Abstract
Altered expression of long noncoding RNAs (lncRNAs) has shown to associate with human cancer development and progression and drug resistance. LncRNA HOX antisense intergenic RNA (HOTAIR) regulates chromatin state and highly expressed in various human cancers. This study analyzed HOTAIR expression in gastric cancer cells and tissues and then assessed the effects of HOTAIR on modulation of gastric cancer cell sensitivity to cisplatin and the underlying molecular events. The data showed that HOTAIR was significantly upregulated in cisplatin-resistant gastric cancer cells and tissues compared with control cells and noncancerous gastric tissues. Overexpression of HOTAIR enhanced gastric cancer cell proliferation, promoted cell cycle G1/S transition, but decreased tumor cell apoptosis. Furthermore, HOTAIR was shown to directly bind to and inhibit miR-126 expression and then to promote VEGFA and PIK3R2 expression and activate the PI3K/AKT/MRP1 pathway. In conclusion, the data demonstrated that high HOTAIR expression acted as a competitive endogenous RNA to promote cisplatin resistance in gastric cancer. Further study will evaluate HOTAIR expression as a biomarker to predict treatment response of cisplatin and explore inhibition of HOTAIR expression as a novel strategy for anti-cisplatin resistance in human gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fourth most frequently diagnosed human malignancy and the second cause of cancer death globally [1]. Gastric cancer is mostly diagnosed at advanced stages of disease, and chemotherapeutic cisplatin is one of the major options in treating the advanced gastric cancer patients. However, development of cisplatin resistance becomes the obstacle to an effective control of gastric cancer clinically. As the most important agent in chemotherapy of various human cancers, previous studies demonstrated that the molecular mechanisms of cisplatin resistance included reduction of intracellular cisplatin accumulation, ineffective induction of cell death, and enhancement of DNA damage repair [2, 3]. The factors that regulate chemoresistance of gastric cancer remain poorly understood. Thus, better understanding the molecular mechanisms of cisplatin resistance in gastric cancer could help medical oncologists to obtain alternative strategies to conquer gastric cancer and prolong survival of patients or simply, predict the treatment effectiveness of cisplatin.
To date, the human genome project has identified that more than 90 % of human genome is actively transcribed into RNA, but only approximately 2 % of these RNA products encodes proteins [4, 5]. These nonprotein-coding RNA molecules longer than 200 nucleotides are named as long noncoding RNAs (lncRNAs), and lncRNAs have shown to significantly contribute to human homeostasis and pathological conditions, including chemotherapy resistance in cancer patients [6–8]. HOTAIR (HOX antisense intergenic RNA) is a lncRNA, gene of which is localized in chromosome 12 and transcribed from the antisense strand of the HOXC locus [9]. Previous studies showed that HOTAIR overexpression was associated with chemoresistance; for example, Xue et al. demonstrated that HOTAIR enhanced estrogen receptor (ER) signaling and promoted tamoxifen resistance in breast cancer [10], while HOTAIR overexpression led to chemoresistance of ovarian cancer by activation of the Wnt/β-catenin pathway [11]. Recently, HOTAIR was reported to be able to regulate cancer cell malignant phenotypes by acting as a competing endogenous RNA (ceRNA), a completely novel mechanism of gene regulation. In particular, lncRNA and messenger RNA (mRNA) transcripts can affect each other by competitively combining with a microRNA (miRNA) response element (MRE) to influence post-transcriptional regulation of mRNA translation [12]. However, it could be interesting to assess whether HOTAIR is bale promote cisplatin resistance in gastric cancer.
Therefore, in this study, we first analyzed HOTAIR expression in gastric cancer cells and tissues. Then we assessed the effects of HOTAIR on modulation of gastric cancer cell sensitivity to cisplatin and the underlying molecular events by exploring whether HOTAIR could act as a ceRNA in regulate cisplatin resistance.
Materials and methods
Tissue samples
Thirty paired primary gastric cancer and corresponding adjacent noncancerous gastric tissues were collected from The First Affiliated Hospital of Nanjing Medical University. None of the patients had received radiotherapy or chemotherapy before surgical resection. Specimens were snap-frozen in liquid nitrogen and stored at −80 °C immediately after resection. This study protocol was approved by the ethical committee of the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), and informed consent was obtained from all patients before the study was initialed.
Cell lines and culture
Human gastric cancer cell lines SGC-7901 and BGC-823 and their corresponding cisplatin resistance cell lines SGC-7901/DDP and BGC-823/DDP were obtained from the Shanghai Institute of Cell Biology (Shanghai, China) and maintained in PRIM-1640 (GIBCO, Rockville, MD, USA) with 10 % fetal bovine serum (GIBCO), 100 U/ml penicillin, and 100 μg/ml streptomycin in humidified incubator with 5 % CO2 at 37 °C.
Quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated from cells and tissues using a Trizol reagent (Takara, Otsu, Japan) following the manufacturer’s instruction and reversely transcribed into complementary DNA (cDNA) using the reverse transcription kit (Takara) for mRNA and using the miRNA First-Strand cDNA Synthesis Kit (GeneCopoeia, Guangzhou, China) for miRNA. qPCR was then conducted in triplicate using the SYBR Green PCR Kit (Takara) on the Applied Biosystems Step One Plus Real-Time PCR System (Applied Biosystems, Foster city, CA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6 were used as internal controls for mRNA and miRNA, respectively. Comparative quantification was determined using the method of 2−ΔΔCT. The primer sequences are listed in Table 1.
Gene transfection
Plasmids and small interfering RNA (siRNA) constructs were purchased from Invitrogen (Nanjing, China). Both the miRNA mimic and miRNA inhibitor were synthesized by GenePharma Company (Shanghai, China). Plasmid and oligonucleotide transfection was conducted using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols. After 48 h or 72 h of gene transfection, cells were collected and used for further experiments.
Dual-luciferase reporter assay
The cDNA fragments from HOTAIR containing the predicted miR-126 binding sites were amplified by PCR and then cloned into the downstream of the firefly luciferase gene in pGL3 (Promega, Madison, WI, USA). In brief, cells grown in the 24-well plates were transfected with luciferase reporters and miR-126 mimic, miR-126 inhibitor, or corresponding negative controls using Lipofectamine 2000 according to the manufacturers’ instruction for 48 h. After that, cells were harvested and subjected to luciferase reporter assays using the kit from Promega and the ratios of firefly luciferase to Renilla luciferase were calculated with a Promega Glomax 2020 Single Tube Luminometer instrument (Promega) for each well. Each experiment was performed in triplicate and repeated at least once.
Protein extraction and Western blot
The total cellular protein was extracted using the RIPA buffer containing the complete protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Protein lysates were quantified and separated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were then blocked with 5 % bovine serum albumin (BSA) in phosphate buffered saline (PBS) for 2 h and were incubated with an antihuman-VEGFA, PIK3R2, AKT, p-AKT, MRP1, or GAPDH antibody (Abcam, Cambridge, MA, USA) at 4 °C overnight. On the next day, the membranes were washed with PBS-Tween-20 (PBS-T) for three times and further incubated with horseradish peroxidase-conjugated anti-mouse or rabbit IgG at the room temperature for 1 h. After washing, the signal was visualized through a chemiluminescent detection system (Pierce ECL Substrate Western blot detection system, Thermo, Rockford, IL, USA) and then exposed in a Molecular Imager ChemiDoc XRS System (Bio-Rad, Hercules, CA, USA).
Cell viability assay
The in vitro chemosensitivity of gastric cancer cells to cisplatin was determined by the CCK-8 (Dojindo, Kumamoto, Japan) assay. Briefly, cells were seeded into 96-well plates at a density of 3 × 103 cells/well and grown overnight. Next, cells were treated with various concentrations (0, 1, 5, 10, 12, 16, 18, 20, 22, and 24 μg/ml) of cisplatin. After 24 h, cell vitality was assessed using CCK-8 solution. Each experiment was performed in triplicate and repeated at least thrice. For assaying the effect of gene transfection, CCK-8 cell viability assay was also performed after gene transfection.
Colony formation assay
Cells were trypsinized to single cell suspensions and were plated on 6-well plates and either treated with different concentration of cisplatin or gene transfection. After incubation in a humidified atmosphere of 5 % CO2 at 37 °C for 10 days, colonies were stained with Crystal violet (Sigma, St Louis, MO, USA) and counted under a dissecting microscope and quantified using MacBiophotonics ImageJ (www.macbiophotonics.ca).
Flow cytometric cell cycle and apoptosis assay
For the cell cycle distribution assay, cells (1 × 106) after treatment and gene transfection were fixed with 70 % ethanol at −20 °C for 24 h and treated with RNase A at 37 °C for 30 min and then added with propidium iodide solution (PI; KeyGen, Nanjing, China). The cell suspension mixture was then incubated at the room temperature for 30 min in the dark. For the apoptosis assay, the Annexin V-FITC Apoptosis Detection Kit (Vazyme, Nanjing, China) was utilized to double stain cells with FITC-Annexin V and PI according to the manufacturer’s instruction. Cells in both assays were analyzed using the BD Biosciences FACSCalibur Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
The data were presented as the mean ± S.D. and statistically analyzed using Student’s t test. The correlation significance was determined by means of Spearman and Pearson correlation analyses. A two side value of p < 0.05 was considered statistically significant. All statistical analyses were performed by using SPSS 20.0 (SPSS, Chicago, IL, USA) or Graphpad Prim 5 (GraphPad Software, La Jolla, CA, USA).
Results
Upregulated HOTAIR expression in cisplatin-resistant gastric cancer cells and tissue specimens
In this study, we first confirmed the cisplatin sensitivity of gastric cancer cells using cell viability CCK-8 assay and confirmed higher cisplatin IC50 in cisplatin-resistant gastric cancer SGC-7901/DDP and BGC-823/DDP cells than in cisplatin-sensitive gastric cancer SGC-7901 and BGC-823 cells (Fig. 1a, b). We then detected HOTAIR expression in these cell lines and tissue samples. We found that HOTAIR expression was significantly upregulated in cisplatin-resistant gastric cancer cells (Fig. 1c, d). Moreover, compared to the normal gastric epithelial cells GES-1, HOTAIR was obviously overexpressed in SGC-7901 than in GES-1 (Fig. 1e). HOTAIR was also overexpressed in 30 pairs of human gastric cancer compared with the corresponding noncancerous tissue specimens (Fig. 1f). After 24 h cisplatin treatment, HOTAIR expression was also upregulated in gastric cancer cells (Fig. 1g). Collectively, these results suggested that upregulated HOTAIR expression might be involved in cisplatin resistance and development of gastric cancer.
Upregulated HOTAIR expression in cisplatin-resistant gastric cancer cell lines and gastric cancer tissues. a Cell viability CCK-8 assay. Gastric cancer SGC-7901 and SGC-7901/DDP cells were treated with various concentrations of cisplatin for 24 h and then subjected to the CCK-8 assay. The IC50 was then calculated for SGC-7901 and SGC-7901/DDP cells. b Cell viability CCK-8 assay. Gastric cancer BGC-823 and BGC-823/DDP cells were treated with various concentrations of cisplatin for 24 h and then subjected to the CCK-8 assay. The IC50 was then calculated for BGC-823 and BGC-823/DDP. c qRT-PCR. Levels of HOTAIR mRNA were assayed using qRT-PCR in SGC-7901 and SGC-7901/DDP cells. d qRT-PCR. Levels of HOTAIR mRNA were assayed using qRT-PCR in BGC-823 and BGC-823/DDP. e qRT-PCR. Levels of HOTAIR mRNA were assayed using qRT-PCR in GES-1, SGC-7901, and BGC-823 cell lines. f qRT-PCR. Levels of HOTAIR mRNA were assayed using qRT-PCR in gastric cancer and the paired normal tissues. g qRT-PCR. Levels HOTAIR mRNA were assayed using qRT-PCR in SGC-7901 cells stimulated by cisplatin for 0, 12, 24, 36, and 48 h. (*p < 0.05)
HOTAIR promotion of gastric cancer SGC-7901 cell resistance to cisplatin
Next, we transfected HOTAIR cDNA and empty vector (pc-control), respectively, into cisplatin-sensitive gastric cancer SGC-7901 cells to assess the effect of HOTAIR on regulation of cisplatin resistance in gastric cancer. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis revealed that HOTAIR expression was significantly increased in pc-HOTAIR-transfected SGC-7901 cells compared with cells transfected with pc-control (Fig. 2a). Moreover, the CCK-8 assay showed that viability of SGC-7901 cells transfected with pc-HOTAIR was significantly higher than that of pc-control-transfected tumor cells (Fig. 2b), while the colony formation assay also showed the similar results (Fig. 2c). Moreover, compared with the empty vector-transfected cells, the apoptosis rate, including early and later apoptosis, was decreased in cells transfected with pc-HOTAIR (Fig. 2d). The percent of SGC-7901 cells in S phase was increased after HOTAIR overexpression (Fig. 2e). Thus, upregulation of HOTAIR expression could promote cisplatin resistance of SGC-7901 cells.
Effects of HOTAIR expression on promotion of SGC-7901 cell resistance to cisplatin. a qRT-PCR. SGC-7901 cells were transfected with plasmid of HOTAIR cDNA (pc-HOTAIR) or control plasmid (pc-control) and then subjected to qRT-PCR analysis of HOTAIR levels. b Cell viability CCK-8 assay. SGC-7901 cells were transfected with pc-HOTAIR cDNA (pc-HOTAIR) or control plasmid (pc-control), exposed to cisplatin (4.0 μg/ml), and then subjected to CCK-8 assay. c Colony formation assay. The duplicated cells were subjected to colony formation assay. The graph is summarized data of the colony formation assay. d Flow cytometric apoptosis assay. The same prepared SGC-7901 cells were subjected to flow cytometric assay. The graph is summarized data. e Flow cytometric cell cycle distribution assay. The same prepared SGC-7901 cells were subjected to flow cytometric cell cycle analysis. The graph is summarized data. All data are expressed as the mean ± S.D. of three individual experiments. *p < 0.05 compared with the controls
Sensitization of gastric cancer SGC7901/DDP cells to cisplatin after HOTAIR knockdown
To further confirm the effect of HOTAIR expression on cisplatin resistance in gastric cancer cells, we knocked down HOTAIR expression in SGC7901/DDP cells using small interfering RNA (sirna) targeting HOTAIR (si-HOTAIR) and sirna targeting control (si-control) as a negative control. The qRT-PCR assay confirmed si-HOTAIR knocked down HOTAIR expression in tumor cells (Fig. 3a). The cell viability assay showed that si-HOTAIR transfection reduced tumor cell viability regardless treatment with or without cisplatin compared with si-control transfected cells (Fig. 3b, c). Moreover, the flow cytometric apoptosis assay showed that si-HOTAIR transfection promoted tumor cells to undergo apoptosis in SGC7901/DDP cells regardless treatment with or without cisplatin compared with those transfected with si-control (Fig. 3d). The percentage of cells in the S phase was declined in si-HOTAIR transfected tumor cells (Fig. 3e). These results therefore suggested that reduced HOTAIR expression sensitized SGC7901/DDP cells to cisplatin treatment.
Effects of HOTAIR knockdown on sensitization of SGC7901/DDP GC cells to cisplatin. a qRT-PCR. SGC-7901 cells were transfected with si-HOTAIR or si-control and then subjected to qRT-PCR analysis of HOTAIR levels. b Cell viability CCK-8 assay. SGC-7901/DDP cells were transfected with si-HOTAIR or si-control, then treated with or without cisplatin (4.0 μM) for up to 96 h, and then subjected to CCK-8 assay. c Colony formation assay. The duplicated cells were subjected to colony formation assay. The graph is summarized data of the colony formation assay. d Flow cytometric apoptosis assay. The same prepared SGC-7901 cells were subjected to flow cytometric assay. The graph is summarized data. e Flow cytometric cell cycle distribution assay. The same prepared SGC-7901 cells were subjected to flow cytometric cell cycle analysis. The graph is summarized data. All data are expressed as the mean ± S.D. of three individual experiments. *p < 0.05 compared with the controls
HOTAIR-regulated cisplatin resistance by directly targeting miR-126
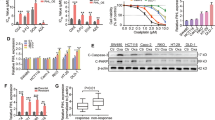
Recently, emerging evidence suggests that lncRNA could regulate microRNA expression because lncRNA sequences are complementary to miRNAs and have a regulatory effect on miRNAs and miRNA targeted mRNA [13, 14]. To explore whether HOTAIR has a similar regulatory mechanism of miRNA in gastric cancer cells, we performed online software Diana Tool analysis and predicted HOTAIR targeting miRNAs and selected miR-126, which has four predicted binding sites with high binding scores (Fig. 4a).
HOTAIR-regulated cisplatin resistance by directly targeting of miR-126. a Diana Tool prediction of cDNA sequences of four different miR-126 binding sites within the HOTAIR 3′-untranslation region (3′-UTR). b Illustration of plasmids for luciferase reporter assay. cDNA sequences of the first miR-126-binding site within the HOTAIR 3′-UTR were PCR-amplified and then cloned into the downstream of the luciferase reporter gene in pGL3-luc vector. Mutation of HOTAIR 3′-UTR was also generated. c Luciferase reporter assay. Four different HOTAIR 3′-UTR fragments were co-transfected into SGC-7901 cells together with miR-126 mimic or inhibitor and then subjected to the luciferase assay. Mutated HOTAIR 3′-UTR corresponding to the first miR-126-binding site was generated and co-transfected into SGC-7901 cells together with miR-126 mimic or inhibitor and then subjected to the luciferase assay. (d, e) qRT-PCR. SGC-7901 cells were transfected with HOTAIR cDNA or si-HOTAIR or their negative controls and then subjected to qRT-PCR analysis of miR-126 expressionT-PCR. (f, g) qRT-PCR. SGC-7901 cells were transfected with HOTAIR cDNA or si-HOTAIR or their negative controls and then subjected to qRT-PCR analysis of miR-126 expression. h qRT-PCR. Level of miR-126 expression was detected in gastric cancer tissues and noncancerous tissues. i, j qRT-PCR. Level of miR-126 expression was detected in SGC-7901, SGC-7901/DDP, BGC-823, and BGC-823/DDP cells. GAPDH was used as an internal control. *p < 0.05 compared to the controls
We then investigated whether HOTAIR is able to directly target miR-126 using the dual-luciferase reporter assay (Fig. 4b). Among these four pGL3-HOTAIR plasmids, the relative luciferase activities of the first reporter plasmid were decreased by 30 % in miR-126 mimic-transfected SGC-7901 cells. In contrast, miR-126 inhibitor transfection significantly elevated the luciferase activities. The first putative miR-126-biding site mutation plasmid-carried luciferase reporter assay showed that co-transfection of miR-126 mimic and inhibitor abrogated the inhibition and promotion effects, respectively (Fig. 4c). Moreover, in comparison with those pc-control groups, miR-126 expression was decreased in pc-HOTAIR-transfected cells (Fig. 4d), whereas transfection with si-HOTAIR upregulated miR-126 expression (Fig. 4e). In addition, similar inverse association between HOTAIR and miR-126 expression occurred when cells were transfected with miR-126 mimic and miR-126 inhibitor compared to their respective controls (Fig. 4f, g).
We then assessed miR-126 expression in gastric cancer cells and tissues and found that miR-126 was significantly downregulated in gastric cancer tissues and SGC-7901/DDP cells compared with noncancerous gastric tissues and SGC-7901, respectively (Fig. 4h, i), although there was no significant difference in miR-126 expression between BGC-823 and BGC-823/DDP cells (Fig. 4j). We found an inverse association between miR-126 and HOTAIR expression in 30 cases of gastric cancer tissues, although it did not reach statistically significant. Taken together, our results demonstrated that HOTAIR could regulate miR-126 expression by acting as an endogenous sponge.
HOTAIR promotion of cisplatin resistance by activation of the PI3K/AKT/MRP1 proteins
Previous studies revealed that miR-126 enhanced sensitivity of non-small cell lung cancer cells to anticancer agents by targeting vascular endothelial growth factor A (VEGFA) [15]. miR-126 was able to inhibit expression of both VEGFA and PIK3R2 in human breast cancer cells [16]. Indeed, VEGAF and PIK3R2 are known to play a crucial role in chemotherapy resistance through activation of the PI3K/AKT pathway and promotion of multidrug resistance-associated protein 1 (MRP1) expression [15, 17]. Thus, we explored whether HOTAIR-regulated cisplatin resistance was through activation of the PI3K/AKT/MRP1. We assessed levels of VEGFA, PIK3R2, PI3K, AKT, phospho-AKT (p-AKT), and MRP1 expression using qRT-PCR and Western blot, respectively (Fig. 5a–d). The results showed that levels of VEGFA, PIK3R2, PI3K, AKT, p-AKT, and MRP1 expression were associated with level of HOTAIR expressions in cells transfected with pc-HOTAIR or si-HOTAIR compared with the corresponding negative controls. We, thus, speculated the mechanism underlying cisplatin resistance of human gastric cancer schematically in Fig. 5e. Taken together, these findings indicated that HOTAIR promoted cisplatin resistance by binding to miR-126 and to promote expression of the miR-126 targeting genes (VEGFA and PIK3R2) and then to activate the PI3K/AKT/MRP1.
HOTAIR-regulated cisplatin resistance by activation of the PI3K/AKT/MRP1 pathway genes. a qRT-PCR. SGC-7901 cells were transfected with pc-HOTAIR or pc-control for 48 h and then subjected to qRT-PCR analysis of gene expression. b qRT-PCR. SGC-7901 cells were transfected with si-HOTAIR or si-control for 48 h and then subjected to qRT-PCR analysis of gene expression. c Western blot. SGC-7901 cells were transfected with pc-HOTAIR or pc-control for 48 h and then subjected to Western blot analysis of protein expression. d Western blot. SGC-7901 cells were transfected with si-HOTAIR or si-control for 48 h and then subjected to Western blot analysis of protein expression. GAPDH was used as an internal control. e Speculation of the mechanism underlying cisplatin resistance in gastric cancer. *p < 0.05 compared to controls
Discussion
Cisplatin is the most important chemotherapeutic agent in clinical treatment of gastric cancer patients, especially in patient unresectable advanced gastric cancer. Cisplatin is able to trigger tumor cells to undergo apoptosis by induction of DNA damage and modulation of gene expression [18]. Despite its initial success in improvement of patients’ survival, cisplatin therapy eventually leads drug resistance of tumor cells [19]. Clinically, the mechanism underlying the drug resistance involves in a series of pathological changes in tumor cells by expression or inhibition of gene expression or alteration of drug distribution and availability in cancer cells [20, 21]. Thus, it is clinically important to identify expression of cisplatin-altered genes, evaluate them as predictive markers of the therapeutic response, and develop novel strategies to improve gastric cancer treatment.
Thus, in the current study, we linked altered expression lncRNA HOTAIR in gastric cancer resistance to cisplatin treatment. Indeed, aberrant expression of various lncRNAs did associate with the development and prognosis of gastric cancer [22]. Moreover, altered expression of lncRNA HOTAIR was reported to promote drug resistance in breast, lung, and ovarian cancers [10, 23, 24]. Our current data showed that HOTAIR expression was upregulated in cisplatin-resistant gastric cancer cells as well as in gastric cancer tissue specimens. HOTAIR expression promoted gastric cancer SGC-7901 cell resistance to cisplatin treatment in vitro, whereas knockdown of HOTAIR expression using siRNA sensitized gastric cancer SGC7901/DDP cells to cisplatin treatment in vitro. Furthermore, HOTAIR enhanced cisplatin resistance by directly targeting and inhibiting miR-126 expression and then activated expression and activity of the PI3K/AKT/MRP1 in gastric cancer cells. Our ex vivo data also confirmed the inverse association of miR-126 and HOTAIR expression in gastric cancer tissues. These findings suggest that HOTAIR plays a role in cisplatin resistance of gastric cancer.
HOTAIR was firstly identified in the nucleus of breast cancer cells, especially metastasized breast cancers [25] and HOTAIR was able to bind to polycomb repressive complex 2 (PRC2) complex and alter H3K27 methylation and gene expression to induce tumor cell invasiveness and metastasis [25]. Moreover, HOTAIR was reported to act as a scaffold in the cytoplasm to induce ubiquitin-mediated proteolysis by facilitating the ubiquitination of ataxin-1 and snurportin-1 [26]. A previous study recently speculated that HOTAIR could be able to communicate with other RNA transcripts through miRNA response elements through this “competing endogenous RNA” (ceRNA) activity and, therefore, to play a role in cancer development [27]. Indeed, a previous study verified that HOTAIR was able to competitively bind to miR-331-3p and in turn to regulate HER2 expression in gastric cancer [28]. In our current study, we found that HOTAIR was able to bind to miR-126 and inhibit miR-126 expression in vitro and our ex vivo data confirmed the inverse association of their expression levels in gastric cancer tissue specimens. Our data surely validated the prediction of the Diana Tool analysis. Our reason to select miR-126 among other miRNAs was based on previous data showing that miR-126 expression could sensitize cervical and lung cancer cells to chemotherapeutic agents [15, 29]. We also showed that transfection of HOTAIR cDNA significantly suppressed miR-126 expression, whereas knockdown of HOTAIR expression significantly upregulated miR-126 expression in gastric cancer cells. Also, transfection of miR-126 mimic into gastric cancer cells decreased HOTAIR expression, which was consistent with a previous study revealing that lncRNA and miRNA were inversely associated and RNA-induced silencing complex (RISC) was recruited to cleave miRNA [14].
Furthermore, miR-126 has been reported to enhance chemotherapy sensitivity in various human cancers, including osteosarcoma, cervical cancer and lung cancer, and osteosarcoma [15, 29–31]. In addition, increasing number of studies focused on the mechanism of miR-126 in drug resistance. For example, Zhu et al. showed that enhanced expression of miR-126 induced the sensitivity of non-small-cell lung cancer to anticancer agents and the mechanism was through a negative regulation of the VEGFA/PI3K/AKT/MRP1 signaling pathway [15]. Multidrug resistance-associated protein 1 (MRP1) is a member of the ATP-binding cassette (ABC) transporter superfamily that can be classified into seven distinct subfamilies and functions as multispecific organic anion transporters, while MRP1 involves in cisplatin-induced multidrug resistance [32, 33]. Previous studies reported that besides VEGFA, PIK3R2 was targeted by miR-126 [16, 34]. Therefore, we hypothesized that HOTAIR could target and inhibit miR-126 to enhance expression of VEGFA and PIK3R2 in gastric cancer cells. Indeed, our data showed that expression and knockdown of HOTAIR in gastric cancer cells modulated expression or activity of VEGFA, PIK3R2, PI3K, AKT, p-AKT, and MRP1 mRNA or proteins. Thus, our current study confirmed the data from previous study and connected this novel HOTAIR-related gene pathway in regulation of cisplatin resistance in gastric cancer. However, the ABC superfamily contains seven subfamilies with dozen proteins and further study is needed to exclude other members of this superfamily in mediation of HOTAIR-promoting cisplatin resistance in gastric cancer.
In conclusion, our current study is for the first time to demonstrate that overexpression of lncRNA HOTAIR was able to promote cisplatin resistance in gastric cancer by induction of tumor cell proliferation and reduction of apoptosis. At the gene level, HOTAIR functioned as the endogenous sponge to activate expression and activity of the PI3K/AKT/MRP1 pathway by competitively binding to and inhibition of miR-126 expression to induce expression of the miR-126 targets VEGFA and PIK3R2 in gastric cancer cells. However, our current study is preliminary and further study is needed to validate or exclude other molecules in mediation of HOTAIR-induced cisplatin resistance in gastric cancer. The better understanding of the precise molecular mechanism of cisplatin resistance could help us to predict the responses of gastric cancer patients to cisplatin-based chemotherapy and may provide novel strategies for effective control of gastric cancer in the future.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer Suppl. 2015;136(5):E359–86.
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nature reviews. Cancer. 2013;13(10):714–26.
Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497(7447):108–12.
Bertone P, Stolc V, Royce TE, Rozowsky JS, Urban AE, Zhu X, et al. Global identification of human transcribed sequences with genome tiling arrays. Science (New York, NY). 2004;306(5705):2242–6.
Finishing the euchromatic sequence of the human genome. Nature. 2004;431(7011):931–45.
Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477(7364):295–300.
Hayes EL, Lewis-Wambi JS. Mechanisms of endocrine resistance in breast cancer: an overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015;17:40.
Sun QL, Zhao CP, Wang TY, Hao XB, Wang XY, Zhang X, et al. Expression profile analysis of long non-coding RNA associated with vincristine resistance in colon cancer cells by next-generation sequencing. Gene. 2015;572(1):79–86.
Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science (New York, NY). 2010;329(5992):689–93.
Xue X, Yang YA, Zhang A, Fong KW, Kim J, Song B, et al. LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene. 2015.
Li J, Yang S, Su N, Wang Y, Yu J, Qiu H, et al. Overexpression of long non-coding RNA HOTAIR leads to chemoresistance by activating the Wnt/beta-catenin pathway in human ovarian cancer. Tumour Biol : J Int Soc Oncodev Biol Med. 2015.
Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–69.
Lu MH, Tang B, Zeng S, Hu CJ, Xie R, Wu YY, et al. Long noncoding RNA BC032469, a novel competing endogenous RNA, upregulates hTERT expression by sponging miR-1207-5p and promotes proliferation in gastric cancer. Oncogene. 2015.
Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–52.
Zhu X, Li H, Long L, Hui L, Chen H, Wang X, et al. miR-126 enhances the sensitivity of non-small cell lung cancer cells to anticancer agents by targeting vascular endothelial growth factor A. Acta Biochim Biophys Sin. 2012;44(6):519–26.
Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu T, et al. Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Mol Cell Biochem. 2011;351(1–2):157–64.
Nath S, Daneshvar K, Roy LD, Grover P, Kidiyoor A, Mosley L, et al. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis. 2013;2:e51.
Xu HB, Shen FM, Lv QZ. Celecoxib enhanced the cytotoxic effect of cisplatin in chemo-resistant gastric cancer xenograft mouse models through a cyclooxygenase-2-dependent manner. Eur J Pharmacol. 2016;776:1–8.
Kimura A, Ogata K, Altan B, Yokobori T, Ide M, Mochiki E, et al. Nuclear heat shock protein 110 expression is associated with poor prognosis and chemotherapy resistance in gastric cancer. Oncotarget. 2016.
Sun Y, Zheng S, Torossian A, Speirs CK, Schleicher S, Giacalone NJ, et al. Role of insulin-like growth factor-1 signaling pathway in cisplatin-resistant lung cancer cells. Int J Radiat Oncol Biol Phys. 2012;82(3):e563–72.
Noel EE, Yeste-Velasco M, Mao X, Perry J, Kudahetti SC, Li NF, et al. The association of CCND1 overexpression and cisplatin resistance in testicular germ cell tumors and other cancers. Am J Pathol. 2010;176(6):2607–15.
Li T, Mo X, Fu L, Xiao B, Guo J. Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget. 2016.
Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W, et al. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21(WAF1/CIP1) expression. PLoS One. 2013;8(10):e77293.
Li J, Yang S, Su N, Wang Y, Yu J, Qiu H, et al. Overexpression of long non-coding RNA HOTAIR leads to chemoresistance by activating the Wnt/beta-catenin pathway in human ovarian cancer. Tumour Biol. 2016;37(2):2057–65.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–6.
Yoon JH, Abdelmohsen K, Kim J, Yang X, Martindale JL, Tominaga-Yamanaka K, et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun. 2013;4:2939.
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PPA. ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–8.
Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92.
Yu Q, Liu SL, Wang H, Shi G, Yang P, Chen XL. miR-126 suppresses the proliferation of cervical cancer cells and alters cell sensitivity to the chemotherapeutic drug bleomycin. Asian Pac J Cancer Prev. 2014;14(11):6569–72.
Jiang L, Tao C, He A, He X. Overexpression of miR-126 sensitizes osteosarcoma cells to apoptosis induced by epigallocatechin-3-gallate. World J Surg Oncol. 2014;12:383.
Jiang L, He A, Zhang Q, Tao C. miR-126 inhibits cell growth, invasion, and migration of osteosarcoma cells by downregulating ADAM-9. Tumour Biol. 2014;35(12):12645–54.
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science (New York, NY). 1992;258(5088):1650–4.
Chang XB. A molecular understanding of ATP-dependent solute transport by multidrug resistance-associated protein MRP1. Cancer Metastasis Rev. 2007;26(1):15–37.
Liu LY, Wang W, Zhao LY, Guo B, Yang J, Zhao XG, et al. Mir-126 inhibits growth of SGC-7901 cells by synergistically targeting the oncogenes PI3KR2 and Crk, and the tumor suppressor PLK2. Int J Oncol. 2014;45(3):1257–65.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (#81270476 and #81470830), Jiangsu Standard Diagnosis and Treatment Research Projects for Key Diseases (#BE2015716), the Priority Academic Program Development of Jiangsu Higher Education Institutions (#JX10231802), and Jiangsu Postgraduate Scientific Research and Innovation Projects (#KYZZ15_0265).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study protocol was approved by the ethical committee of the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), and informed consent was obtained from all patients before the study was initialed.
Conflicts of interest
None
Additional information
Jin Yan, Yini Dang, and Shiyu Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yan, J., Dang, Y., Liu, S. et al. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumor Biol. 37, 16345–16355 (2016). https://doi.org/10.1007/s13277-016-5448-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5448-5