Abstract
Several studies attempted to determine whether there is a relationship between the use of preoperative biliary drainage and morbidity after pancreaticoduodenectomy (PD). We retrospectively evaluated post-PD outcome in patients with and without preoperative biliary drainage and the role of bacteriobilia and antibiotic prophylaxis in post-operative complications. Data relating to the PDs performed at the Hepato-Bilio-Pancreatic Surgical Department of Treviso Hospital between 2010 and 2017 were retrospectively evaluated. Morbidity and intra-hospital mortality related to preoperative biliary stent were the primary outcomes. Between 2010 and 2017, 128 patients (mean age 68 years) underwent PD; 72 were treated with early surgery (ES) and 56 underwent preoperative biliary drainage (PBD). Overall morbidity was 50% in the ES cohort and 43% in the PBD (ns, p = 0.43). In the PBD group, bacteriobilia was found in the 100% of the bile cultures (48; 8 unavailable). The microbiota was represented by: Klebsiella spp (48%), Enterococcus spp (29%), E. coli (27%) and Candida spp (21%). In 52% of cases, at least one of the isolated bacteria was resistant to the perioperative antibiotic prophylaxis (69% of cases Amoxicillin–Clavulanic Ac.). The majority of postoperative surgical complications occurred in patients with prophylaxis-resistant bacteriobilia (68% vs 39%; p = 0.04). Antibiotic resistance is a determining factor in morbidity after PD. We therefore propose to pay particular attention to the preoperative prophylaxis, diversifying it between drained and non-drained patients. In fact, in the former, appropriate broad spectrum preoperative antibiotic coverage is strongly suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “periampullary tumor” defines a heterogeneous group of neoplasms that may originate from the pancreatic head (40–60%), the ampulla of Vater (10–20%), the distal bile duct (10%), the duodenal wall (5–10%) or the uncinate process of pancreas (2%) [1, 2]. The symptomatology of these patients is dominated by neoplastic obstructive jaundice and pancreaticoduodenectomy (PD) is the only curative treatment. Only between 14 and 30% of patients are amenable of this surgical treatment [3]. In the last decades, the surgical outcome has improved due to the development of surgical technique, anesthesiology and peri-operative care. Although high-volume centers reported a decline in intra-hospital mortality, which is currently less than 5%, post-operative morbidity is still high ranging from 25 to 60% [3, 4].
Many organs are involved in the deleterious effects of a prolonged and worsening obstructive jaundice. Biliary stasis can lead to: hepatic failure, opportunistic infections, renal failure, heart failure, coagulation disorders and immune deficiencies [5, 6]. The preoperative restoration of biliary outflow can be performed by percutaneous trans-hepato-duodenal drainage (PTBD) or by endoscopic retrograde cholangiopancreatography (ERCP) [7, 8].
An element of discussion is the balance between risks and benefits of the preoperative biliary drainage in resectable patients. Despite the efficacy of biliary drainage in reducing serum bilirubin levels, the majority of studies have not shown an improvement in postoperative outcome but, rather, an increase in infectious complications [6, 9]. The meta-analysis that investigated this topic concluded that preoperative biliary drainage should not be performed routinely, given the absence of difference in mortality and the increased associated morbidity [7, 10,11,12]. Stenting is recommended in patients with cholangitis, pruritus, coagulopathy, renal failure or for whom the surgical treatment is delayed for at least one week [13]. The preoperative biliary drainage (PBD) can lead to ascending bacterial colonization and consequently to an increased risk of infections, including those of surgical site (wound or intra-abdominal), cholangitis and sepsis. Several studies have shown that patients undergoing PBD have an increased rate of positive intraoperative bile cultures and have an increased infection-related morbidity and mortality rate. The study of the susceptibility to antibiotic prophylaxis in patients with preoperative biliary drainage is a matter of recent debate in surgical literature. Recent studies demonstrated that bile culture is useful to diagnose bacteriobilia, to determine the organisms involved and to provide the antibiogram for modulation of antibiotic therapy [14,15,16,17,18].
Implementation of measures to reduce and control infectious complications after PD may be the key to a new improvement in this surgery. Adequate treatment of documented bacteriobilia in patients undergoing preoperative biliary drainage can lead to a reduction in morbidity.
The objective of this study is the assessment of patients undergoing PD with and without preoperative drainage to determine if biliary drainage influences the complication rate (general and specific) and if bacteriobilia has a role in post-operative complications.
Materials and methods
The operative series of pancreaticoduodenectomies performed at the Department of Hepato-Bilio-Pancreatic (HBP) Surgery of Treviso Hospital between January 2010 (the year of beginning of routine intra-operative bile cultures) and December 2017 were retrospectively analyzed. Demographic information, anthropomorphic data, performance status, past medical history (with attention to pulmonary disease, cardiovascular disease, diabetes, smoking, pancreatitis, obesity) and blood chemistry values were recorded. Obesity was defined for a BMI > 30 kg/m2; smoking has been registered as current, past or non-smoker; renal failure was defined for a value of creatinine > 1.2 mg/dL; hypoalbuminemia for a value < 3.2 mg/dL. The American Society of Anesthesiologists (ASA) score was obtained from anesthesiologist’s records. The presence of biliary stent was recorded taking into account the date of ERCP or PBD, the type of material (self-expanding metallic covered versus plastic) and possible substitution. All procedures were performed at our Hospital by the gastroenterology or radiology unit. For each intervention, we recorded: the antibiotic prophylaxis, the bile culture and the following antibiogram; in the post-operative period, the continuation of the prophylaxis or not, the switch or the association of further therapy in the light of the microbial culture or of any subsequent complication. All the complications that occurred within 90 days after surgery were analyzed and classified according to Clavien–Dindo score: fistula (pancreatic, biliary, gastro-jejunal); blood loss; Delayed Gastric Emptying (DGE); abdominal abscess; wound infection; pneumonia; cholangitis; heart attack; portal thrombosis and re-laparotomy. Continuous variables were reported as median (range) and categorical variables as frequency and percentage. Continuous variables were compared across groups using the Wilcoxon test and categorical variables with the χ2 test. Statistical analysis was performed with the SPSS Statistics Software (IBM® v. 25.0), the significance was set at p < 0.05.
Results
A total of 128 patients with a lesion of the peri-ampullary region underwent PD. Most patients were male (M/F: 77/58) with a mean age of 67.3 ± 10.3 years (maximum 85 years, minimum 32.7 years). Histological examination showed in most cases pancreatic adenocarcinoma (62/128, 48.4%), followed by distal cholangiocarcinoma (17/128, 13.2%) and intraductal papillary mucinous neoplasia (IPMN) (15/128, 11.7%). Other diagnoses in the remaining 26.3% of cases: adenocarcinoma of Vater’s ampulla, neuroendocrine tumor, serous cystadenoma, squamous carcinoma, metastatic localizations and duodenal cystic dystrophy. In case of pancreatic adenocarcinoma, the staging of the disease (where available) was in most cases 2B (43 patients—33.5%) or 2A (31 patients—24.2%). The overall post-operative complication rate was 46.8% and 30-day mortality was 3.1%. 72 patients out of 128 underwent immediate surgery; 56 underwent preoperative biliary drainage. In accordance with NCCN guidelines [13], PBD was placed in symptomatic patients (cholangitis, pruritus, coagulopathy, renal failure) or for whom the surgical treatment was scheduled after at least one week, no drop out from surgery was recorded. The general characteristics of the two populations are summarized in Table 1. Statistical analysis revealed no statistically significant differences between the two subgroups analyzed.
The intra- and postoperative course was analyzed for each group of patients depending on the preoperative biliary drainage variable—absent or present—and the respective co-variables to determine which could be related (Table 2). Data analysis shows that the two groups, immediate surgery vs. preoperative drainage, are comparable regarding blood loss (585 ± 423 ml vs 578 ± 423 ml, p = 0.93), duration of surgery (354 ± 51 min vs 370 ± 61 min, p = 0.12) and complications (50% vs 43%, p = 0.43). The antibiotic prophylaxis was prescribed by the surgeon assigned to the check-list in the Operating Room (OR), according to the Hospital Guidelines drawn up in their different editions (from revision 2 in 2010 up to revision 4 since 2015). Except for the combination of piperacillin + tazobactam and metronidazole + gentamicin, the other extra-protocol prophylactic regimens prescribed were justified on a therapy already in place at home or in pre-operative hospitalization. The regimens, however, did not differ between the two groups (Table 2).
Overall morbidity was 50% in the early surgery group and 43% in the PBD one. No significant differences emerged between the two populations (p = 0.43). RR due to the PBD was 0.85 (IC95% 0.57–1.24). Complications were recorded according to the Dindo and Clavien classification [19], demonstrating that among minor complication degrees (1 and 2) and among those of greater severity (3–5) there were no differences between the two groups (grades 1–2, 45.8% vs 33.9%; grades 3a–5, 16.6% vs. 21.4%). Two deaths were registered in both groups. In the early surgery group: a septic shock and a hemorrhagic shock. In the PBD group, they were due to complications of severe cirrhosis and hemorrhagic shock, Table 2.
Eventually, bacteriobilia rate was analyzed. Intra-operative bile culture tests were obtained from a bile sample at the time of bile duct section. Biliary cultures were not performed on 8 patients out of the 56 who underwent biliary drainage. Bacteriobilia was significantly higher in patients that underwent stenting, with a 100% infection rate (48 patients) compared to 8.3% (8 patients) of early surgery, p ˂ 0.001 RR = 9.00 (IC 95% 6.55–16.69) (Table 3).
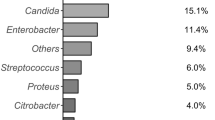
The relationship between antibiotic prophylaxis and resistance revealed a global resistance rate of 52% (25/48). Isolated bacteria are summarized in Table 4. The most frequently responsible for resistant bacteriobilia were: Escherichia spp, Enterobacter spp, Klebsiella spp, Candida spp and Enterococcus spp. PBD patients were, therefore, analyzed based on the resistance to prophylaxis. Data are summarized in Table 5. There were no differences in the stent placement route. The material used was predominantly plastic (95% vs 92%). Registered cases of obstruction or malfunction that required replacement only occurred to plastic stents at a rate of 17.3% vs 16%. The time between stent placement and surgery was not statistically different between the two groups: 29.8 ± 15.0 vs 40.5 ± 30.8 days, p = 0.14. Moreover, in the case of resistance, only in 48% of cases, it was possible to modulate the antibiotic therapy on the basis of the antibiogram due to allergies or, more often, to the time elapsed between the execution and the response of the culture examination which made the data ineffectual. The incidence of complications was statistically different between the two groups with a higher incidence rate in patients with antibiotic-prophylaxis resistance: 68% vs. 39%, p = 0.04 with a RR of 1.73 (IC 95% 1.012–3.214).
Discussion
The placement of preoperative biliary drainage (PBD) can lead to the ascending bacterial colonization of bile and consequently to an increased risk of infections, including those of surgical site, cholangitis and sepsis. Several studies have shown that PBD patients have an increased rate of positive intraoperative bile cultures and have an increased infection-related morbidity and mortality rate [14, 15, 20]. Bacteriobilia is associated with stent placement in up to 80% of cases. Patients with bacteriobilia have an increased rate of major postoperative complications (overall, 71% vs 43% p = 0.012) [15], wound infections (14% vs 2%, p = 0.03) and bacteremia (8% vs 0%, p = 0.04) [14]. The most frequently isolated microorganisms are: Enterococcus species (51%), Escherichia coli species (37%) and Klebsiella species (14%). Furthermore, these microorganisms are predictive of those isolated in infectious complications [14, 15].
The study of the susceptibility to antibiotic prophylaxis in patients with preoperative biliary drainage is a matter of recent debate in the surgical literature. In 2014, Somala and colleagues demonstrated that 83% of the stented patients had bacteriobilia, compared to 13% of the controls. In addition, 37% (vs 2%) had a fungal infection. Targeted antibiotic treatment of patients with positive biliary culture led to a drop in wound infection rates from 12 to 3%; p = 0.036 [16]. In 2017 Scheufele demonstrated that PBD patients in 63.6% of cases had a biliary microbiota resistant to the prophylaxis administered (ampicillin–sulbactam) [18]. Sudo and colleagues proposed that the most suitable antibiotics regimen should be a fourth generation cephalosporin (cefozopran) or the imipenem–cilastatin association which exhibit a resistance after PBD of 9% and 5%, respectively [17].
Published studies report different incidences of fungal biliary infections. The term "biliary candidiasis" has recently entered the surgical lexicon. A recent study by Lenz and colleagues has shown that Candida is commonly isolated from bile in association with the immunosuppression state related to the neoplasm or to ongoing cancer therapies. Biliary candidiasis should be considered a "real" infection and treated [21]. The conclusion of these studies is unanimous: the execution of the bile culture is useful to diagnose bacteriobilia, to determine the organisms involved and to provide the antibiogram to early modulate antibiotic therapy [14,15,16,17,18].
Our study was designed to answer the question of whether preoperative biliary drainage could play a role in post-operative complications after pancreaticoduodenectomy. All PBD patients (100%, n = 48) had bacteriobilia, compared to 8.3% (n = 8) in the early surgery group. The RR associated with stent was 9.00 (95% CI 55–16.69). The rate and bacterial profile of biliary infections detected in our cohort of patients are similar to the ones in literature, with infection rates in stented patients ranging between 80 and 100% [14, 15, 22]. Between different studies, intraoperative bile cultures demonstrate the growth of aerobic and anaerobic bacteria. Fungal infections, including Candida spp and Hafnia spp, should not be underestimated as they vary between 25 and 44% total rate; and in our study, they assess at 21%. The bacteria most responsible for colonization are: Klebsiella spp, Enterococcus spp, Escherichia spp, Enterobacter spp. In most cases, the cultures showed a mixed microbial flora rather than monomicrobial. All these microorganisms (Enterobacteria, Enterococci, Fungi, Lactobacilli) are generally present in the gastrointestinal flora. The fungi of the Candida species are classified as opportunistic pathogens, and they become pathogenic only in some specific conditions. Overgrowth is usually inhibited by specific and nonspecific defense systems (immunity, intestinal flora, peristalsis) which are deflected in the neoplastic or jaundiced patient leading to the virulence of Candida spp strains, which can therefore play a harmful role. Numerous studies have confirmed the hypothesis that positive biliary cultures are associated with a higher rate of infectious complications [14, 15, 20]. The microbiological etiology of these post-operative infections strongly suggests that some of them are due to biliary contamination [15].
We hypothesized that surgical antibiotic prophylaxis may play an important role in the management of infectious complications in this group of patients. Intra-operative bile culture examination showed that in 25 out of 48 cases (52%), bacteriobilia was resistant to the prophylaxis administered. The major rate of complications was experienced in the group of patients with antibiotic resistance 68% vs. 39%; p = 0.04, RR 1.73 (95% CI 1.012–3.214). These results bring under a new light the debate about the role of preoperative biliary stent in post-operative complications after PD. In fact, what has just been presented is an element of novelty in literature. We demonstrated that bacterial infection not treated by an appropriate antibiotic regimen led to an almost double overall complication rate (p = 0.04).
At the conclusion of this study, therefore, we propose that a broad-spectrum antibiotic prophylaxis with a fourth-generation cephalosporin or with the imipenem–cilastatin association should be used for an action directed towards gram + and gram − bacteria. In addition, an antifungal therapy must be associated in defedated and immunosuppressed patients to treat biliary candidiasis. We also propose that the execution of the biliary culture should be applied routinely, if it is not already an integral part of surgical practice, and that adequate antibiotic therapy should be changed on the basis of antibiogram. The overall complication rate in PDs could be equal to the one we found in patients with prophylaxis-sensitive bacteriobilia: 39%. However, it is necessary that further multicenter and randomized studies will investigate the hypothesis illustrated here, to obtain solid and incontrovertible results.
References
Sener SF, Fremgen A, Menck HR, Winchester DP (1999) Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg 189:1–7. https://doi.org/10.1016/S1072-7515(99)00075-7
Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW (2004) Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 91:586–594. https://doi.org/10.1002/bjs.4484
Engelken FJF, Bettschart V, Rahman MQ, Parks RW, Garden OJ (2003) Prognostic factors in the palliation of pancreatic cancer. Eur J Surg Oncol 29:368–373. https://doi.org/10.1053/ejso.2002.1405
Pauli-Magnus C, Meier PJ (2005) Hepatocellular transporters and cholestasis. J Clin Gastroenterol 39:S103–110
Smith RA, Dajani K, Dodd S, Whelan P, Raraty M, Sutton R et al (2008) Preoperative resolution of jaundice following biliary stenting predicts more favourable early survival in resected pancreatic ductal adenocarcinoma. Ann Surg Oncol 15:3138–3146. https://doi.org/10.1245/s10434-008-0148-z
Papadopoulos V, Filippou D, Manolis E, Mimidis K (2007) Haemostasis impairment in patients with obstructive jaundice. J Gastrointest Liver Dis 16:177–86
Sewnath ME, Karsten TM, Prins MH, Rauws EJA, Obertop H, Gouma DJ (2002) A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg 236:17–27. https://doi.org/10.1097/00000658-200207000-00005
Fang Y, Gurusamy KS, Wang Q, Davidson BR, Lin H, Xie X et al (2013) Meta-analysis of randomized clinical trials on safety and efficacy of biliary drainage before surgery for obstructive jaundice. Br J Surg 100:1589–1596. https://doi.org/10.1002/bjs.9260
Wang Q, Gurusamy KS, Lin H, Xie X, Wang C (2008) Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev CD005444. https://doi.org/10.1002/14651858.CD005444.pub2
Fang Y, Wang Q, Ks G, Lin H, Xie X, Wang C et al (2012) Preoperative biliary drainage for obstructive jaundice ( Review ). Cochrane Database Syst Rev 10–3:1. https://doi.org/10.1002/14651858.CD005444.pub3
Chen Y, Ou G, Lian G, Luo H, Huang K, Huang Y (2015) Effect of preoperative biliary drainage on complications following pancreatoduodenectomy: a meta-analysis. Medicine 94:e1199. https://doi.org/10.1097/MD.0000000000001199
Scheufele F, Schorn S, Demir IE, Sargut M, Tieftrunk E, Calavrezos L et al (2017) Preoperative biliary stenting versus operation first in jaundiced patients due to malignant lesions in the pancreatic head: a meta-analysis of current literature. Surgery (United States) 161:939–950. https://doi.org/10.1016/j.surg.2016.11.001
Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW et al (2017) Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 15:1028–1061. https://doi.org/10.6004/jnccn.2017.0131
Howard TJ, Yu J, Greene RB, George V, Wairiuko GM, Moore SA et al (2006) Influence of bactibilia after preoperative biliary stenting on postoperative infectious complications. J Gastrointest Surg 10:523–531. https://doi.org/10.1016/j.gassur.2005.08.011
Cortes A, Sauvanet A, Bert F, Janny S, Sockeel P, Kianmanesh R et al (2006) Effect of bile contamination on immediate outcomes after pancreaticoduodenectomy for tumor. J Am Coll Surg 202:93–99. https://doi.org/10.1016/j.jamcollsurg.2005.09.006
Mohammed S, Evans C, Vanburen G, Hodges SE, Silberfein E, Artinyan A et al (2014) Treatment of bacteriobilia decreases wound infection rates after pancreaticoduodenectomy. HPB 16:592–598. https://doi.org/10.1111/hpb.12170
Sudo T, Murakami Y, Uemura K, Hashimoto Y, Kondo N, Nakagawa N et al (2014) Perioperative antibiotics covering bile contamination prevent abdominal infectious complications after pancreatoduodenectomy in patients with preoperative biliary drainage. World J Surg 38:2952. https://doi.org/10.1007/s00268-014-2688-7
Scheufele F, Aichinger L, Jäger C, Demir IE, Schorn S, Sargut M et al (2017) Effect of preoperative biliary drainage on bacterial flora in bile of patients with periampullary cancer. Br J Surg 104:e182–e188. https://doi.org/10.1002/bjs.10450
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Povoski SP, Karpeh MS, Conlon KC, Blumgart LH, Brennan MF (1999) Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg 230:131–142. https://doi.org/10.1097/00000658-199908000-00001
Lenz P, Conrad B, Kucharzik T, Hilker E, Fegeler W, Ullerich H et al (2009) Prevalence, associations, and trends of biliary-tract candidiasis: a prospective observational study. Gastrointest Endosc 70:480–487. https://doi.org/10.1016/j.gie.2009.01.038
Olsson G, Frozanpor F, Lundell L, Enochsson L, Ansorge C, Del Chiaro M et al (2017) Preoperative biliary drainage by plastic or self-expandable metal stents in patients with periampullary tumors: results of a randomized clinical study. Endosc Int Open 5:E798–808. https://doi.org/10.1055/s-0043-110565
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TS, CN, BP, AG and MM. The first draft of the manuscript was written by TS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Research involving human participants and/or animals
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stecca, T., Nistri, C., Pauletti, B. et al. Bacteriobilia resistance to antibiotic prophylaxis increases morbidity after pancreaticoduodenectomy: a monocentric retrospective study of 128 patients. Updates Surg 72, 1073–1080 (2020). https://doi.org/10.1007/s13304-020-00772-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-020-00772-z