Abstract
Precipitation amounts and patterns at high latitude sites have been predicted to change as a result of global climatic changes. We addressed vegetation responses to three years of experimentally increased summer precipitation in two previously unaddressed tundra types: Betula nana-dominated shrub tundra (northeast Siberia) and a dry Sphagnum fuscum-dominated bog (northern Sweden). Positive responses to approximately doubled ambient precipitation (an increase of 200 mm year−1) were observed at the Siberian site, for B. nana (30 % larger length increments), Salix pulchra (leaf size and length increments) and Arctagrostis latifolia (leaf size and specific leaf area), but none were observed at the Swedish site. Total biomass production did not increase at either of the study sites. This study corroborates studies in other tundra vegetation types and shows that despite regional differences at the plant level, total tundra plant productivity is, at least at the short or medium term, largely irresponsive to experimentally increased summer precipitation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Northern tundra ecosystems are important in the current context of a changing climate as they contain a large part of our global terrestrial soil carbon pool (Tarnocai et al. 2009). Despite their low primary productivity, tundra ecosystems act as a carbon sink due to their low decomposition rates. Climatic changes, which are currently increasing decomposition rates of old carbon, threaten to change the tundra from a carbon sink into a source (Dorrepaal et al. 2009). In contrast, enhanced vegetation productivity in response to climatic changes may increase net storage of carbon in these systems in the near future (Qian et al. 2010). Hence, climatic changes alleviating growth-limiting factors of tundra vegetation have a potential to feed back to the global carbon cycle.
Plant productivity in northern tundra ecosystems is strongly constrained by the adverse physical environment. In addition to low temperature and low nutrient availability (Chapin et al. 1995; Shaver et al. 2001; Aerts et al. 2006; Elmendorf et al. 2012), water shortage is frequently listed as one of the growth-limiting factors for tundra vegetation (e.g. Bliss et al. 1994; Ostendorf and Reynolds 1998; Press et al. 1998a; Hodkinson et al. 1999; Kade et al. 2005; Qian et al. 2010). However, the notion that growth of tundra vegetation is water limited is largely based on observational evidence: the best-developed arctic plant communities are often found at sites where snowmelt water or seasonal streambeds keep the soil moist (Bliss et al. 1984, 1994; Kade et al. 2005). In addition, dendrochronological research suggests summer precipitation as one of the main climate drivers for shrub (Betula nana) growth in northeast-Siberian tundra (Blok et al. 2011). This is supported by modelling approaches, which reveal enhanced precipitation as the major driving factor for increasing growing season leaf area index trends in northeast-Siberian tundra as well as in northern Scandinavia from 1980 to 2000 (Piao et al. 2006).
In contrast with these correlative or modelling studies that postulate water as a limiting factor for arctic plant growth, direct measurements of plant water potential suggest that water is not a growth-limiting factor, at least for Alaskan arctic tundra vegetation (Oberbauer and Miller 1982). In addition, very few positive plant responses to experimentally increased summer precipitation in arctic ecosystems have been documented (for an overview of performed experiments see Table 1). However, this experimental evidence is based on a limited number of experiments (seven) only, which were restricted to an even more limited number of geographic areas (Table 1). Moreover, only three of these increased summer precipitation experiments at higher latitudes were performed in tundra sites, as the other studies were executed in subarctic forest ecosystems (Table 1). As such, the tundra types covered in these three studies do not reflect the full diversity of tundra ecosystems (Walker et al. 2005).
Climate change predictions include increasing precipitation at high latitudes for the coming decades (IPCC 2007; Bengtsson et al. 2011), while long-term trends of both the increased precipitation amounts and the intensity have already been observed (IPCC 2007; Callaghan et al. 2010). If low water availability indeed poses a limitation to plant productivity, this may critically affect carbon uptake of northern ecosystems. Thus, there is clearly a need for additional studies on tundra plant water limitation in previously unaddressed tundra types in order to corroborate the conclusions of previous experiments (Table 1). Two tundra types, which have not been previously addressed, are northeast-Siberian shrub tundra (‘dry heath’, van Huissteden et al. 2005) and Swedish permafrost-underlain ombrotrophic bog tundra (‘dry elevated palsa’, Rydén 1976). Together, these dry tundra types represent a large part of the tundra biome (~30 %) (Gorham 1991; Walker et al. 2005). Moreover, both the shrub tundra and the bog tundra are important actors in the global carbon cycle: shrub tundra because of its long-term C-storage in woody biomass, and bog tundra because of their efficient C-storage capacity in slowly decomposing peat. Their responsiveness to increased moisture availability in a changing climate therefore needs to be known.
In this study, we assessed how increased summer precipitation, through modulating water availability, will affect vegetation productivity and species-specific growth responses in dry heath and dry elevated palsa types. Hence, we performed increased summer precipitation experiments in both the habitats for three consecutive growing seasons and approximately doubled the ambient summer precipitation. If water indeed inhibits growth of tundra vegetation, we hypothesize vegetation productivity to increase in response to additional summer precipitation. Given the high spatial heterogeneity that is commonly observed in biomass sampling studies (Whittaker and Marks 1975) (and accordingly low statistical power), total annual vascular production might not represent the most sensitive vegetation variable. We therefore also determined more sensitive species-specific stem growth responses of dominant species. Traits allow sensitive detection of vegetation responses to environmental drivers (e.g. Wright et al. 2005; Ordonez et al. 2009). Commonly measured traits that are known to be particularly sensitive to drought stress include leaf size and specific leaf area (SLA) of dominant individual species (Cornwell and Ackerly 2009). Therefore, we hypothesized that leaf size would increase and SLA would decrease if moisture is indeed inhibiting growth in dry elevated palsa and dry heath. In addition, because stem growth of subarctic shrub species has been shown to respond strongly to a release of inhibiting factors (Bret-Harte et al. 2001), we additionally determined more sensitive species-specific stem growth responses of dominant species.
Material and Methods
Study Sites
Siberia
The first site (Fig. 1a) is a low-Arctic tundra site within the Kytalyk nature reserve in NE-Siberia, Russia (70°49′N, 147°28′E, elevation 48 m.a.s.l.), previously described by Van Huissteden et al. (2005). Mean annual precipitation is 205 mm, most of which falls during the summer months. July is the wettest month with an average precipitation of 32 mm (Tank et al. 2002). Regional climate data [Chokurdakh weather station (WMO station 21946), http://climexp.knmi.nl/, 1948–2006] show mean annual air temperatures of −13.9 °C and average July temperatures of 10.5 °C. In 2007, the growing season was longer than usual, with snowmelt occurring 3 weeks earlier than the other measured years and average temperatures were higher than normal. The following years were more typical for this region, with 2009 being the coldest of the studied 3-year period (Parmentier et al. 2012). The same holds true for wetness, where 2007 was the wettest, 2008 in between and 2009 the driest (Parmentier, pers. obs.). The experiment was set up on an elevated dry palsa, with a maximum active layer thickness of around 25 cm (measured at the end of the growing season, in each plot at three replicate points, by probing a 0.5-cm diameter graduated stainless steel rod into the soil to refusal). The subsoil is silt clay overlain by 10–15 cm of highly organic soil, carpeted with an ~4- to 5-cm thick moss layer [consisting of 35–45 % Aulacomnium turgidum (Wahlenb.) Schwägr.]. The vascular plant community consists of the deciduous shrubs B. nana subsp exilis (93 % of total aboveground biomass) and Salix pulchra (4 %), the graminoid Arctagrostis latifolia (2 %), and the forbs Saxifraga spp. (<1 %) and Pyrola rotundifolia (<1 %) and has been classified as dry heath (van Huissteden et al. 2005).
Sweden
The second study site (Fig. 1b) is at the Stordalen nature reserve in northern Sweden (68°21′N, 19°03′E, 351 m.a.s.l.) which has been described in full detail by Sonesson (1980). Annual precipitation in the area is around 300 mm year−1 (1913–2006: Johansson et al. 2009). Summer precipitation at the site in the years of the experiment equalled 287 mm in 2007, 228 mm in 2008 and 190 mm in 2009; with a mean of 235 mm ± 49 SD (Olefeldt and Roulet 2012). The mean summer temperature at the site is 7 °C, the mean winter temperature is −6 °C. The experiment was set up on an elevated, dry (Rydén 1976; Sonesson 1980), ombrotrophic Sphagnum fuscum (Schimp.)-dominated palsa, with an active layer thickness of around 50 cm. The vascular plant community consists of the evergreen dwarf shrubs Empetrum hermaphroditum L. (56 % of total aboveground biomass) and Andromeda polifolia L. (9 %), the deciduous dwarf shrubs B. nana L. subsp. nana (6 %) and Vaccinium uliginosum (7 %), the forb Rubus chamaemorus L. (15 %), and the graminoid Eriophorum vaginatum L. (8 %) and has been classified as dry-growing elevated palsa vegetation by Madsen and Widell (1974).
Treatment and Experimental Design
At both sites, 16 visually similar plots of 1 m2 were laid out at the beginning of the growing season in 2007. Following a completely randomized design, the plots were assigned to one of the precipitation treatments (ambient or increased precipitation, n = 8). The plots with increased precipitation were evenly watered 16 times during each growing season with 12.5 l of water per plot per time. This resulted in a total of 200 mm extra precipitation per growing season, i.e. an approximate doubling of the ambient summer precipitation. Both the experiments lasted three growing seasons.
At the Siberian site, water for additional precipitation was taken from nearby clear-water pools and the Berelekh (Yelon) River, a tributary of the Indigirka River. Total inorganic nitrogen was 0.4 ± 0.2 mg l−1 (data based on water samples used for the treatment, taken in 2007 and analysed using a SA-40 auto-analyser, Skalar, Breda, The Netherlands).
At the Swedish site, water for additional precipitation was taken from the stream adjacent to the experimental site and had a conductivity of 50.0 ± 11.5 μS cm−1, pH 6.7 ± 0.2, dissolved organic carbon content of (DOC) 10.6 ± 2.6 mg l−1, and total nitrogen of (TN) 0.3 ± 0.2 mg l−1 (data based on measurements from two separate spots in the stream from the end of April until the end of September 2008) (Olefeldt and Roulet 2012). Given these low nutrient concentrations in neither site, nutrient addition does not seem to have been a confounding factor in our set-up.
Vegetation Measurements
Total Annual Productivity
We chose to present annual vascular production instead of total aboveground biomass, as the latter is bound to be largely unresponsive to perturbations due to dilution of the current season’s growth by material produced in previous years (cf. Parsons et al. 1994). After three growing seasons, all aboveground biomass, defined as all plant material emerging above the moss layer, was harvested. The total annual vascular production was subsequently calculated as the sum of: (1) the aboveground green biomass of all graminoids and forbs (A. latifolia, Saxifraga spp., P. rotundifolia for the Siberian site and E. vaginatum and R. chamaemorus for the Swedish site), (2) the leaf biomass and length increment biomass of all deciduous shrubs (B. nana subsp. exilis and S. pulchra for the Siberian site and B. nana subsp. nana and V. uliginosum for the Swedish site) and (3) all new growth of the evergreen species, which were only present at the Swedish site. Total biomass of the evergreen’s new growth was calculated by multiplying the total aboveground biomass with the ratio of current-year’s growth biomass to total shoot biomass. This ratio was determined on samples of 20 randomly picked shoots for E. hermaphroditum, whereas the ratio for A. polifolia was estimated to be 1:3 (Aerts, pers. comm., data from a site 200 m from the present study site).
Because the most productive species at the Swedish site is the peat moss S. fuscum, productivity of this bryophyte was measured separately. The length growth of S. fuscum was measured in all plots, using a modification (Dorrepaal et al. 2004) of the cranked wire technique (Clymo 1970), with four wires per plot. Although length growth was measured in last 2 years of the experiments, bulk density of the moss was determined (destructively, on the top 1 cm of the moss carpet), in the last year of the experiment (on two samples per plot, dry weight per volume). Therefore, annual production was calculated only for the last year of the experiment (by multiplying bulk density with length growth). However, to check for temporal consistency, length growth was analysed with repeated measures (RM)-ANOVA (within-subject factor year and between-subject factor treatment) for the last 2 years of the experiment.
Species-Specific Growth Responses
In addition to the integrative measure of total annual production, several more sensitive key variables of species-specific plant performance were measured (leaf size, SLA, length increments and biomass per length increment).
Two traits known to be particularly responsive to drought, leaf size and SLA, were measured in the last year of the experiment on randomly picked leaves (number of leaves per plot) of the most abundant broad-leaved species [B. nana subsp. exilis (twenty), S. pulchra (five) and A. latifolia (five) at the Siberian site, and B. nana subsp. nana (ten), V. uliginosum (ten) and A. polifolia (ten) at the Swedish site]. Leaf size and SLA were measured and calculated according to the standard protocol described in Cornelissen et al. (2003).
Current-year apical stem length increments of the dominant shrub species were determined as measures of growth responses. At the Siberian site, all stem length increments of five randomly selected individual shrubs of B. nana subsp. exilis per plot were measured of the last two years’ growth. The length of all shoots (both the short and the long shoots) of these individuals was determined in order to obtain a reliable measurement. Apical stem length increments of all S. pulchra plants per plot (min. three, max. nine) were measured during the last year of the experiment. Current-year length increments were identified using colour differences of the stem and scars of the terminal bud. At the Swedish site, length increments of ten randomly selected E. hermaphroditum shoots per plot were measured during the last 2 years of the experiment. Current-year growth was identified using colour differences of the stem, bud scars and changes in leaf length between the growth segments of the different years (Keuper et al. 2011). B. nana subsp. nana length increments at the Swedish site were measured as the distance from a marked point on the stem until the tip of the shoot, both at the beginning (early June) and at the end (early September) of the growing season. Only the first 2 years were measured because the destructive end harvest in the last year of the experiment interfered with these measurements.
Statistics
All data were tested for normality and homogeneity of residual variances by visual inspection of residual and probability plots. Log transformation improved the homogeneity of residual variances for the total vascular production data and for the B. nana subsp. nana length increment data. For all other variables residual variance was approximately normal and homogeneous.
Total vascular production in the last year of the experiment at both the sites was analysed simultaneously with a two-factor ANOVA (with treatment and site as independent factors) in order to reduce the chance of Type I error.
Leaf size and SLA were analysed with separate MANOVAs for the two sites, with treatment as independent factor and the leaf traits (leaf size and SLA) as dependent variables with the species identities acting as multivariates.
Differences in length increments and biomass per new stem length of the dominant shrubs were analysed using separate RM-ANOVAs for species and site when data for 2 years were available (B. nana, E. hermaphroditum), with year as a within-subject factor and treatment as a between-subject factor. A one-way ANOVA was used when only 1 year of measurements was available (stem length increments of S. pulchra, number of B. nana leaves on the new stem, weight per unit new stem length, and production of the dominant peat moss S. fuscum for the Swedish site).
Before statistical analysis, values for each variable were averaged per plot, and all analyses were performed with SPSS 15.0 for Windows. Given the high intrinsic variability of many of the vegetation response measures, the statistical power is generally low and therefore we also discuss results with P < 0.1.
Results
Total Aboveground Biomass Production
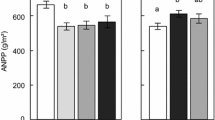
Total annual aboveground biomass production of the vascular plant communities at the Siberian and the Swedish site was not affected by precipitation addition (F = 2.0; p > 0.1). Although vascular production was higher at the Siberian site (Fig. 2; F = 85.7; p < 0.001), there was no interaction (F = 0.35; p > 0.1) between site and treatment (Fig. 2).
Species-Specific Responses to the Precipitation Treatment
Leaf Traits
At the Siberian study site, leaf size of B. nana subsp. exilis was not affected by the treatment (F = 0.068; p > 0.1), but leaf size of both S. pulchra (F = 5.4; p < 0.05) and A. latifolia (F = 11.1; p < 0.01) were 25 and 113 % larger in the plots with additional precipitation, respectively (Fig. 3a; Table 2). In contrast to leaf size, SLA (cm2 g−1) of B. nana subsp. exilis was only slightly larger in the irrigated plots (F = 3.75; p < 0.1). SLA of A. latifolia was even ~25 % larger in the irrigated plots (F = 12.9; p < 0.001). In contrast, SLA of S. pulchra was not affected by treatment (F = 3.04; p > 0.1) (Fig. 3c). At the Swedish site, neither leaf size nor SLA of any of the examined species (B. nana subsp. nana, V. uliginosum, A. polifolia) were affected by treatment (p > 0.05 for all species) (Fig. 3b, d).
Mean (±1 SE) leaf size and SLA at the Siberian (a, c) and the Swedish (b, d) study site in the third year of the experiment in water addition (black bars) compared to control (grey bars) plots (n = 8). Significant differences between water addition and controls per species are indicated with + P < 0.1, *p < 0.05 and **p < 0.01 (see main text for details on statistical analysis)
Stem Traits
At the Siberian study site, length increments of the dominant shrub B. nana subsp. exilis were about 30 % larger in the irrigated plots both in the penultimate and in the last year of the experiment (F = 17.1; p < 0.01). There was a marginally significant difference in growth among the years (F = 3.6; p < 0.1), while there was no interaction between treatment and year (p > 0.1) (Fig. 4a). These elongated B. nana subsp. exilis stems produced about one leaf per cm stem length less (−25 %, last experimental year data only) (F = 13.7; p < 0.01). The treatment did not affect biomass per unit length of the new stem growth (F = 2.4; p > 0.1). Length increment of the subdominant shrub S. pulchra at the Siberian site was also 32 % larger in the last year of the experiment (18.1 cm ± 3.9 SD for the irrigated plots vs. 13.7 cm ± 3.4 SD for the control plots, F = 4.8; p < 0.05), but the number of leaves per new stem length remained unchanged (3 per cm stem, F = 2.4; p > 0.1) and biomass of the new stem growth was not affected either (F = 0.73; p > 0.1).
Mean (±1 SE) apical annual growth of the dominant dwarf shrubs in both the study sites in response to summer water addition (black bars) or control treatment (grey bars) in the last 2 years of the experiment: a Betula nana subsp. exilis at the Siberian site and b Empetrum hermaphroditum at the Swedish site (n = 8). Significant differences between water addition and controls per species are indicated with + P < 0.1, *P < 0.05 and **P < 0.01 (see main text for details on statistical analysis)
At the Swedish study site, the length increments of the dominant shrub E. hermaphroditum were not affected by additional precipitation (F = 0.059; p > 0.1) and there was no effect of year, nor an interaction between year and treatment (p > 0.1) (Fig. 4b). Biomass per unit length of the new growth was neither affected by treatment (1.49 ± 0.24 SD mg mm−1 year−1 for control and 1.36 ± 0.26 SD mg mm−1 year−1 for plots with additional precipitation; F = 0.89; p > 0.1) and no effect of year nor an interaction between year and treatment was observed (p > 0.1). Similarly with the other Swedish results but in contrast to the Siberian results for this species, B. subsp. nana length increments were not affected by additional precipitation at the Swedish site (5.9 ± 5.1 SD mm−1 year−1 for control and 3.6 ± 2.7 SD mm−1 year−1 for plots with additional precipitation; F = 0.93; p > 0.1), nor was there an effect of year (F = 0.66; p > 0.1) or an interaction between year and treatment (F = 0.77; p > 0.1). Length growth of the dominant bryophyte S. fuscum was not different from the control in the plots with additional precipitation during the last 2 years of the experiment (4.10 ± 2.2 SD mm year−1 in the control plots and 4.73 ± 1.9 SD mm year−1 in the treatment plots: F = 0.57; p > 0.1). Sphagnum length growth was significantly different in the two measured years (F = 20.8; p = 0.00), but there was no significant interaction between treatment and year (F = 0.11; p > 0.1). Biomass production of the peat moss was not affected by the precipitation treatment (288 ± 170 SD g m−2 in the control plots and 313 ± 141 SD g m−2 in the plots with additional precipitation: F = 0.10; p > 0.1).
Discussion
Water Limited Plant Growth in Tundra? No Response in Total Annual Productivity
Three years of experimentally increased summer precipitation did not affect total aboveground vascular biomass production at either of the studied tundra sites. Total annual production is the integrative measure of all species-specific growth responses. Hence, this finding shows that overall, primary production of Siberian B. nana heath and Swedish ombrotrophic bog tundra vegetation is at the short to medium term not limited by water availability, even though some specific-species growth responses to the treatment were observed. Our results extend the established pattern of negligible tundra plant productivity responses to experimentally increased summer precipitation (reviewed by Dormann and Woodin 2002) with two previously unaddressed tundra types.
Although the investigated systems generally receive little natural precipitation (between 150 and 300 mm on average, Remer 2009), there are several potential explanations for their resistance in plant productivity to changes in summer precipitation. First, plant productivity may be more limited by other factors such as nutrient availability and/or temperature to such extent that positive responses to alleviation of water limitation are hampered or masked. This explanation is supported by the study of Parsons et al. (1995) (Table 1), who only found a significant effect of watering on graminoid biomass if watering was applied in combinations with either increased temperature or fertilizer. Second, although summer precipitation in tundra may be low, total evapotranspiration is also low, ensuring that in general tundra soils remain relatively wet throughout the summer (polar deserts excluded). Moreover, soils are driest in late summer (Rydén 1976), while most growth takes place in early summer (Sonesson 1980; Karlsson and Callaghan 1996). At the beginning of the growing season, moisture may still be available from snowmelt, while drainage is limited due to the shallowness of the active layer (McGraw 1985). Finally, total productivity responses to water addition might be hard to detect due to spatial heterogeneity in initial biomass and a relatively short duration of the experiment (three years).
Species-Specific Responses: Signs of Drought Stress-Release and Growth Responses
While the overall productivity was unaffected, species-specific growth responses to increased summer precipitation differed remarkably between species and sites (Table 2). The increased leaf size in response to irrigation observed for the deciduous shrub S. pulchra and the grass A. latifolia at the Siberian study site suggests that growth of these species is water limited, in line with our hypotheses. SLA of S. pulchra remained constant, but for A. latifolia the increased leaf size co-occurred with thinner leaves, again in line with our hypotheses. Leaves of the dominant shrub B. nana subsp. exilis (93 % of aboveground biomass) also became slightly thinner (p < 0.1) (Fig. 3b) while its leaf size remained unchanged in response to irrigation (Fig. 3a). While nutrient deficiency and drought stress are known to have very similar effects on vegetative plant characteristics (Small 1973), the very low nutrient concentrations in our irrigation water decrease possible interferences by increased nutrient availability as confounding factor. Together, the species-specific vegetation responses to the water addition suggest that growth of the vegetation is water limited at the Siberian dry heath. Alleviation of this water limitation allowed length increments of B. nana subsp. exilis to increase with around 30 %, while length increments of S. pulchra increased as well (Fig. 4a). The fact that these growth responses were not sufficient to increase the overall productivity at the Siberian site might be explained by the relatively small contribution of B. nana subsp. exilis length increments (4 %) to total annual productivity, compared to the contribution of total B. nana subsp. exilis leaf biomass (84 %, results not shown), which did not differ among treatments (results not shown). However, note should be taken that we did not take growth (thickening of the stems) into account. Such secondary growth can amount up to an annual production of ~15 % of total standing biomass (Bret-Harte et al. 2002) and has previously been reported responsive to natural summer precipitation patterns (Blok et al. 2011). Given the relatively large contribution of the ‘old’ woody biomass to the total standing biomass at the Siberian site (84 %, results not shown) secondary growth might have contributed considerably to annual total productivity in the plots with additional precipitation.
In contrast, at the Swedish site, the absence of any responses in leaf size and SLA and the absence of increased length increments of all measured species, including S. fuscum, suggests that in this habitat, drought stress was not a condition that limited the plants during the period of leaf and stem development (Figs. 3b, d, 4b). Empetrum (56 % of aboveground biomass at the Swedish site) is assumed to be tolerant to variations in soil water content (Bell and Tallis 1973) and has previously been found unresponsive to experimentally increased summer precipitation (Shevtsova et al. 1997) (Table 1). Although S. fuscum is sensitive to water availability and has previously been shown to respond to small increases in summer precipitation (up to 20 mm year−1) (Sonesson et al. 2002), larger increases, such as provided in this study, may also negatively affect productivity through increased leaching of nutrients (Sonesson et al. 2002). This would explain the absence of net effects of increased summer precipitation.
The difference in B. nana responsiveness among the sites may be due to the occurrence of different ‘ecotypes’ of B. nana at the two sites: different responses to experimental manipulation in different geographical areas (Alaska, Northern Sweden) have previously been observed for this species (Van Wijk et al. 2004). Previous observations of plant responses to water drainage patterns in Alaskan tundra (Matthes-Sears et al. 1988) showed that both B. nana as well as S. pulchra had higher aboveground biomass in water tracks. Growth of B. nana at the Siberian site might also have been more drought stressed ambiently, and might have contributed to drying of the soil because it comprised 93 % of the total biomass and thus had a relatively large transpiring leaf area. Finally, at the Siberian site, B. nana subsp. exilis supposedly had superficial roots only due to the shallow active layer thickness (max. 25 cm compared to 50 cm at the Swedish site) and could thus only exploit a much smaller reservoir of soil water.
Long-Term Implications for Species Composition
Despite species-specific responses to the treatment at the Siberian study site, no changes in species composition indices were found on either site (results not shown). Hence, this study shows that even doubling of yearly summer precipitation does not induce a shift in species composition during the first three years. For the Swedish site, impacts on vegetation composition through species interactions are not expected due to the low total species cover in the plots and the complete absence of responses. However, at the Siberian site, the increased leaf size of S. pulchra and A. latifolia suggests that these species are drought stressed and may, if the tundra gets wetter, on the long-term benefit from wetter conditions. Enhanced responses in tundra ecosystems in terms of species composition and total productivity to additional precipitation seem even more likely in a future climate when changes in temperature and nutrient availability are predicted to co-occur with increased summer precipitation (Parsons et al. 1994) and when permafrost degradation drastically affects local hydrology, the first signs of which are already apparent (Molau 2010).
Conclusions
The plant level responses for the Swedish site indicate that increased summer precipitation alone will not have major effects on ombrotrophic peatland vegetation productivity. For the Siberian B. nana dominated dry heath site on the other hand, the fact that several individual plant measurements show a positive response to the precipitation treatment, suggests that in the long-term biomass production may increase in response to increased summer precipitation. However, over the course of this three-year experiment, primary production did not increase in response to the treatment at either of the study sites. Hence, despite regional differences at the plant level, our study shows that total tundra plant productivity shows at the short term no response to experimentally increased summer precipitation, corroborating previous studies on tundra plant productivity responses to summer precipitation. This contrasts with the results of nutrient addition experiments and suggests that water in most tundra ecosystems not be the primary growth-limiting factor but a co-limiting growth factor. This study extends the available knowledge with two previously unaddressed tundra types.
References
Aerts, R., J.H.C. Cornelissen, and E. Dorrepaal. 2006. Plant performance in a warmer world: General responses of plants from cold, northern biomes and the importance of winter and spring events. Plant Ecology 182: 65–77.
Bell, J.N.B., and J.H. Tallis. 1973. Biological flora of British Isles—Empetrum Nigrum L. Journal of Ecology 61: 289–305.
Bengtsson, L., K.I. Hodges, S. Koumoutsaris, M. Zahn, and N. Keenlyside. 2011. The changing atmospheric water cycle in Polar Regions in a warmer climate. Tellus Series a-Dynamic Meteorology and Oceanography 63: 907–920. doi:10.1111/j.1600-0870.2011.00534.x.
Bliss, L.C., J. Svoboda, and D.I. Bliss. 1984. Polar deserts, their plant cover and plant-production in the Canadian high Arctic. Holarctic Ecology 7: 305–324.
Bliss, L.C., G.H.R. Henry, J. Svoboda, and D.I. Bliss. 1994. Patterns of plant-distribution within 2 polar desert landscapes. Arctic and Alpine Research 26: 46–55.
Blok, D., U. Sass-Klaassen, G. Schaepman-Strub, M.M.P.D. Heijmans, P. Sauren, and F. Berendse. 2011. What are the main climate drivers for shrub growth in Northeastern Siberian tundra? Biogeosciences 8: 1169–1179. doi:10.5194/bg-8-1169-2011.
Bret-Harte, M.S., G.R. Shaver, and F.S. Chapin. 2002. Primary and secondary stem growth in arctic shrubs: Implications for community response to environmental change. Journal of Ecology 90: 251–267.
Bret-Harte, M.S., G.R. Shaver, J.P. Zoerner, J.F. Johnstone, J.L. Wagner, A.S. Chavez, R.F. Gunkelman, S.C. Lippert, & J.A. Laundre. 2001. Developmental plasticity allows Betula nana to dominate tundra subjected to an altered environment. Ecology 82: 18–32.
Callaghan, T.V., F. Bergholm, T.R. Christensen, C. Jonasson, U. Kokfelt, and M. Johansson. 2010. A new climate era in the sub-Arctic: Accelerating climate changes and multiple impacts. Geophysical Research Letters 37: 6. doi:10.1029/2009gl042064.
Chapin III, S.F., G.R. Shaver, A.E. Giblin, K.J. Nadelhoffer, and J.A. Laundre. 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology 76: 694–711.
Clymo, R.S. 1970. Growth of Sphagnum—methods of measurement. Journal of Ecology 58: 13–17.
Cornelissen, J.H.C., S. Lavorel, E. Garnier, S. Diaz, N. Buchmann, D.E. Gurvich, P.B. Reich, H. ter Steege, et al. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51(4): 335–380. doi:10.1071/bt02124.
Cornwell, W.K., and D.D. Ackerly. 2009. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecological Monographs 79: 109–126. doi:10.1890/07-1134.1.
Dormann, C.F., and S.J. Woodin. 2002. Climate change in the Arctic: Using plant functional types in a meta-analysis of field experiments. Functional Ecology 16: 4–17.
Dorrepaal, E., R. Aerts, J.H.C. Cornelissen, T.V. Callaghan, and R.S.P. van Logtestijn. 2004. Summer warming and increased winter snow cover affect Sphagnum fuscum growth, structure and production in a sub-arctic bog. Global Change Biology 10: 93–104.
Dorrepaal, E., S. Toet, R.S.P. van Logtestijn, E. Swart, M.J. van de Weg, T.V. Callaghan, and R. Aerts. 2009. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460: U616–U679. doi:10.1038/nature08216.
Elmendorf, S.C., G.H.R. Henry, R.D. Hollister, R.G. Björk, A.D. Bjorkman, T.V. Callaghan, L.S. Collier, E.J. Cooper, et al. 2012. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecology Letters 15: 164–175. doi:10.1111/j.1461-0248.2011.01716.x.
Gorham, E. 1991. Northern peatlands—role in the carbon-cycle and probable responses to climatic warming. Ecological Applications 1(2): 182–195.
Henry, G.H.R., B. Freedman, and J. Svoboda. 1986. Effects of fertilization on 3 tundra plant-communities of a polar desert oasis. Canadian Journal of Botany-Revue Canadienne De Botanique 64: 2502–2507.
Hodkinson, I.D., N.R. Webb, J.S. Bale, and W. Block. 1999. Hydrology, water availability and tundra ecosystem function in a changing climate: The need for a closer integration of ideas? Global Change Biology 5: 359–369.
IPCC. 2007. Climate Change 2007—The Physical Science Basis. In Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Johansson, M., T.V. Callaghan, H.J. Akerman, and M. Jackowicz-Korczynsky, T.R. Christensen. 2009. Rapid response of active layer thickness and vegetation in sub-arctic Sweden to experimentally increased snow cover. Changing lowland permafrost in northern Sweden: multiple drivers of past and future trends, PhD thesis, Lund.
Kade, A., D.A. Walker, and M.K. Raynolds. 2005. Plant communities and soils in cryoturbated tundra along a bioclimate gradient in the Low Arctic, Alaska. Phytocoenologia 35: 761–820.
Karlsson, P.S. 1985. Effects of water and mineral nutrient supply on a deciduous and an evergreen dwarf shrub-Vaccinium uliginosum L. and V. vistis-idaea L. Holarctic Ecology 8: 1–8.
Karlsson, P.S., and T.V. Callaghan. 1996. Plant ecology in the subarctic Swedish Lapland. Ecological Bulletin 45: 220–227.
Keuper, F., E. Dorrepaal, P.M. Van Bodegom, R. Aerts, R.S.P. Van Logtestijn, T.V. Callaghan, and J.H.C. Cornelissen. 2011. A Race for Space? How Sphagnum fuscum stabilizes vegetation composition during long-term climate manipulations. Global Change Biology 17: 2162–2171.
Madsen, I.-L., and S. Widell. 1974. A vegetation map of the Stordalen site. In Progress Report 1973. IBP Swedish Tundra Biome Project Tech. Rep. 16:3–15, ed. J.G.K. Flower-Ellis. Lund University.
Matthes-Sears, U., W.C. Matthes-Sears, S.J. Hastings, and W.C. Oechel. 1988. The effects of topography and nutrient status on the biomass, vegetative characteristics, and gas exchange of two deciduous shrubs on an Arctic tundra slope. Arctic and Alpine Research 20: 342–351.
McGraw, J.B. 1985. Experimental ecology of Dryas octopetala ecotypes. 3. Environmental factors and plant-growth. Arctic and Alpine Research 17: 229–239. doi:10.2307/1551013.
Molau, Ulf. 2010. Long-term impacts of observed and induced climate change on tussock tundra near its southern limit in northern Sweden. Plant Ecology & Diversity 3: 29–34. doi:10.1080/17550874.2010.487548.
Oberbauer, S., and P.C. Miller. 1982. Growth of Alaskan tundra plants in relation to water potential. Holarctic Ecology 5: 194–199.
Olefeldt, D., and N.T. Roulet. 2012. Effects of permafrost and hydrology on the composition and transport of dissolved organic carbon in a subarctic peatland complex. Journal of Geophysical Research-Biogeosciences 117: 15. doi:10.1029/2011jg001819.
Ordonez, J.C., P.M. van Bodegom, J.-P.M. Witte, I.J. Wright, P.B. Reich, and R. Aerts. 2009. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecology and Biogeography 18: 137–149. doi:10.1111/j.1466-8238.2008.00441.x.
Ostendorf, B., and J.F. Reynolds. 1998. A model of arctic tundra vegetation derived from topographic gradients. Landscape Ecology 13: 187–201.
Parmentier, F.J.W., M.K. van der Molen, J. van Huissteden, S.A. Karsanaev, A.V. Kononov, D.A. Suzdalov, T.C. Maximov, and A.J. Dolman. 2012. Longer growing seasons do not increase net carbon uptake in the northeastern Siberian tundra. Journal of Geophysical Research-Biogeosciences 116. doi:10.1029/2011jg001653.
Parsons, A.N., J.M. Welker, P.A. Wookey, M.C. Press, T.V. Callaghan, and J.A. Lee. 1994. Growth-responses of four sub-arctic dwarf shrubs to simulated environmental-change. Journal of Ecology 82: 307–318.
Parsons, A.N., M.C. Press, P.A. Wookey, J.M. Welker, C.H. Robinson, T.V. Callaghan, and J.A. Lee. 1995. Growth-responses of Calamagrostis lapponica to simulated environmental-change in the sub-arctic. Oikos 72: 61–66.
Phoenix, G.K., D. Gwynn-Jones, T.V. Callaghan, D. Sleep, and J.A. Lee. 2001. Effects of global change on a sub-Arctic heath: Effects of enhanced UV-B radiation and increased summer precipitation. Journal of Ecology 89: 256–267. doi:10.1046/j.1365-2745.2001.00531.x.
Piao, S., P. Friedlingstein, P. Ciais, L. Zhou, and A. Chen. 2006. Effect of climate and CO(2) changes on the greening of the Northern Hemisphere over the past two decades. Geophysical Research Letters 33. doi:10.1029/2006gl028205.
Potter, J.A., M.C. Press, T.V. Callaghan, and J.A. Lee. 1995. Growth responses of Polytrichum commune and Hylocomium splendens to simulated environmental change in the sub-arctic. New Phytologist 131: 533–541. doi:10.1111/j.1469-8137.1995.tb03089.x.
Press, M.C., T.V. Callaghan, and J.A. Lee. 1998a. How will European arctic ecosystems respond to projected global environmental change? AMBIO 27: 306–311.
Press, M.C., J.A. Potter, M.J.W. Burke, T.V. Callaghan, and J.A. Lee. 1998b. Responses of a subarctic dwarf shrub heath community to simulated environmental change. Journal of Ecology 86: 315–327.
Qian, H., R. Joseph, and N. Zeng. 2010. Enhanced terrestrial carbon uptake in the northern high latitudes in the 21st century from the coupled carbon cycle climate model intercomparison project model projections. Global Change Biology 16: 641–656. doi:10.1111/j.1365-2486.2009.01989.x.
Remer. 2009. Temperature and Precipitation Graphs. NASA Earth Observatory. http://earthobservatory.nasa.gov/Experiments/Biome/biotundra.php. Accessed 18 Feb 2012.
Robinson, C.H., P.A. Wookey, J.A. Lee, T.V. Callaghan, and M.C. Press. 1998. Plant community responses to simulated environmental change at a high arctic polar semi-desert. Ecology 79: 856–866.
Rydén, B.E. 1976. Water availability to some arctic ecosystems. Nordic Hydrology 7: 73–80.
Shaver, G.R., S.M. Bret-Harte, M.H. Jones, J. Johnstone, L. Gough, J. Laundre, and F.S. Chapin. 2001. Species composition interacts with fertilizer to control long-term change in tundra productivity. Ecology 82: 3163–3181.
Shevtsova, A., E. Haukioja, and A. Ojala. 1997. Growth response of subarctic dwarf shrubs, Empetrum nigrum and Vaccinium vitis-idaea, to manipulated environmental conditions and species removal. Oikos 78: 440–458. doi:10.2307/3545606.
Small, E. 1973. Xeromorphy in plants as a possible basis for migration between arid and nutritionally-deficient environments. Botaniska Notiser 126: 534–539.
Sonesson, M. 1980. Ecology of a subarctic mire. Ecological Bulletins. Stockholm: Ecological Bulletins 30.
Sonesson, M., B.A. Carlsson, T.V. Callaghan, S. Halling, L.O. Bjorn, M. Bertgren, and U. Johanson. 2002. Growth of two peat-forming mosses in subarctic mires: Species interactions and effects of simulated climate change. Oikos 99: 151–160.
Tank, A., J.B. Wijngaard, G.P. Konnen, R. Bohm, G. Demaree, A. Gocheva, M. Mileta, S. Pashiardis, et al. 2002. Daily dataset of 20th-century surface air temperature and precipitation series for the European climate assessment. International Journal of Climatology 22: 1441–1453. doi:10.1002/joc.773.
Tarnocai, C., J.G. Canadell, E.A.G. Schuur, P. Kuhry, G. Mazhitova, and S. Zimov. 2009. Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochemical Cycles 23: 11. doi:10.1029/2008gb003327.
van Huissteden, J., T.C. Maximov, and A.J. Dolman. 2005. High methane flux from an arctic floodplain (Indigirka lowlands, eastern Siberia). Journal of Geophysical Research-Biogeosciences 110. doi:10.1029/2005JG000010.
Van Wijk, M.T., K.E. Clemmensen, G.R. Shaver, M. Williams, T.V. Callaghan, F.S. Chapin, J.H.C. Cornelissen, L. Gough, et al. 2004. Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: Generalizations and differences in ecosystem and plant type responses to global change. Global Change Biology 10: 105–123.
Walker, D.A., M.K. Raynolds, F.J.A. Daniels, E. Einarsson, A. Elvebakk, W.A. Gould, A.E. Katenin, S.S. Kholod, et al. 2005. The circumpolar arctic vegetation map. Journal of Vegetation Science 16: 267–282.
Welker, J.M., P.A. Wookey, A.N. Parsons, M.C. Press, T.V. Callaghan, and J.A. Lee. 1993. Leaf carbon-isotope discrimination and vegetative responses of Dryas Octopetala to temperature and water manipulations in a high Arctic polar semidesert, Svalbard. Oecologia 95: 463–469.
Whittaker, R.H., and P.L. Marks. 1975. Methods of assessing terrestrial productivity. In Primary productivity of the biosphere, ed. H. Lieth, and R.H. Whittaker, 55–118. New York: Springer.
Wookey, P.A., A.N. Parsons, J.M. Welker, J.A. Potter, T.V. Callaghan, J.A. Lee, and M.C. Press. 1993. Comparative responses of phenology and reproductive development to simulated environmental-change in sub-arctic and high arctic plants. Oikos 67: 490–502.
Wookey, P.A., C.H. Robinson, A.N. Parsons, J.M. Welker, M.C. Press, T.V. Callaghan, and J.A. Lee. 1995. Environmental constraints on the growth, photosynthesis and reproductive development of Dryas Octopetala at a high Arctic polar semidesert, Svalbard. Oecologia 102: 478–489.
Wright, I.J., P.B. Reich, J.H.C. Cornelissen, D.S. Falster, P.K. Groom, K. Hikosaka, W. Lee, C.H. Lusk, et al. 2005. Modulation of leaf economic traits and trait relationships by climate. Global Ecology and Biogeography 14: 411–421. doi:10.1111/j.1466-822x.2005.00172.x.
Acknowledgments
We are grateful to the staff of the BioGeoChemical Cycles of Permafrost Ecosystems Lab in Yakutsk for logistic support and to the staff of the Kytalyk State Resource Reservation for their permission and hospitality to conduct research in the Kytalyk reserve. Special thanks to Sergei Karsanaev, Roman Sofronov, Elena Ivanova, Lena Paryadina, Stanislav Ksenofontov and Trofim Maximov. We also thank the staff of the Abisko Research station for technical assistance and Milo Keuper for practical support. The helpful comments of two anonymous reviewers greatly improved the manuscript. Financial support was offered to FK by the Darwin Centre for Biogeosciences (Grant 142.161.042) and ANS Scholarship 2008, and to RA by the Dutch Polar Program (ALW-NPP Grant 851.30.023) and the EU-ATANS (Grant FP6 506004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keuper, F., Parmentier, FJ.W., Blok, D. et al. Tundra in the Rain: Differential Vegetation Responses to Three Years of Experimentally Doubled Summer Precipitation in Siberian Shrub and Swedish Bog Tundra. AMBIO 41 (Suppl 3), 269–280 (2012). https://doi.org/10.1007/s13280-012-0305-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13280-012-0305-2