M, Fondevila C, Garcia-Valdecasas JC, Bruix J, Fuster J (2016) Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology 63:839–849
Cillo U, Giuliani T, Polacco M, Herrero Manley LM, Crivellari G, Vitale A (2016) Prediction of hepatocellular carcinoma biological behavior in patient selection for liver transplantation. World J Gastroenterol 22:232–252
Yamashita Y, Tsuijita E, Takeishi K, Fujiwara M, Kira S, Mori M, Aishima S, Taketomi A, Shirabe K, Ishida T, Maehara Y (2012) Predictors for microinvasion of small hepatocellular carcinoma ≤ 2 cm. Ann Surg Oncol 19:2027–2034
Cucchetti A, Piscaglia F, Grigioni AD, Ravaioli M, Cescon M, Zanello M, Grazi GL, Golfieri R, Grigioni WF, Pinna AD (2010) Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol 52:880–888
Colecchia A, Scaioli E, Montrone L, Vestito A, Di Biase AR, Pieri M, D’Errico-Grgioni A, Bacchi-Reggiani ML, Ravaioli M, Grazi GL, Festi D (2011) Preoperative liver biopsy in cirrhotic patients with early hepatocellular carcinoma represents a safe and accurate diagnostic tool for tumour grading assessment. J Hepatol 54:300–305
Zhao J, Mao J, Li W (2019) Association of tumor grade with long-term survival in patients with hepatocellular carcinoma after liver transplantation. Transplant Proc 51:813–819
Shen J, Liu J, Li C, Wen T, Yan L, Yang J (2018) The impact of tumor differentiation on the prognosis of HBV-associated solitary hepatocellular carcinoma following hepatectomy: a propensity score matching analysis. Dig Dis Sci 63:1962–1969
Sasaki K, Matsuda M, Ohkura Y, Kawamura Y, Inoue M, Hashimoto M, Ikeda K, Kumada H, Watanabe G (2015) The influence of histological differentiation grade on the outcome of liver resection for hepatocellular carcinomas 2 cm or smaller in size. World J Surg 39:1134–1141
Iguchi T, Shirabe K, Aishima S, Wang H, Fujita N, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, Oda Y, Maehara Y (2015) New pathologic stratification of microvascular invasion in hepatocellular carcinoma: predicting prognosis after living-donor liver transplantation. Transplantation 99:1236–1242
Xu X, Zhang HL, Liu QP, Sun SW, Zhang J, Zhu FP, Yang G, Yan X, Zhang YD, Liu XS (2019) Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol 70:1133–1144
Chen J, Zhou J, Kuang S, Zhang Y, Xie S, He B, Deng Y, Yang H, Shan Q, Wu J, Sirlin CB, Wang J (2019) Liver imaging reporting and data system category 5: MRI predictors of microvascular invasion and recurrence after hepatectomy for hepatocellular carcinoma. AJR 213:821–830
Hu HT, Wang Z, Huang XW, Chen SL, Zheng X, Ruan SM, Xie XY, Lu MD, Yu J, Tian J, Liang P, Kuang M (2019) Ultrasound-based radiomics score: a potential biomarker for the prediction of microvascular invasion in hepatocellular carcinoma. Eur Radiol 29:2890–2901
Santambrogio R, Cigala C, Maggioni BM, M, Scifo G, Bruno S, Bertolini E, Opocher E, Bulfamante G, (2018) Intraoperative ultrasound for prediction of hepatocellular carcinoma biological behavior: prospective comparison with pathology. Liver Int 38:312–320
Santambrogio R, Barabino M, De Nicola E, Galfrascoli E, Giovenzana M, Zappa MA (2020) Laparoscopic ablation therapies for hepatocellular carcinoma: could specific indications for the laparoscopic approach influence the effectiveness? Surg Endosc 16:349–354
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet 391:1301–1314
Santambrogio R, Kluger MD, Costa M, Belli A, Barabino M, Laurent A, Opocher E, Azoulay D, Cherqui D (2013) Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh’s A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB 15:78–84
Pugh RNH, Murray-Lyon M, Dawson JL, Pietroni MC, Williams R (1973) Transection of the esophagus for bleeding esophageal varices. Br J Surg 60:646–649
Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, Lock AS (2005) Prognosis of hepatocellular carcinoma: Comparison of seven staging systems in an American cohort. Hepatology 41:707–716
Santambrogio R, Costa M, Strada D, Bertolini E, Zuin M, Barabino M, Opocher E (2011) Intraoperative ultrasound score to predict recurrent hepatocellular carcinoma after radical treatments. Ultrasound Med Biol 37:7–15
Santambrogio R, Bruno S, Kluger CM, Salceda J, Belli A, Laurent A, Barabino M, Opocher E, Azoulay D, Cherqui D (2016) Laparoscopic ablation therapies or hepatic resection in cirrhotic patients with small hepatocellular carcinoma. Dig Liver Dis 48:189–196
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications. A new proposal with evaluation in a cohort of 633 patients and results of a survey. Ann Surg 240:205–213
Ahmed M (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 273:241–260
Hajage D, Chauvet G, Belin L, Lafourcade A, Tubach F, De Rycke Y (2018) Closed-form variance estimator for weighted propensity score estimators with survival outcome. Biom J 60:1151–1163
Dong YI, Zhou L, Xia W, Zhao XY, Zhang Q, Jian JM, Gao X, Wang WP (2020) Preoperative prediction of microvascular invasion in hepatocellular carcinoma: initial application of a radiomic algorithm based on grayscale ultrasound images. Front Oncol 10:353. https://doi.org/10.3389/fonc.2020.00353
Zhu YJ, Feng B, Wang S, Wang LM, Wu JF, Ma XH, Zhao XM (2019) Model-based three-dimensional texture analysis of contrast-enhanced magnetic resonance imaging as a potential tool for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Oncol Let 18:720–732
Wang H, Wu MC, Cong WM (2019) Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res 49:344–354
Han J, Li ZL, Xing H, Wu H, Zhu P, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Wu MC, Shen F, Yang T (2019) The impact of resection margin and microvascular invasion on long-term prognosis after curative resection of hepatocellular carcinoma: a multi-institutional study. HPB 21:962–971
Hidaka M, Eguchi S, Okuda K, Beppu T, Shirabe K, Kondo K, Takami Y, Ohta M, Shiraishi M, Ueno S, Nanashima A, Noritomi T, Kitahara K, Fujioka H (2020) Impact of anatomical resection for hepatocellular carcinoma with microportal invasion (vp1). A multi-institutional study by the Kyushu Group of Liver Surgery. Ann Surg 271:339–346
Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, Kim SY, Sinn DH, Kim JM, Kim K, Sy Ha (2019) Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. https://doi.org/10.1097/SLA.0000000000003268
Chen J, Peng K, Hu D, Shen J, Zhou Z, Xu L, Chen J, Pan Y, Wang J, Zhang Y, Chen M (2018) Laparoscopic ablation therapies for hepatocellular carcinoma: could specific indications for the laparoscopic approach influence the effectiveness? Cancers 10:378. https://doi.org/10.3390/cancers10100378
Rossi S, Ravetta V, Rosa L, Ghittoni G, Torello Viera F, Garbagnati F, Silini EM, Dionigi P, Calliada F, Quaretti P, Tinelli C (2011) Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study 53:136–147
Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, Han JK, Choi BI (2014) Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270:900–909
Sparchez Z, Mocan T, Radu P, Mocan LP, Sparchez M, Leucuta DC, Al HN (2018) Prognostic factors after percutaneous ablation in the treatment of hepatocellular carcinoma. Impact of incomplete ablation on recurrence and overall survival rates. J Gastrointestin Liver Dis 27:399–407
Xu HX, Lu MD, Xie XY, Yin XY, Kuang M, Chen JW, Xu ZF, Liu GJ (2005) Prognostic factors for long-term outcome after percutaneous thermal ablation for hepatocellular carcinoma: a survival analysis of 137 consecutive patients. Clin Radiol 60:1018–1025
Santambrogio R, Salceda J, Costa M, Kluger MD, Barabino M, Laurent A, Opocher E, Azoulay D, Cherqui D (2013) External validation of a simplified BCLC staging system for early hepatocellular carcinoma. EJSO 39:850–857
Cillo U, Bertacco A, Fasolo E, Carandina R, Vitale A, Zanus G, Gringeri E, D’Amico F, Bassi D, Neri D, Dadduzio V, Farinati F, Aliberti C (2019) Videolaparoscopic microwave ablation in patients with HCC at a European high-volume cnter: results of 815 procedures. J Surg Oncol 120:956–965
Vibert E, Schwartz M, Olthoff KM (2020) Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol 72:262–276
Fan H, Zhou C, Yan J, Meng W, Zhang W (2020) Treatment of solitary hepatocellular carcinoma up to 2 cm. A PRISMA-compliant systematic review and meta-analysis. Medicine 99:e20321. https://doi.org/10.1097/MD.0000000000020321
Funding
No funding.
Author information
Authors and Affiliations
Contributions
RS acts as the submission's guarantor. Each author participated with substantial contributions to conception, analysis and interpretation of data, active participate in drafting and revising it, with a final approval for publication. RS: project development, data analysis & collection, interpretation of data, manuscript writing/editing. MB: project development, data analysis & collection, interpretation of data, manuscript editing. VD’A: data analysis & collection, manuscript editing. GI: data analysis & collection, manuscript editing. EO: project development, interpretation of data, manuscript editing. MG: project development, interpretation of data, manuscript writing/editing. MAZ: project development, interpretation of data, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest or financial ties to disclose.
Ethics approval
This retrospective study protocol was approved by our Institutional Review Board and waived the requirement for informed consent.
Research involving human participants and/or animals Institutional review research board approval was granted by ASST Fatebenefratelli Sacco, and appropriate good clinical and research practices were followed.
Informed consent
We have obtained consent to publish from the participants to report individual patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper has been accepted by the EAES Congress 2020 and it is eligible to be fast-tracked.
Rights and permissions
About this article
Cite this article
Santambrogio, R., Barabino, M., D’Alessandro, V. et al. Micronvasive behaviour of single small hepatocellular carcinoma: which treatment?. Updates Surg 73, 1359–1369 (2021). https://doi.org/10.1007/s13304-021-01036-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-021-01036-0