Abstract
We review known and hypothesized effects of nitrogen (N) deposition owing to human activities on the chemistry, organisms, and ecosystem processes of remote oligotrophic freshwaters. Acidification is the best-known effect of N deposition on water chemistry, but additional effects include increased nutrient availability and alteration of the balance between N and other nutrients. Our synthesis of the literature, framed in a comprehensive model for the effects of N deposition on natural ecosystems, shows that all these effects can reduce biological diversity and alter ecosystem processes in remote freshwaters. N deposition is projected to grow worldwide in the near future and will interact with other global changes. Present effects on these fragile ecosystems may be only early signs of more radical impacts ahead.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since pre-industrial times, human activities have more than doubled the global input of N into the environment (Vitousek et al. 1997; Galloway et al. 2004). In freshwaters, anthropogenic (human-induced) inputs have become the main driver of variation in N concentration among rivers worldwide (Meybeck 1982; Kroeze and Seitzinger 1998) and are still increasing (Seitzinger et al. 2010). Because N is a nutrient, and N compounds can acidify streams and lakes, alterations of N availability have far-reaching effects on freshwater ecosystems (Vitousek et al. 1997; Schlesinger 2009).
The main anthropogenic sources of N are runoff from urban and agricultural land due to fertilizers, N-fixing crops, and sewage, and atmospheric deposition due to combustion of fossil fuels and animal husbandry (Wellburn 1994; Vitousek et al. 1997). N inputs from these two sources span different magnitudes and affect different ecosystems. In populated watersheds, urban and agricultural runoff can add 10–25 mg L−1 of dissolved inorganic N (DIN) to freshwaters, whose natural concentrations are typically ≤1 mg L−1 (Meybeck 1982; Heathwaite et al. 1996). In comparison, deposition usually contributes <1 mg L−1 of DIN to freshwaters (Lepori et al. 2003a; Bergström and Jansson 2006). Such contribution is easily overshadowed by other N inputs in populated watersheds. However, atmospheric N can travel hundreds of kilometers (Fay et al. 1987) and also affect regions barely or not affected by direct human impact (hereafter, “remote”). Freshwaters in these regions, which notably include high-altitude and high-latitude areas (Fig. 1; Table 1), tend to be naturally oligotrophic (nutrient-poor; usually <0.1 mg L−1 of DIN) and lack acid-neutralizing capacity (ANC) (Table 1). Here, therefore, even small inputs of N from the atmosphere can cause substantial ecological effects (Baron et al. 2011).
In remote oligotrophic freshwaters, N deposition affects organisms and ecological processes in several ways, among which the best known is acidification (Davies et al. 1992; Wigington et al. 1996; Lepori et al. 2003a). Recently, freshwater ecologists have also become interested in effects that arise through nutrient enrichment and nutrient imbalance, i.e., the alteration of the balance between N and other nutrients (Baron et al. 2000; Bergström et al. 2005). Interest in nutrient-enriching effects was probably hindered by a widespread belief that freshwater communities are more likely to be limited by phosphorous (P) than by N (“the P-limitation paradigm”; see Lewis and Wurtsbaugh 2008). In contrast with this view, recent research suggests that N limitation is common in oligotrophic freshwaters (Bergström et al. 2005; Elser et al. 2007, 2009b). Moreover, hypotheses from ecological stoichiometry and limited empirical work suggest that nutrient imbalance because of excess N can alter the transfer of energy and materials across the food web (Elser et al. 2009a, b, 2010). Therefore, N deposition impacts remote freshwaters beyond acidification, although the additional effects are poorly understood.
The prospect that N inputs have multiple, potentially severe effects on remote freshwater ecosystems should be all the more concerning considering that N deposition is increasing at the global scale. At present, N deposition is high enough to affect freshwaters in mountain ranges in Europe (Alps, Scandes, and Tatras), the USA (Adirondacks, Catskill Mountains, Sierra Nevada, and Rocky Mountains), Asia (Central Mountains of Japan), and possibly in Arctic archipelagos (Table 1). However, large parts of South Asia, South and Central America, and Central Africa will probably join this the list of affected regions by 2050 (Galloway et al. 2004; Dentener et al. 2006), turning an issue so far characteristic of industrialized or industrializing countries into a global ecological threat.
This review aims to raise the awareness of other scientists, policy makers, and the general public on the multiple effects of N deposition on remote oligotrophic freshwater ecosystems. Remote freshwaters including mountain lakes and streams harbor a diversity of specialized and often endemic species (Brown et al. 2007), provide ecosystem services including clean water, and are highly valued for their recreational and esthetic value. Moreover, these freshwaters are ecologically fragile and could change rapidly when faced with environmental impacts (Williams et al. 1996). Yet, we suggest that the effects of N deposition on freshwater ecosystems are under-recognized. Whereas there is a wealth of information on the effects on terrestrial ecosystems (e.g., Aber et al. 1989; Matson et al. 2002; Bobbink et al. 2010), syntheses on the effects on freshwater ecosystems are few, regionally focused, and incomplete regarding the type of effects considered (Driscoll et al. 2003; Fenn et al. 2003). In this paper we present a comprehensive model for the effects of N deposition on natural ecosystems, use the model to frame a synthesis of the effects on oligotrophic remote freshwaters, and suggest priorities for future research.
Model and Synthesis
The Model
Our model summarizes the pathways through which N deposition affects the structure and functioning of ecosystems (Fig. 2). First, deposited N reaches ecosystems either directly (terrestrial ecosystems) or following mediation by the watershed (aquatic ecosystems). Second, the chemistry of the receiving soil and water is altered. We distinguish two types of chemical alterations relevant to ecosystems: the alteration of the acid–base status, and alteration of the nutrient status. In turn, the alteration of the nutrient status includes an increase in the total availability of nutrients, and a shift in the relative availability of nutrients; notably a shift in soil or water N:P ratio. Third, changes in acid–base status, total nutrient availability, relative nutrient availability, and/or interactions among these changes drive effects on ecosystem structure (i.e., species composition, richness, evenness, and chemical composition of the organisms) and processes. Below, we discuss these effects in turn for remote oligotrophic freshwater ecosystems. We exclude freshwaters in densely settled watersheds, where atmospheric N inputs are likely to be overshadowed by direct N inputs. We use numbers in square brackets, e.g. [X], to link our explanations to the possible effects on ecological targets shown in Fig. 2.
Effects on Water Chemistry
Transfer of Atmospheric N to Freshwaters
Atmospheric N is deposited on watersheds mainly in form of ammonium (NH4 +) and nitrate (NO3 −), but transfer of N to freshwaters concerns predominantly NO3 − (Campbell et al. 2004). NH4 + and NO3 − face different fates once they reach watershed soils. NH4 + ions are readily adsorbed onto soil particles, where they are made available for uptake by vegetation, microbial immobilization, and nitrification (Dillon and Molot 1990; Campbell et al. 2004). In comparison, NO3 − ions move more freely in the soil solution. Therefore, any NO3 − not retained through biological uptake becomes liable to leach into groundwater first, and into surface freshwaters in due course [1].
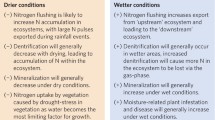
The capacity of a watershed to retain deposited N depends on soil cover, vegetation composition, vegetation successional status, land-use history, water-retention time, and time of exposure to high deposition—among other factors (Kopáček et al. 1995; Campbell et al. 2004; Bergström 2010). As a result, similar levels of N deposition can result in substantially different rates of N leaching among watersheds (Kopáček et al. 1995; Bergström 2010). By the same token, the level of N deposition that exceeds N retention capacity varies among watershed types: it can be as high as 10 kg ha−1 year−1 for forested watersheds (Dise and Wright 1995; Aber et al. 2003) or as low as 1–3 kg ha−1 year−1 for sparsely vegetated mountain watersheds (Williams et al. 1996; Baron et al. 2011). Nonetheless, N deposition is currently sufficiently high to drive substantial N leaching in watershed of different types in a number of regions across Europe, the USA, and Japan (Mitchell et al. 1997; Wright et al. 2001; Aber et al. 2003; Lepori et al. 2003a; Bergström and Jansson 2006).
In addition to varying among watersheds, N leaching varies also seasonally within watersheds, depending on biological activity and precipitation patterns (Lepori et al. 2003a; Bergström et al. 2008). In watersheds receiving high N deposition in Europe, the USA, and Japan, some leaching occurs throughout the year, indicating that the capacity for N retention is close to saturation (Van Migroet 1994; Wigington et al. 1996; Mitchell et al. 1997; Wright et al. 2001). However, in most watersheds, N leaching alternates seasonal highs and lows, whose timing depends on climate. In Europe and the USA, highs occur during the dormant seasons, and lows during the summer growing season, when biological uptake is greater (Stoddard 1994). In Japan, where the climate is wetter and warmer, the pattern is reversed (Mitchell et al. 1997). Moreover, short-lived surges in N export into freshwaters during hydrological events can overlay the seasonal patterns. To date, only in a few European watersheds N deposition is so high that the seasonal leaching cycle is disrupted, and nitrogen concentrations in freshwaters remain constantly high (Wright et al. 2001). However, high, aseasonal leaching could become common in the future if watershed soils become increasingly saturated with N (Stoddard 1994; Van Migroet 1994).
Effects on Acid–Base Status
In the atmosphere, N compounds from combustion and animal husbandry form nitric acid (HNO3) and NH4 + (Wellburn 1994). Both HNO3 and NH4 + acidify freshwaters (Dillon and Molot 1990). HNO3 acidifies waters directly, NH4 + following biological uptake, which causes release of protons (H+) into the soil solution (Vitousek et al. 1997). However, acidity from either source can be partly or fully neutralized in watershed soils before it reaches freshwaters. The degree of neutralization depends on the ANC of the watershed, which reflects factors such as bedrock geology, elevation, aspect, and slope steepness ([2]; Nanus et al. 2009). Bedrock geology is of almost-universal importance. In watersheds underlain by biogenic sedimentary rocks, acidity is usually neutralized fully by dissolution of calcium carbonate or certain silicates (Wellburn 1994). In comparison, in watersheds underlain by hard magmatic or volcanic rocks, weaker buffer systems (e.g., cation exchange systems and hydrated aluminum hydroxide exchange) can only buffer a small amount of acidity before they are exhausted. The importance of altitude and aspect varies regionally (Nanus et al. 2009). Nonetheless, the influence of these factors means that vulnerability to the acidifying effects of N deposition can vary within (Lepori et al. 2003a; Nanus et al. 2009) as well as among regions (Table 1).
Freshwater acidification from N deposition can be episodic or chronic. Episodic acidification arises for days to weeks following hydrological events (especially spring floods), during which large amounts of NO3 − reach freshwaters, driving H+ ions with them (Davies et al. 1992; Wigington et al. 1996). In watersheds of low ANC, acid episodes can be severe. Here, acid runoff traveling through (/above) soil can exhaust (/bypass) buffer systems completely, causing pH to drop below 5.6 in receiving lakes and streams ([3]; Wigington et al. 1996; Lepori et al. 2003a, b). Moreover, acid runoff activates the hydrated aluminum hydroxide exchange buffer system (Wellburn 1994), triggering surges of toxic inorganic aluminum (Lepori et al. 2003b; [4]). Chronic acidification from N deposition has been documented in Europe and the USA (Williams et al. 1996; Henriksen and Brakke 1988; Lepori et al. 2003a), although sulfate (SO4 2−) is usually mainly responsible for this type of acidification (Stoddard et al. 2001). Acidification from SO4 2− has a more chronic character than acidification from N because SO4 2− is not taken up by vegetation (Van Migroet 1994). The contribution of NO3 − to chronic acidification is usually too small to exhaust buffer systems completely (Williams et al. 1996; Lepori et al. 2003a; Baron et al. 2011). Nonetheless, such contribution makes streams and lakes more sensitive to acid episodes, and is therefore indirectly responsible for some of their ecological effects.
Effects on Nutrient Status
Because N is a key nutrient, N leaching into freshwaters increases the total concentration of nutrients available to the biological community [5]. Equally important, because atmospheric deposition of P is usually much lower than that of N, N leaching also tends to increase the N:P ratio of the water [6]. For example, spatial gradients of N deposition across Sweden and the Alps are associated with increasing water N:P ratios in lakes and streams (Bergström et al. 2005; F.L., unpublished results). Similarly, in the Rocky Mountains and Scandinavia, lakes receiving high N deposition (>6 kg ha−1 year−1) have water N:P ratios 2–5 times greater than lakes receiving low deposition (~<4.5 kg ha−1 year−1; Elser et al. 2009b).
Effects on Ecosystem Structure and Processes
Effects Driven by Changes in Acid–Base Status
Acidity from N deposition reduces the richness of fish and macroinvertebrate species in streams and lakes [7]. For example, in the Adirondacks, the richness of fish and macroinvertebrates decreases with declining water pH (Driscoll et al. 2003; Baldigo et al. 2009). Similarly, in the Alps, richness of stream macroinvertebrates reflects vulnerability to acid episodes, with significantly fewer taxa occurring in streams prone to acid episodes than in neutral or alkaline streams (Lepori et al. 2003b). The loss of richness probably owes to the toxicity of acidity and associated high aluminum concentrations. In particular, pH values <5.6 and aluminum concentrations >100 μg L−1 are thresholds of lethal toxicity for a variety of aquatic organisms, because they cause loss of physiologically important ions such as Na+ from the blood plasma (Simpson et al. 1985; Driscoll and Schecher 1990; Baker et al. 1996; Lepori et al. 2003b; Felten and Guérold 2006). Thus, in Adirondack streams, brook trout (Salvelinus fontinalis) and forage fish (blacknose dace, Rhinichthys atratulus; mottled sculpin, Cottus bairdi; and slimy sculpin, Cottus cognatus) suffer high mortality during acid episodes (Baker et al. 1996). In Alpine streams, nymphs of the mayfly Baetis alpinus suffer mortality as high as 90% within 2 weeks when exposed to acid episodes during spring snowmelt (Lepori and Ormerod 2005).
Another apparent effect of acidification is the increased abundance of acid-tolerant invertebrates, such as certain stoneflies (Amphinemura, Leuctra, and partly Isoperla), caddisflies (Rhyacophila), and blackflies (Simulium) in acidic or episodically acidic streams (Simpson et al. 1985; Lepori et al. 2003b). The causes of these increases are unclear, although they suggest release from competition or predation by species eliminated or reduced by acidity. The apparent replacement of acid-sensitive by acid-tolerant forms amplifies changes in assemblage composition [8], and explains why invertebrate density is not necessarily reduced in streams affected by acidity despite losses of richness (Lepori et al. 2003b).
In addition to reducing the diversity of fish and invertebrates, acidification usually affects the species composition of algae [9] and slows down the decomposition of organic detritus ([10]; review by Ormerod 1992). However, evidence for these effects arises mostly from freshwaters where NO3 − is not necessarily, and probably is not, the main driver of acidification. Therefore, the extent of these effects remains to be determined with regard to freshwaters acidified by NO3 −. Indeed, chronic acidification driven by sulfate and episodic acidification driven by NO3 − can have substantially different effects on communities of benthic diatoms (Passy 2006).
Effects Driven by Changes in Absolute Nutrient Availability
Evidence that N limits the biomass of primary producers (algae, cyanobacteria, and other autotrophs) in freshwaters may be at odds with the long-standing paradigm of P limitation (Schindler 1977), but appears to be accumulating fast (Dodds and Welch 2000; Bergström et al. 2005; Elser et al. 2007; Lewis and Wurtsbaugh 2008). N-limitation is particularly common in unpolluted lakes, suggesting that it might even be the natural state of freshwater ecosystems (Bergström and Jansson 2006; Lewis and Wurtsbaugh 2008). For example, a synthesis of the results of nutrient assays across Scandinavia and the Rocky Mountains showed that N limited phytoplankton biomass in 43% of the lakes receiving low amounts of N deposition (≤4.5 kg ha−1 year−1; Elser et al. 2009b). Therefore, inputs of N from the atmosphere should increase the biomass of primary producers in a large proportion of otherwise unimpacted freshwaters [11]. Consistent with this expectation, observational studies indicate that the biomass of phytoplankton and benthic algae in remote freshwaters increases along gradients of N deposition in Scandinavia (Bergström et al. 2005; Liess et al. 2009), the Rocky Mountains (Elser et al. 2009a), and more in general across the northern hemisphere (Bergström and Jansson 2006).
Increased absolute availability of NO3 − from leaching also influences ecosystem processes. In lakes, NO3 − not stored or exported downstream is eventually removed by production of nitrous oxide (N2O) or denitrification, the microbially mediated production of nitrogen gas (N2) from NO3 − (Seitzinger et al. 2006). McCrackin and Elser (2010) found that in Norway NO3 − removal by these processes was greater in lakes receiving high N deposition than in lakes receiving low deposition [12]. In these lakes, the enhanced removal of NO3 − by production of N2O and denitrification is bound to mitigate excess N and export of N to downstream ecosystems (Seitzinger et al. 2006). However, no similar effects were found in Rocky Mountain lakes with contrasting N deposition (McCrackin and Elser 2011), perhaps because N deposition rates were lower than in Europe (Table 1).
Effects Driven by Changes in Relative Nutrient Availability
Changes in water N:P ratio are expected to affect the composition of primary producers because optimal N:P ratios for growth vary among species (Rhee and Gotham 1980), and species with optimal N:P ratio matching the water N:P ratio can reduce other species through competition ([13]; Stelzer and Lamberti 2001). Shifts in composition may occur especially where increases in water N:P ratio cause ecosystems to tip over from N-limitation to P-limitation (Bergström and Jansson 2006; Elser et al. 2009b). In these cases, primary producers thriving under N limitation, such as cyanobacteria, may be replaced by others more competitive at higher N:P ratios, such as certain diatoms (Schindler 1977; Smith 1983; Tilman et al. 1986). However, observations and most experiments of nutrient enrichment do not allow to separate the effects of total nutrient availability from the effects of N:P ratio unequivocally. Among the few that do, Stelzer and Lamberti (2001) showed that the stream-dwelling diatoms Achnanthidium minutissima, Amphipleura pellucida, and Cymbella affinis increase in abundance with increasing water N:P ratio. Concerning lentic ecosystems, a model and an empirical test by Bulgakov and Levich (1999) supported the prediction that cyanobacteria dominate phytoplankton at low water N:P of 5–10:1 (by weight), whereas N:P ratios to 20–50:1 favor greater abundance of the green algae chlorococcales.
Changes in water N:P ratios are also expected to influence the diversity of the primary producers based on resource-ratio theory (Tilman 1980). This theory predicts that if a resource is supplied at a much greater rate than other, equally essential ones, the single best competitor for that resource out-competes other species and becomes dominant in the community. If so, by pushing the N:P ratio to unnaturally skewed levels, N deposition should reduce the richness and the evenness of communities of primary producers [14]. Paleolimnological studies from lakes in the western USA provide circumstantial support to this prediction by showing that over the last century, as N deposition increased, diatoms communities have become increasingly dominated by a few species (Baron et al. 2000; Wolfe et al. 2001).
Resource-ratio theory assumes exploitative competition for shared nutrients. Therefore, it probably applies to lake ecosystems, which act essentially as closed systems with regard to nutrient supply, in the short term (Newbold 1992). It is less clear whether it also applies to streams and rivers. In these systems nutrients are supplied continuously from the watershed, and algae (represented by benthic forms) presumably have less influence on their concentrations (Stelzer and Lamberti 2001). However, benthic algae can deplete nutrients within algal biofilms (Bothwell 1989) and might therefore compete for nutrients at this microscale even if they do not reduce nutrient concentrations across the water column. Dominance by species with skewed optimal N:P ratios may also be promoted by mechanisms other than competitive exploitation. For example, in the experiment of Stelzer and Lamberti (2001) a diatom that became dominant at high N:P ratios (Cymbella affinis) produced a gelatinous mat that appeared to inhibit other algae, suggesting that this species reached high relative abundance partly through interference competition.
Changes in water N:P ratio can also influence the cellular quotas of N and P taken up by primary producers [15], and therefore their elemental (C, N, P) composition. First, algae store in their cells nutrients that are supplied in excess to their optimal N:P ratio (a process known as “luxury uptake”); as a result, the N:P of algal cells tend to mirror that of the water (Sterner and Hessen 1994; Stelzer and Lamberti 2001). Second, algal communities in freshwaters with high N:P may be dominated by species of algae best suited to grow with low P cellular quotas relative to carbon (C) and N (Elser et al. 2009a). Thus, in Rocky Mountain and Scandinavian lakes the N:P and C:P ratios of seston (the suspended organic material, including phytoplankton) and benthic algae tend to be significantly higher in lakes receiving high N deposition than in low-deposition lakes (Elser et al. 2009a, 2010; Liess et al. 2009).
Alterations of cell N:P and C:P ratios in primary producers set the stage for indirect effects that could influence the transfer of energy through the algae-based food web. In planktonic food webs, key herbivores such as the cladoceran Daphnia have high dietary demand of P relative to N (Sterner et al. 1993). Therefore, by favoring algae with high cell N:P and C:P, inputs of atmospheric N should promote stoichiometric imbalance between herbivores and primary producers in lakes (Elser and Urabe 1999; Elser et al. 2010). Such imbalance could reduce not only the growth and biomass of herbivores ([16]; Lafrancois et al. 2004), but also that of consumers at higher trophic levels, including fish (Elser et al. 2009a). However, the effects of N deposition on herbivory are poorly studied in freshwater ecosystems, and any generalization would be premature. In terrestrial systems, where research on this topic is more developed, N deposition tend to have positive effects on herbivore insects, because increased N content is thought to improve the quality of leaves as food for these organisms (Throop and Lerdau 2004). Therefore, the effects on herbivory might be partly idiosyncratic, and depend on the taxonomic identity of the dominant herbivores.
Concerning effects on ecosystem processes, an experimental addition of N to a small watershed in Maine resulted in faster decomposition of detritus (litter) in the draining stream ([17]; Chadwick and Huryn 2003). The effect was caused mainly by an increase in the N content of the leaves, which stimulated the decomposing activity of microbes and, possibly, that of leaf-shredding invertebrates. In comparison, the increase in NO3 − concentration in the water associated with the N addition had little effect. Again, there is no basis to assess the generality of this response at present. Research on the effects of N deposition on litter decomposition in terrestrial ecosystems reveals contrasting, apparently ecosystem-specific responses. A framework for explaining the variation in results suggests that N deposition affects negatively the decomposition of recalcitrant litter, mediated by white rot-type fungi, but affects positively the decomposition of labile litter mediated by other fungi (Waldrop et al. 2004). Future studies may determine whether effects are similarly variable in freshwaters. In any case, the available evidence suggests that N deposition can alter not only the algae-based food web of aquatic ecosystems, but also the detritus-based food web.
Effects Driven by Interactions Between Changes in Acid–Base and Nutrient Status
In poorly buffered watersheds, changes in nutrient status because of N deposition can interact with acidification, causing cumulative ecosystem effects that might not be easily predicted from the responses to each change alone [18]. For example, in lakes of the Rocky Mountains, an experimental addition of N plus hydrochloric acid caused more striking shifts in phytoplankton composition than the addition of N alone (Lafrancois et al. 2004). Furthermore, either N or acidity promoted greater abundance of green algae, which are thought to be poorer food to herbivore zooplankton than the algae replaced (golden algae and diatoms). Therefore, the combined, apparently synergistic effect could have exacerbated food-web effects on zooplankton. Despite this study, the interactions among the different effects of N deposition (Fig. 2) and between N deposition and other stressors remain one of the least understood threats to remote freshwaters.
Discussion and Conclusions
Our review shows that N deposition can cause profound alterations to the structure and processes of remote oligotrophic freshwater ecosystems. These ecological impacts have been affecting, and will continue to affect industrialized regions in North America, Western Europe, and Japan for a long time. In these regions, N emissions are projected to remain high and steady at least until 2050 (Galloway et al. 2004; Dentener et al. 2006). This means that atmospheric N will contribute to episodic acidification, nutrient enrichment, and nutrient imbalance in remote freshwaters for the foreseeable future. Moreover, even if N deposition will remain steady, the ecological effects might worsen because of continuing watersheds saturation (Stoddard 1994; Dise and Wright 1995; Driscoll et al. 2003) and synergistic effects between N saturation and climate change. The effects of climate may be pronounced at northern latitudes, where soils store large pools of N. In Norway, for example, exposing watersheds to experimental warming resulted in increased NO3 − leaching (Wright 1998). Moreover, NO3 − export through streams and rivers is expected to increase under scenarios of increased precipitation (Howarth et al. 2006). However, not all effects of climate change might work in the same direction. Predicted increases in temperature are also expected to (i) reduce acidification via increased mineral weathering, (ii) increase watershed N retention via increased winter uptake, and (iii) reduce nutrient limitation via increased leaching of dissolved organic matter, which promotes light limitation (Eisenreich 2005; Karlsson et al. 2009). Give these possibly conflicting effects, the net outcome of climate change on N leaching remains difficult to predict.
All other effects aside (and ignoring interactions with other environmental changes), continuing acidification from N deposition might not create new issues, but might work against present efforts to manage old ones. Over the last three decades, Europe and North America have undertaken considerable efforts to reduce acid deposition, and have been particularly successful at reducing S emissions (Driscoll et al. 2001; Fischer et al. 2007). In turn, reduced S emissions have been followed by an almost-universal decrease in lake and stream sulfate concentrations (Stoddard et al. 1999). At the same time, in part of these regions increasing N saturation has increased acidification from N deposition (Williams et al. 1996; Lepori et al. 2003a; Baron et al. 2011). Where this has happened, N has either given rise to new acidification or has replaced S as the main culprit of long-standing acidification (Henriksen and Brakke 1988). As an illustration of the latter case, a decline in S deposition from 1982 to 1992 in the Adirondack Mountains failed to reduce acidification because lake N concentrations increased over the same period (Sullivan et al. 1997). Moreover, the increased contribution of N to acidification probably exacerbated acid episodes, which are particularly toxic to the biota. Clearly, any ecological recovery from S-driven acidification risks to be delayed for as long as N emissions are not reduced with equal success.
While the ecological impacts of N deposition in Europe and North America are cause for continuing concern, more worrying is the probable rise of these same impacts at the global scale in the future. In most developing countries, especially in Asia, Central Africa, and Latin America, N emissions and deposition are projected to increase by 2050 (Galloway et al. 2004; Dentener et al. 2006). In large part of Asia, deposition is expected to exceed 50 kg ha−1 year−1, which is roughly the double of what might be considered very high deposition at present (Table 1), and up to 30 times the load at which ecological effects may begin to arise in some mountain watersheds (Baron et al. 2011). Therefore, high N deposition will increase not only in geographic extent, but also in magnitude; and so presumably will the ecological effects.
What can scientists do to limit the growing impact of N deposition on remote freshwaters? We suggest that scientists are only beginning to unravel the some of the more complex and indirect effects of nitrogen inputs on the structure and functioning of freshwater ecosystems. The total impact might remain underestimated or ignored. A first important step towards management would, therefore, involve a better understanding of these effects. Three areas in particular seem to deserve prior attention:
-
1.
Beyond the better-known effects owing to acidification, effects of N deposition via changes in nutrient status need much further research. Moreover, most of the research available on the effects of nutrient enrichment and nutrient imbalance concerns planktonic communities; with few exceptions (Liess et al. 2009; McCrackin and Elser 2010, 2011) the effects on benthic communities remain almost unexplored. The present focus on planktonic rather than benthic communities reflects a greater interest in nutrient effects on food webs among lake ecologists (who often focus on plankton), not a different vulnerability between communities.
-
2.
N deposition affects streams already threatened climate change, UV-B exposure, and micropollutant contamination. Therefore, it is important to assess not only the effects of deposition in isolation, but also the interactions between deposition and other stressors. This assessment is important to evaluate and predict the overall ecological impact, which may be worse than the impact of any one stressor, and establish priorities for stressor abatement or ecosystem remediation.
-
3.
As patterns of N deposition are shifting globally, we should better understand the ecological effects outside Europe and North America, where most research has been conducted in the past. For example, although N deposition is predicted to increase dramatically in the tropics, the response of tropical freshwater ecosystems to N inputs is poorly investigated. Yet, tropical and northern ecosystems may be essentially different with regard to N cycling, and the responses to N additions may vary likewise (Phoenix et al. 2006).
Finally, we suggest that threats to remote freshwaters warrants greater attention among scientists, policy makers, and the general public. Acidification no longer makes headline news, and reviews of the global status of freshwaters place emphasis on the effects of habitat alteration, pollution, and overexploitation in settled watersheds (e.g., Dudgeon et al. 2006). Although these impacts represent the main current threats to freshwater ecosystems, emerging impacts, including N deposition and climate change, will extend the span of marked human footprint to remote freshwaters (Hobbs et al. 2010). Present effects on remote freshwaters, such as the ones reviewed here, may be only early signs of rapid and radical changes ahead.
References
Aber, J.D., K.J. Nadelhoffer, P. Steudler, and J.M. Melillo. 1989. Nitrogen saturation in northern forest ecosystems. BioScience 39: 378–386.
Aber, J.D., C.L. Goodale, S.V. Ollinger, M.-L. Smith, A.H. Magill, M.E. Martin, R.A. Hallett, and J.L. Stoddard. 2003. Is nitrogen deposition altering the nitrogen status of northeastern forests? BioScience 53: 375–389.
Baker, J.P., J. Van Sickle, C.J. Gagen, D.R. DeWalle, W.E. Sharpe, R.F. Carline, B.P. Baldigo, P.S. Murdoch, et al. 1996. Episodic acidification of small streams in the northeastern United States: Effects on fish populations. Ecological Applications 6: 422–437.
Baldigo, B.P., G.B. Lawrence, R.W. Bode, H.A. Simonin, K.M. Roy, and A.J. Smith. 2009. Impacts of acidification on macroinvertebrate communities in streams of the western Adirondack Mountains, New York, USA. Ecological Indicators 9: 226–239.
Baron, J.S., H.M. Rueth, A.M. Wolfe, K.R. Nydick, E.J. Allstott, J.T. Minear, and B. Moraska. 2000. Ecosystem responses to nitrogen deposition in the Colorado Front Range. Ecosystems 3: 352–368.
Baron, J.S., C.T. Driscoll, J.L. Stoddard, and E.E. Richer. 2011. Empirical critical loads of atmospheric nitrogen deposition for nutrient enrichment and acidification of sensitive US lakes. BioScience 61: 602–613.
Bergström, A.-K. 2010. The use of TN:TP and DIN:TP ratios as indicators for phytoplankton nutrient limitation in oligotrophic lakes affected by N deposition. Aquatic Sciences 72: 277–281.
Bergström, A.-K., and M. Jansson. 2006. Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Global Change Biology 12: 635–643.
Bergström, A.-K., P. Blomqvist, and M. Jansson. 2005. Effects of atmospheric nitrogen deposition on nutrient limitation and phytoplankton biomass in unproductive Swedish lakes. Limnology and Oceanography 50: 987–994.
Bergström, A.-K., A. Jonsson, and M. Jansson. 2008. Phytoplankton responses to nitrogen and phosphorus enrichment in unproductive Swedish lakes along a gradient of atmospheric nitrogen deposition. Aquatic Biology 4: 55–64.
Bobbink, R., K. Hicks, J. Galloway, T. Spranger, R. Alkemade, M. Ashmore, M. Bustamante, S. Cinderby, et al. 2010. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecological Applications 20: 30–59.
Bothwell, M.L. 1989. Phosphorus-limited growth dynamics of lotic periphytic diatom communities: Aerial biomass and cellular growth rate responses. Canadian Journal of Fisheries and Aquatic Sciences 46: 1293–1301.
Brown, L.E., A.M. Milner, and D.M. Hannah. 2007. Hydroecology of alpine rivers. In Hydroecology and ecohydrology: Past, present and future, ed. P.J. Wood, D.M. Hannah, and J.P. Sadler, 339–360. Chichester: Wiley.
Bulgakov, N.G., and A.P. Levich. 1999. The nitrogen:phosphorus ratio as a factor regulating phytoplankton community structure. Archiv für Hydrobiologie 146: 3–22.
Campbell, J.L., J.W. Hornbeck, M.J. Mitchell, M.B. Adams, M.S. Castro, C.T. Driscoll, J.S. Kahl, J.N. Kochenderfer, et al. 2004. Input-output budgets of inorganic nitrogen for 24 forest watersheds in the Northeastern United States: a review. Water, Air, and Soil pollution 151: 373–396.
Chadwick, M.A., and A.D. Huryn. 2003. Effect of a whole-catchment N addition on stream detritus processing. Journal of the North American Benthological Society 22: 194–206.
Davies, T.D., M. Tranter, P.J. Wigington Jr, and K.N. Eshleman. 1992. ‘Acidic episodes’ in surface waters in Europe. Journal of Hydrology 132: 25–69.
Dentener, F., J. Drevet, J.F. Lamarque, I. Bey, B. Eickhout, A.M. Fiore, D. Hauglustaine, and L.W. Horowitz, et al. 2006. Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Global Biogeochemical Cycles 20:GB4003. doi:10.1029/2005GB002672.
Dillon, P.J., and L.A. Molot. 1990. The role of ammonium and nitrate retention in the acidification of lakes and forested catchments. Biogeochemistry 11: 23–43.
Dise, N.B., and R.F. Wright. 1995. Nitrogen leaching from European forests in relation to nitrogen deposition. Forest Ecology and Management 71: 153–162.
Dodds, W.K., and E. Welch. 2000. Establishing nutrient criteria in streams. Journal of the North American Benthological Society 19: 186–196.
Driscoll, C.T., and W.D. Schecher. 1990. The chemistry of aluminum in the environment. Environmental Health Perspectives 12: 28–49.
Driscoll, C.T., G.B. Lawrence, A.J. Bulger, T.J. Butler, C.S. Cronan, C. Eagar, K.F. Lambert, G.E. Likens, et al. 2001. Acidic deposition in the Northeastern United States: Sources and inputs, ecosystem effects, and management strategies. BioScience 51: 180–198.
Driscoll, C.T., K.M. Driscoll, M.J. Mitchell, and D.J. Raynal. 2003. Effects of acidic deposition on forest and aquatic ecosystems in New York State. Environmental Pollution 123: 327–336.
Dudgeon, D., A.H. Arthington, M.O. Gessner, Z.-I. Kawabata, M.O. Gessner, D.J. Knowler, C. Lévêque, R.J. Naiman, et al. 2006. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Eisenreich, S.J., ed. 2005. Climate change and the European Water Dimension. Joint Research Centre of the European Commission, EU Report No. 21553, Ispra, Italy.
Elser, J.J., and J. Urabe. 1999. The stoichiometry of consumer-driven nutrient recycling: Theory, observations, and consequences. Ecology 80: 745–751.
Elser, J.J., M.E.S. Bracken, E.E. Cleland, D.S. Gruner, W.S. Harpole, H. Hillebrand, J.T. Ngai, E.W. Seabloom, et al. 2007. Global analysis of nitrogen and phosphorus limitation of primary production in freshwater, marine, and terrestrial ecosystems. Ecology Letters 10: 1135–1142.
Elser, J.J., M. Kyle, L. Steger, K.R. Nydick, and J.S. Baron. 2009a. Nutrient availability and phytoplankton nutrient limitation across a gradient of atmospheric nitrogen deposition. Ecology 90: 3062–3073.
Elser, J.J., T. Andersen, J.S. Baron, A.-K. Bergström, M. Jansson, M. Kyle, K.R. Nydick, L. Steger, et al. 2009b. Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326: 835–837.
Elser, J.J., A.L. Peace, M. Kyle, M. Wojewodzic, M.L. McCrackin, T. Andersen, and D.O. Hessen. 2010. Atmospheric nitrogen deposition is associated with elevated phosphorus limitation of lake zooplankton. Ecology Letters 13: 1256–1261.
Fay, J.A., D. Golomb, and K. Subramanyam. 1987. Anthropogenic nitrogen oxide transport and deposition in eastern North America. Atmospheric Environment 21: 61–68.
Felten, V., and F. Guérold. 2006. Short-term physiological responses to a severe acid stress in three macroinvertebrate species: A comparative study. Chemosphere 63: 1427–1435.
Fenn, M.E., and M.A. Poth. 1999. Temporal and spatial trends in streamwater nitrate concentrations in the San Bernardino Mountains, Southern California. Journal of Environmental Quality 28: 822–836.
Fenn, M.E., J.S. Baron, E.B. Allen, H.M. Rueth, K.R. Nydick, L. Geiser, W.D. Bowman, J.O. Sickman, et al. 2003. Ecological effects of nitrogen deposition in the western United States. BioScience 53: 404–420.
Fischer, R., V. Mues, E. Ulrich, G. Becher, and M. Lorenz. 2007. Monitoring of atmospheric deposition in European forests and an overview on its implication on forest condition. Applied Geochemistry 22: 1129–1139.
Galloway, J.N., F.J. Dentener, D.G. Capone, E.W. Boyer, R.W. Howarth, S.P. Seitzinger, G.P. Asner, C.C. Cleveland, et al. 2004. Nitrogen cycles: Past, present, and future. Biogeochemistry 70: 153–226.
Goldman, C.R. 1988. Primary productivity, nutrients, and transparency during the early onset of eutrophication in ultra-oligotrophic Lake Tahoe, California-Nevada. Limnology and Oceanography 33: 1321–1333.
Heathwaite, A.L., P.J. Johnes, and N.E. Peters. 1996. Trends in nutrients and water quality. Hydrological Processes 10: 263–293.
Henriksen, A., and D.F. Brakke. 1988. Increasing contributions of nitrogen to the acidity of surface waters in Norway. Water, Air, and Soil pollution 42: 183–201.
Henriksen, A., J. Kämäri, M. Posch, and A. Wilander. 1992. Critical loads in Nordic countries. Ambio 21: 356–363.
Hobbs, W.O., R.J. Telford, H.J.B. Birks, J.E. Saros, R.R.O. Hazewinkel, B.B. Perren, É. Saulnier-Talbot, and A.P. Wolfe. 2010. Quantifying recent ecological changes in remote lakes of North America and Greenland using sediment diatom assemblages. PLoS ONE 5: e10026. doi:10.1371/journal.pone.0010026.
Holmgren, S.U., C. Bigler, Ó. Ingólfsson, and A.P. Wolfe. 2010. The Holocene–Anthropocene transition in lakes of western Spitsbergen, Svalbard (Norwegian High Arctic): Climate change and nitrogen deposition. Journal of Paleolimnology 43: 393–412.
Howarth, R.W., D.P. Swaney, E.W. Boyer, R. Marino, N. Jaworski, and C. Goodale. 2006. The influence of climate on average nitrogen export from large watersheds in the Northeastern United States. Biogeochemistry 79: 163–186.
Karlsson, J., P. Byström, J. Ask, P. Ask, L. Persson, and M. Jansson. 2009. Light limitation of nutrient-poor lake ecosystems. Nature 460: 506–509.
Kopáček, J., L. Procházková, E. Stuchlík, and P. Blažka. 1995. The nitrogen–phosphorus relationship in mountain lakes: Influence of atmospheric input, watershed, and pH. Limnology and Oceanography 40: 930–937.
Kroeze, C., and S.P. Seitzinger. 1998. Nitrogen inputs to rivers, estuaries, and continental shelves and related nitrous oxide emissions in 1990 and 2050: A global model. Nutrient Cycling in Agroecosystems 52: 195–212.
Lafrancois, B.M., K.R. Nydick, B.M. Johnson, and J.S. Baron. 2004. Cumulative effects of nutrients and pH on the plankton of two mountain lakes. Canadian Journal of Fisheries and Aquatic Sciences 61: 1153–1165.
Lepori, F., and S.J. Ormerod. 2005. Population data and in situ toxicity tests reveal consistent effects of spring acid episodes on macroinvertebrates. Freshwater Biology 50: 1568–1577.
Lepori, F., A. Barbieri, and S.J. Ormerod. 2003a. Causes of episodic acidification in Alpine streams. Freshwater Biology 48: 175–198.
Lepori, F., A. Barbieri, and S.J. Ormerod. 2003b. Effects of episodic acidification on macroinvertebrate assemblages in Swiss Alpine streams. Freshwater Biology 48: 1873–1885.
Lewis Jr., W.M., and W.A. Wurtsbaugh. 2008. Control of lacustrine phytoplankton by nutrients: Erosion of the phosphorus paradigm. International Review of Hydrobiology 93: 446–465.
Liess, A., S. Drakare, and M. Kahlert. 2009. Atmospheric nitrogen-deposition may intensify phosphorus limitation of shallow epilithic periphyton in unproductive lakes. Freshwater Biology 54: 1759–1773.
Marchetto, A., A. Barbieri, R. Mosello, and G.A. Tartari. 1994. Acidification and weathering processes in high mountain lakes in Southern Alps. Hydrobiologia 274: 75–81.
Matson, P.A., K. Lohse, and S. Hall. 2002. The globalization of nitrogen deposition: Consequences for terrestrial ecosystems. Ambio 31: 113–119.
McCrackin, M.L., and J.J. Elser. 2010. Atmospheric nitrogen deposition influences denitrification and nitrous oxide production in lakes. Ecology 91: 528–539.
McCrackin, M.L., and J.J. Elser. 2011. Denitrification kinetics and denitrifier abundances in sediments of lakes receiving atmospheric nitrogen deposition (Colorado, USA). Biogeochemistry. doi:10.1007/s10533-011-9571-5.
Meybeck, M. 1982. Carbon, nitrogen, and phosphorous transport by world rivers. American Journal of Science 282: 401–450.
Mitchell, M.J., G. Iwatsubo, K. Ohrui, and Y. Nakagawa. 1997. Nitrogen saturation in Japanese forests: An evaluation. Forest Ecology and Management 97: 39–51.
Nanus, L., M.W. Williams, D.H. Campbell, K.A. Tonnessen, T. Blett, and D.W. Clow. 2009. Assessment of lake sensitivity to acidic deposition in national parks of the Rocky Mountains. Ecological Applications 19: 961–973.
Newbold, J. 1992. Cycles and spirals of nutrients. In Rivers Handbook, Vol. 1: Hydrological and Ecological Principles, ed. P. Calow and G.E. Petts, 379–408. Oxford: Blackwell Science.
Ohte, N., M.J. Mitchell, H. Shibata, N. Tokuchi, H. Toda, and G. Iwatsubo. 2001. Comparative evaluation on nitrogen saturation of forest catchments in Japan and northeastern United States. Water, Air, and Soil pollution 130: 649–654.
Ollinger, S.V., J.D. Aber, G.M. Lovett, S.E. Millham, R.G. Lathrop, and J.M. Ellis. 1993. A spatial model of atmospheric deposition for the northeastern U.S. Ecological Applications 3: 459–472.
Ormerod, S.J. 1992. Effects on aquatic ecosystems. In Atmospheric acidity sources, consequences and abatement, ed. M. Radojević, and R.M. Harrison, 363–404. London: Elsevier.
Passy, S.I. 2006. Diatom community dynamics in streams of chronic and episodic acidification: The roles of environment and time. Journal of Phycology 42: 312–323.
Phoenix, G.K., W.K. Hicks, S. Cinderby, J.C.I. Kuylenstierna, W.D. Stock, F.J. Dentener, K.E. Giller, A.T. Austin, et al. 2006. Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts. Global Change Biology 12: 470–476.
Rhee, G.Y., and I.J. Gotham. 1980. Optimum N:P ratios and coexistence in phytoplankton. Journal of Phycology 16: 486–489.
Robinson, C.T., U. Uehlinger, and M.O. Gessner. 2003. Nutrient limitation. In Ecology of a glacial floodplain, ed. J.V. Ward, and U. Uehlinger, 231–241. Dordrecht: Kluwer Academic Publishers.
Rogora, M., A. Marchetto, and R. Mosello. 2001. Trends in the chemistry of atmospheric deposition and surface waters in the Lake Maggiore catchment. Hydrology and Earth System Sciences 5: 379–390.
Saunders, P.A., W.H. Shaw, and P.A. Bukaveckas. 2000. Differences in nutrient limitation and grazer suppression of phytoplankton in seepage and drainage lakes of the Adirondack region, NY, U.S.A. Freshwater Biology 43: 391–407.
Schindler, D.W. 1977. Evolution of phosphorous limitation in lakes. Science 195: 260–262.
Schlesinger, W.H. 2009. On the fate of anthropogenic nitrogen. Proceedings of the National Academy of Sciences 106: 203–208.
Seitzinger, S.P., E. Mayorga, A.F. Bouwman, C. Kroeze, A.H.W. Beusen, G. Billen, G. Van Drecht, E. Dumont, et al. 2010. Global river nutrient export: A scenario analysis of past and future trends. Global Biogeochemical Cycles 24:GB0A08. doi:10.1029/2009GB003587.
Seitzinger, S., J.A. Harrison, J.K. Böhlke, A.F. Bouwman, R. Lowrance, B. Peterson, C. Tobias, and G. Van Drecht. 2006. Denitrification across landscapes and waterscapes: A synthesis. Ecological Applications 16: 2064–2090.
Simpson, K.W., R.W. Bode, and J.R. Colquhoun. 1985. The macroinvertebrate fauna of an acid-stressed headwater stream system in the Adirondack Mountains, New York. Freshwater Biology 15: 671–681.
Smith, V.H. 1983. Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science 221: 669–671.
Stelzer, R.S., and G.A. Lamberti. 2001. Effects of N:P ratio and total nutrient concentration on stream periphyton community structure, biomass, and elemental composition. Limnology and Oceanography 46: 356–367.
Sterner, R.W., and D.O. Hessen. 1994. Algal nutrient limitation and the nutrition of aquatic herbivores. Annual Review of Ecology and Systematics 25: 1–29.
Sterner, R.W., D.D. Hagemeier, W.L. Smith, and R.F. Smith. 1993. Phytoplankton nutrient limitation and food quality for Daphnia. Limnology and Oceanography 38: 857–871.
Stoddard, J.L. 1994. Long-term changes in watershed retention of nitrogen, its causes and aquatic consequences. In Environmental chemistry of lakes and reservoirs, ed. L.A. Baker, 223–284, Washington, DC: American Chemical Society.
Stoddard, J.L. 1995. Episodic acidification during snowmelt of high elevation lakes in the Sierra Nevada mountains of California. Water, Air, and Soil pollution 85: 353–358.
Stoddard, J.L., D.S. Jeffries, A. Lükewille, T.A. Clair, P.J. Dillon, C.T. Driscoll, M. Forsius, M. Johannessen, et al. 1999. Regional trends in aquatic recovery from acidification in North America and Europe. Nature 401: 575–578.
Stoddard, J.L., T.S. Traaen, and B.L. Skjelkvåle. 2001. Assessment of nitrogen leaching at ICP-waters sites (Europe and North America). Water, Air, and Soil pollution 130: 781–786.
Sullivan, T.J., J.M. Eilers, B.J. Cosby, and K.B. Vaché. 1997. Increasing role of nitrogen in the acidification of surface waters in the Adirondack Mountains, New York. Water, Air, and Soil pollution 95: 313–336.
Throop, H.L., and M.T. Lerdau. 2004. Effects of nitrogen deposition on insect herbivory: implications for community and ecosystem processes. Ecosystems 7: 109–133.
Tilman, D. 1980. A graphical-mechanistic approach to competition and predation. American Naturalist 116: 362–393.
Tilman, D., R.L. Kiesling, R. Sterner, S.S. Kilham, and F.A. Johnson. 1986. Green, bluegreen and diatom algae: Taxonomic differences in competitive ability for phosphorus, silicon, and nitrogen. Archiv für Hydrobiologie 106: 473–485.
Van Migroet, H. 1994. The relative importance of sulfur and nitrogen compounds in the acidification of fresh water. In Acidification of freshwater ecosystems: Implications for the future, ed. C.E.W. Steinberg and R.F. Wright, 33–49, Chichester: Wiley.
Vertucci, F.A., and P.S. Corn. 1996. Evaluation of episodic acidification and amphibian declines in the Rocky Mountains. Ecological Application 6: 449–457.
Vitousek, P.M., J.D. Aber, R.W. Howarth, G.E. Likens, P.A. Matson, D.W. Schindler, W.H. Schlesinger, and D.G. Tilman. 1997. Human alteration of the global nitrogen cycle: Sources and consequences. Ecological Applications 7: 737–750.
Waldrop, M.P., D.R. Zak, and R.L. Sinsabaugh. 2004. Microbial community response to N deposition in northern forest ecosystems. Soil Biology and Biochemistry 36: 1443–1451.
Wellburn, A. 1994. Air pollution and climate change: The biological impact, 2nd ed. Harlow: Longman Scientific & Technical.
Wigington Jr, P.J., D.R. DeWalle, P.S. Murdoch, W.A. Kretser, H.A. Simonin, J. Van Sickle, and J.P. Barker. 1996. Episodic acidification of small streams in the northeastern United States: Ionic controls of episodes. Ecological Applications 6: 389–407.
Williams, M.W., J. Baron, N. Caine, R. Sommerfeld, and R. Sanford. 1996. Nitrogen saturation in the Colorado Front Range. Environmental Science and Technology 30: 640–646.
Wolfe, A.P., J.S. Baron, and R.J. Cornett. 2001. Anthropogenic nitrogen deposition induces rapid ecological changes in alpine lakes of the Colorado Front Range (USA). Journal of Paleolimnology 25: 1–7.
Wolfe, A.P., C.A. Cooke, and W.O. Hobbs. 2006. Are current rates of atmospheric nitrogen deposition influencing lakes in the Eastern Canadian Arctic? Arctic, Antarctic, and Alpine Research 38: 465–476.
Wright, R.F. 1998. Effect of increased CO2 and temperature on run-off chemistry at a forested catchment in southern Norway (CLIMEX project). Ecosystems 1: 216–225.
Wright, R.F., C. Alewell, J.M. Cullen, C.D. Evans, A. Marchetto, F. Moldan, A. Prechtel, and M. Rogora. 2001. Trends in nitrogen deposition and leaching in acid-sensitive streams in Europe. Hydrology and Earth System Sciences 5: 299–310.
Acknowledgments
We thank Sibel Şatıroğlu, Florian Mermillod-Blondin, and three anonymous reviewers for providing comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lepori, F., Keck, F. Effects of Atmospheric Nitrogen Deposition on Remote Freshwater Ecosystems. AMBIO 41, 235–246 (2012). https://doi.org/10.1007/s13280-012-0250-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13280-012-0250-0