Abstract
Altered microRNA (miRNA) expression plays a role in cholangiocarcinoma (CCA) development; thus, detection of blood-circulating miRNAs could be useful as CCA markers. This study profiled serum miRNA levels in patients with primary sclerosing cholangitis (PSC) and CCA and then assessed the role of miR-150-5p in CCA progression in vitro. Three samples were randomly selected from each of 50 sera of healthy controls, 30 PSC sera, and 28 CCA sera with matched bile samples for miRNA microarray profiling. The dysregulated miRNAs were confirmed using qRT-PCR, and miR-150-5p was selected for further in vitro and ex vivo studies. The miRNA microarray identified three dysregulated miRNAs in both CCA and PSC samples, while miR-150-5p level was consistently lower in CCA sera, bile, and tissues than in normal control and PSC sera (P < 0.05). Furthermore, levels of miR-150-5p were associated with serum carbohydrate antigen 19-9 (CA19-9) levels and CCA pathological grade. Bioinformatic Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analyses showed that miR-150-5p could regulate hand-full gene pathways, including cancer pathway (P < 0.01). However, overexpression of miR-150-5p inhibited proliferation, migration, and invasion capability of CCA cells (P < 0.05). Luciferase reporter assay showed that miR-150-5p bound to an oncogene Ets including gene-1 (ELK1), and Western blot data confirmed that miR-150-5p suppressed ELK1 expression in CCA cell lines. These results suggest that reduced miR-150-5p expression could contribute to CCA development and progression due to uncontrolled ELK1 expression. Thus, further study could evaluate miR-150-5p as a novel target and predictor for CCA prevention and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholangiocarcinoma (CCA) is an aggressive and fatal malignancy in the intra- and extra-hepatic biliary tract with increase in incidence and dismal prognosis [1, 2]. CCA is a biologically and clinically challenging disease due to the poor understanding of the etiology, risk factors, and pathophysiology as well as limited treatment options [3]. To date, primary sclerosing cholangitis (PSC) is considered as a premalignant lesion [4], which is caused by inflammation and obliterative fibrosis of the bile ducts and therefore impedes the bile flow into the intestine and lead to liver cirrhosis, CCA, or liver cancer [5]. Thus, it is urgently needed to better understand the molecular mechanisms of CCA development and progression to prevent this now deadly disease, improve early detection, and/or predict treatment responses and survival of patients.
MicroRNAs (miRNAs) are a class of small-sized non-coding RNAs in the human body and function to posttranscriptional regulation of gene expression by either degradation of the targeting protein-coding RNAs or inhibition of their translation into protein [6]. Previous studies demonstrated that aberrant miRNA expression was associated with human cancer development and progression [7]. Thus, detection of miRNA levels in tissues, cells, and body fluids has been evaluated as biomarkers for tumor early diagnosis, therapy, and prediction of prognosis [8], which could also provide a novel tool for CCA. For example, a previous study showed that altered serum level of miR-192 was involved in CCA development and detection of serum miR-192 level could be useful as a prognostic indicator for CCA [9], while another study reported a bile-based CCA diagnostic panel of miRNAs, which are stable, reproducible, and suitable for clinical use as biomarkers [10]. Thus, in this study, we first profiled serum miRNA levels in CCA patients vs. normal control sera and sera from PSC patients and then selected and assessed the role of miR-150-5p in CCA progression in vitro. We assessed the effects of miR-150-5p knock-in or knockdown on regulation of CCA cell proliferation, migration, and invasion in vitro. Moreover, we searched TargetScan and Sanger miRNA databases [11] to identify potential miR-150-5p-targeting genes and performed luciferase reporter assay to confirm some of these genes. After that, we performed the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analyses [12, 13] to predict the miRNA-regulated gene pathways according to previous studies [14, 15]. We expected to provide novel insightful information regarding the role of altered miRNAs in CCA development and progression and to use miR-150-5p as a novel target and predictor for CCA prevention and treatment in future clinical studies.
Materials and methods
Collection of human body liquid and tissue samples
Blood samples from 50 healthy controls and serum samples of 28 CCAs and 30 PSCs were collected from Wuxi People’s Hospital and The First People’s Hospital of Xuzhou (Jiangsu, China). The diagnosis of PSC and CCA were according to the 2013 Mayo clinic criteria [16, 17], and all 28 CCAs were extra-hepatic. Moreover, we also collected bile samples from these 28 CCA patients during endoscopic retrograde cholangiopancreatography (ERCP). In addition, among 28 CCA cases, we also obtained 13 human CCA tissue specimens with matched tumor-adjacent tissues from Wuxi People’s Hospital and The First People’s Hospital of Xuzhou. All samples were snap-frozen upon collection and stored at −80 °C until use.
RNA isolation and miRNA microarray analysis
Three serum samples of each group were randomly selected based on the shortest sampling time for miRNA microarray analysis. In particular, total RNA was isolated from these frozen body fluids using an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The quality and quantity of the isolated RNAs were then assessed using agarose gel electrophoresis and a spectrophotometer (Sangon Biotech, Shanghai, China) and then labeled with biotin and hybridized to a GeneChip miRNA 3.0 Array from Affymetrix (Santa Clara, CA, USA) according to the manufacturer’s protocol. Following hybridization, the images were digitized and analyzed using a laser scanner interfaced with Affymetrix GeneChip Command Console (AGCC). The most differentially expressed miRNAs in CCA serum samples were identified by comparing them to those of normal control and PSC samples. The microarray data were submitted to ArrayExpress with the accession number of E-MTAB-2642.
Quantitative reverse transcription-polymerase chain reaction
miRNA microarray-identified differentially expressed miRNAs were further confirmed using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) using serum, bile, and tissue samples. In brief, RNA samples were converted into complementary DNA (cDNA) using 0.5 μg RNA sample and a reverse transcription kit (Takara, Otsu, Shiga, Japan). qPCR was performed using the SYBR Premix Ex Taq II (Takara) in an ABI 7500 qPCR system (Applied Biosystems, Foster City, CA, USA). The optimal dilution and melting curves were utilized to quantify each of the amplified productions using specific primer sets. Bulge-Loop™ miRNA qRT-PCR primer sets (one RT primer and a pair of qRT-PCR primers for each miRNA) were designed and produced by RiboBio (Guangzhou, China), and 5S ribosomal RNA was used as an internal control for serum and bile miR-150-5p level, while U6 was used as an internal control for tissue miR-150-5p detection. The relative quantification of each gene was measured using the 2(−DDCT) method. We performed qRT-PCR in 13 CCA tissue samples with matched tumor-adjacent tissues, 50 healthy control sera, 30 PSC sera, and 28 CCA sera with matched bile samples in triplicate and repeated three times.
Cell lines and culture
Human CCA cell lines HCCC-9810 and RBE were cultured in RPMI-1640 containing 10 % fetal bovine serum, 100 μg/mL streptomycin, and 100 U/mL penicillin in a humidified incubator with 5 % CO2 at 37 °C.
miRNA mimic and inhibitor and transfection
The mature miR-150-5p sequences are 5′-UCUCCCAACCCUUGUACCAGUG-3′ and used to chemically synthesize oligonucleotides for generation of a double-stranded RNA that mimics native mature miR-150-5p to regulate cellular processes in vitro. Moreover, the miR-150-5p inhibitor is a single-stranded RNA that was chemically synthesized to inhibit endogenous miR-150-5p activity through specifically binding to miR-150-5p. These synthesized non-specific mimic (NSM), miR-150-5p mimic (150-5pM), non-specific inhibitor (NSI), and miR-150-5p inhibitor (150-5pI) were all from RiboBio. For cell transfection, we seeded and grew CCA cell lines to 50–70 % confluency and transiently transfected 20 nM 150-5pM or NSM and 50 nM miR-150-5pI or NSI into cells using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA). After that, cells were assayed using Western blot as well as cell viability, migration, and invasion assays.
Protein extraction and Western blot
Total cellular protein was extracted using a lysis buffer from Pierce (Rockford, IL, USA) and quantified using bicinchoninic acid (BCA) method (Pierce). Protein samples were then separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 10 % SDS-PAGE gels and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). For Western blotting, the membranes were first blocked in 5 % dry milk for 1 h in Tris–saline buffer and 0.1 % Tween-20 (TBST) and then incubated with a rabbit anti-human phospho-ELK1 (p-ELK1) antibody at a dilution of 1:2000 (Cell Signaling Technology, Danvers, MA, USA) or a rabbit anti-human β-actin antibody at a dilution of 1:5000 (Cell Signaling Technology) at 4 °C overnight. On the next day, the membranes were washed with TBST thrice and then further incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG at a dilution of 1:2000 (Sigma Aldrich, St. Louis, MO, USA) for 1 h at room temperature. The target protein band on the membrane was detected using the SuperSignal West Pico Chemiluminescent Substrate (Pierce) and quantified using Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA).
Cell growth, proliferation, invasion, and wound healing assays
Cell growth was counted using a hemocytometer (Sangon Biotech). Briefly, 10,000 transfected cells were placed into each well of 24-well plates (Corning, Corning, NY, USA) and grown in the completed medium, and the number of cells was counted every other day with a hemocytometer under an inverted-light microscope. We also performed the 5-ethynyl-20-deoxyuridine-Apollo 488 (EdU-Apollo 488) incorporation assay to assay cell proliferation capacity, i.e., CCA cells were seeded into 96-well plates at a density of 3 × 104 cells/mL and 24 h later, cells were transfected with 20 nM of NSM or 150-5pM or 50 nM of NSI or 150-5p for 6 h. The cells were stained with EdU and Hoechst 33342 separately from a kit from RiboBio according to the manufacturer’s instructions. The proliferating cells were checked under fluorescence microscope and summarized as the rate of cell proliferation (proliferation rate = the number of proliferating cells/total cells × 100 %).
Tumor cell invasion assay was performed using Transwell chambers coated with Matrigel (R&D Systems, Minneapolis, MN, USA), i.e., 2.5 × 104 transfected cells were placed into the upper chamber of 24-well Transwell inserts, and the bottom chambers were filled with the completed growth medium. Cells were allowed to grow for 24 h, and non-invaded cells on the upper filter surface were removed using a cotton swab, while cells that invaded into the bottom side of the filter were fixed with 10 % buffer formalin and stained with 0.5 % of crystal violet and then photographed under an inverted-light microscope for 10 random fields. The cell numbers were counted on the pictures and summarized as percentage of control.
For the wound healing assay, we first seeded 2.5 × 104 transfected cells in each well of 24-well plates and allowed them to grow until approximately 90 % confluency. After that, we utilized a 200-μL pipette tip to wound the cell monolayer and washed the plates with ice-cold phosphate-buffered saline (PBS) to remove cell debris and then continuously incubated the cells for up to 48 h. At 0 and 48 h, photographs were taken for 10 randomly selected fields to record cell wound healing. At the end of experiments, the wounds were measured and summarized as percentage of control.
Plasmid construction and luciferase reporter assay
The pmiR-RB-REPORT™ vector (RioBio) was used to clone the wild-type and mutated 3′-untranslated region (3′-UTR) of ELK1 cDNA. In brief, the 3′-UTR of human ELK1 cDNA (780 bp in length) containing the putative target site of miR-150-5p was amplified from genomic DNA of 293T cell lines using PCR and cloned into the pmiR-RB-REPORT™ vector downstream of the Renilla luciferase reporter gene. The primers were wild-type, 5′-GGCGGCTCGAGTTTTAGGGTGGTTAGGACA-3′ and 5′-AATGCGGCCGCAGACAG GACAGGCTTTATG-3′ with XhoI and NotI sites, respectively. The mutants were generated by substitution of seven nucleotides of the miR-150-5p-binding sites using the QuikChange XL Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA) with primers to substitute a 541–547-bp position (5′-TTAATGGGAACCCTCTCTTGGCCAAGGAAGAAT-3′ and 5′-GGCCAAGAGAGGGTTCCCATTAAATGGTACAAA-3′) and substitute a 1051–1057-bp position (5′-TTAATGGGAACCCTCTCTTGGCCAAGGAAGAAT-3′ and 5′-AATGCGGCC GCAGACAGGACAGGCTTTATGTCCCCCATTCCTCCGAGGGTTCTCCAGGGACATTGAAAG-3′). These constructed plasmids were amplified and the DNA sequence was confirmed before being used in luciferase reporter assay.
For luciferase reporter assay, 293T cells (1.5 × 104) were seeded into each well of a 96-well plate and grown for 24 h and then transiently transfected with pmiR-RB-REPORT™ luciferase reporter plasmids (80 ng per well) containing either the wild-type or the mutated ELK1 3′-UTR and 20 nM miRNA mimic, 50 nM inhibitor, 20 nM NSM, or 50 nM NSI using Lipofectamine 2000 (Invitrogen) for 48 h. Luciferase activity in cells was detected with the Dual-Glo Luciferase Assay System (Promega, Madison, WI, USA) in a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA, USA). Firefly luciferase level was used to normalize the Renilla luciferase activity. All experiments were performed in triplicate and repeated thrice.
TargetScan search and Kyoto Encyclopedia of Genes and Genomes and Gene Ontology analyses
To predict potential targeting genes of miRNAs, we performed a search on TargetScan miRNA database using TargetScan (Release 6.2) and Sanger databases. We then performed KEGG and GO term analyses to build up an miRNA-messenger RNA (mRNA) network.
Statistical analysis
The data were expressed as mean ± SEM and analyzed using one-way and two-way ANOVAs with GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA). The association of miR-150-5p with clinicopathological features was analyzed by Pearson correlation coefficient test. Differences between the group with P < 0.05 were considered statistically significant.
Results
Profiling of dysregulated miRNAs in body fluids among health control, PSC, and CCA
In this study, we first profiled differentially expressed miRNAs in serum samples of health control, PSC, and CCA and found that 30 dysregulated serum miRNAs in PSCs were not observed in healthy control sera (P < 0.01). Of them, 15 were upregulated and 15 were downregulated (Fig. 1a). Moreover, there were 19 dysregulated serum miRNAs in CCAs that were not found in healthy control sera (P < 0.01), of which 5 were upregulated and 14 were downregulated (Fig. 1b). There were 66 dysregulated bile miRNAs in CCAs that were not found in healthy control sera (P < 0.01), of which 36 were upregulated and 30 were downregulated (Fig. 1c). In addition, there were nine miRNAs dysregulated in both CCA sera and bile (Fig. 1d), while three miRNAs were altered in both PSC and CCA compared to the health control, i.e., miR-4484 and miR-3178 (upregulated) and miR-150-5p (downregulated; Fig. 1e). It is therefore speculated that these three miRNAs could play a key role in CCA development and progression. We then further pursued miR-150-5p in this study.
Profiling of differentially expressed miRNAs in CCA sera. Differentially expressed miRNAs were profiled in three cases of each of healthy control (normal), PSC, and CCA with matched bile samples (CCAB) using miRNA microarrays. a Thirty deregulated serum miRNAs in PSCs compared to healthy controls (P < 0.01). b Nineteen deregulated serum miRNAs in CCAs, which were not found in healthy controls (P < 0.01). c Sixty-six deregulated bile miRNAs in CCAs (CCAB) that were not found in sera of healthy controls (P < 0.01). d Nine deregulated miRNAs in CCA sera and bile (P < 0.01). e Three altered miRNAs in both PSC and CCA. The top bar represents the levels of miRNA expression from −1.99 (green) to 1.99 (red). The individual identity of significantly deregulated miRNAs is shown on the right
Association of downregulated miR-150-5p expression with serum CA-19-9 level and CCA pathological grade
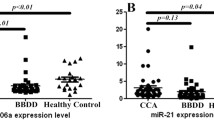
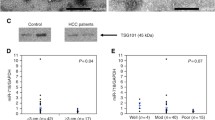
Next, we verified the downregulation of miR-150-5p expression in 13 human CCA tumor tissues with matched tumor-adjacent tissues, 50 sera from healthy controls, 30 sera from PSC, and 28 sera with matched bile samples from CCA using qRT-PCR. Our data confirmed that the level of miR-150-5p expression was markedly lower in both PSCs (serum) and CCAs (serum and bile) than in healthy controls (P < 0.01) and the reduction was also obvious in both serum and bile of CCAs compared to that of PSCs (P < 0.05), whereas there was no significant difference between CCA sera and bile (P > 0.05; Fig. 2a). Expression of miR-150-5p was also lower in CCA tissue samples than in the matched tumor-adjacent tissues (P < 0.01; Fig. 2b). We then associated miR-150-5p expression in tissues with clinicopathological data from 13 CCA patients and found that miR-150-5p was inversely associated with serum level of tumor marker CA-19-9 and pathological grade with the correlation coefficient R of −0.6783 and −0.7774, respectively (Fig. 2c, d).
qRT-PCR verification of miR-150-5p expression and its association with clinicopathological data from CCA patients. a qRT-PCR verification of downregulated miR-150-5p expression in PSC sera and CCA sera and matched bile samples. qRT-PCR was performed in 30 PSC serum samples, 28 CCA serum and matched bile samples, and 30 healthy control serum samples. The horizontal bars represent the mean ± SEM of miR-150-5p level. qRT-PCR was in triplicate and repeated three times.*P < 0.05, **P < 0.01, ***P < 0.001. b miR-150-5p expression was assessed in 13 tumor (CCA) and matched adjacent-tumor tissues (normal) from CCA patients. The horizontal bars represent the mean ± SEM of miR-150-5p level. c The correlation between miR-150-5p expression in tumor tissues and carbohydrate antigen 19-9 (CA19-9) levels from CCA patients. d The correlation between miR-150-5p expression in tumor tissues and pathological grade from CCA patients. R represents a correlation coefficient; a negative R value suggests a negative correlation
Effect of miR-150-5p expression on decrease in growth and proliferation of CCA cell lines in vitro
Thus far, we showed downregulation of miR-150-5p expression in CCA sera, bile, and tissue specimens, which was associated inversely with serum level of CA-19-9 and CCA pathological grades ex vivo. Next, we assessed the effect of miR-150-5p expression on regulation of growth and proliferation of CCA cell lines in vitro. Our cell counting and EdU incorporation assays showed that transfection of CCA cell HCCC-9810 and RBE lines with 150-5pM reduced tumor cell growth and proliferation rate compared to those of NSM-transfected cells, whereas 150-5pI transfection induced tumor cell growth and proliferation rate compared to the cells transfected with NSI (P < 0.05; Fig. 3a–e).
Effect of miR-150-5p expression on regulation of CCA cell growth and proliferation rate. a, b Cell counting assay. Ten thousand HCCC-9810 and RBE cells were transiently transfected with miR-150-5p mimic (150-5pM), miR-150-5p inhibitor (150-5pI), non-specific mimic (NSM), and non-specific inhibitor (NSI), respectively, and the number of cells was counted in 0, 1, 3, 5, and 7 days. Data are expressed as the mean value of three independent experiments ± SEM. **P < 0.01. c, d EdU incorporation assay. Cells (3 × 104 cells/mL) were seeded into 96-well plates and grown for 24 h and then transiently transfected with 150-5pM, 150-5pI, NSM, or NSI for 48 h. Cells were stained with EdU-Apollo 488 and Hoechst 33342; the Hoechst 33342 blue fluorescence represented all of the live cells, and the Edu-Apollo 488 green fluorescence represented the proliferating cells. e Cells were then counted in 10 randomly selected microscopic fields under inverted microscope. The proliferation ratio was proliferating cell number/total cell number × 100 %. Data are expressed as the mean value of three independent experiments ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
Effect of miR-150-5p expression on decrease in migration and invasion capacity of CCA cell lines in vitro
We further assessed the effect of miR-150-5p expression on regulation of CCA cell migration and invasion capacity and found that miR-150-5p overexpression in HCCC-9810 and RBE cells significantly inhibited tumor cell wound healing and invasion capacity compared to those of NSM-transfected cells, whereas transfection of the miR-150-5p inhibitor upregulated tumor cell wound healing and invasion capacity compared to those of NSI-transfected cells (P < 0.01; Fig. 4a, b).
Effect of miR-150-5p expression on regulation of CCA cell migration and invasion capacity in vitro. a Invasion of CCA cells. After 48 h of transfection, 2.5 × 104 cells were trypsinized and seeded in 24-well plates with Matrigel-coated membranes for the invasion assays. Cells that invaded through the Matrigel and reached the bottom of the insert were fixed with formalin, stained with crystal violet, and counted in 10 random view fields under inverted microscope at 48 h after incubation. The upper panel represents the HCCC-9810 cells and the lower panel represents the RBE cells. Data represents the mean value of three independent experiments. Bars represent the mean ± SEM of number of invaded cells per field. *P < 0.05, **P < 0.01. b Wound healing study. After 48 h of transfection, 2.5 × 104 cells were trypsinized and seeded in 24-well plates. A line was scratched using a sterile 50-μL pipette tip after the cells reached 90 % confluence. The cells were observed in the same 10 random views under inverted microscope, and pictures were taken at 0 and 48 h after scratching, respectively. The upper panel represents HCCC-9810 cells and the lower panel represents the RBE cells. Data represents the mean value of three independent experiments. Bars represent the mean ± SEM of wound area percentage per field. *P < 0.05, **P < 0.01, ***P < 0.001
miR-150-5p-targeting and suppression of ELK1 expression in CCA cells
Functions of miRNAs are to regulate expression of their targeting genes through degradation and inhibition of mRNA translation in vivo. Thus, we searched TargetScan and Sanger [11] miRNA databases and predicted 26 potential miR-150-5p-targeting genes, including ELK1. miR-150-5p is able to target the oncogenic ELK1 by binding to two potential binding sites at the ELK1 3′-UTR (521–527 and 1032–1038 bp, respectively; Fig. 5a). We then performed the KEGG and GO term analyses [12, 13], and the miRNA-mRNA network was generated using the three overlapping miRNAs (Figs. S2 and S3).
miR-150-5p targeting ELK1. a Illustration of potential binding sites of miR-150-5p to the ELK1 3′-UTR. Verification of the target gene predicted by TargetScan and Sanger databases. The binding sites of miR-150-5p to the ELK1 mRNA 3′-UTR were highlighted in red (human and mouse). The seed sequence of human miR-150-5p (in red) displays complementarity to positions 541–547 and 1051–1057 bp of the ELK1 3′-UTR. b Luciferase reporter assay. 293T cells were grown and co-transfected with miR-150-5p or non-specific mimic (NSM) plus wild type of the ELK1 3′-UTR (ELK1-WT), the 541–547 mutant 3′-UTR (ELK1-Mut1), the 1051–1057 mutant 3′-UTR (ELK1-Mut2), or both mutants (ELK1-Mut1 + Mut2) and then subjected to the Renilla luciferase activity assay. Data represent mean value of three independent experiments ± SEM. *P < 0.05, **P < 0.01. c Western blot analysis. HCCC-9810 and RBE cells were grown and co-transfected with 150-5pM, 150-5pI, NSM, or NSI for 48 h and then subjected to Western blotting analysis of ELK1 expression. d Quantified data of Western blots. The Western blot data were quantified using Image-Pro Plus software, and data was normalized to beta-actin and represents the mean value of three independent experiments ± SEM. **P < 0.01, ***P < 0.001
We then performed luciferase reporter assay to confirm the direct binding of miR-150-5p to these two sites at the ELK1 3′-UTR and found that luciferase activity was decreased in cells co-transfected with 150-5pM plus wild type, Mut1, or Mut2 of the ELK1 3′-UTR compared to the control cells, whereas luciferase activity in cells co-transfected with 150-5pM plus Mut1 + Mut2 of the ELK1 3′-UTR had no significant change compared to cells transfected with NSM plus Mut1 + Mut2 of the ELK1 3′-UTR (Fig. 5b). It was speculated that miR-150-5p can directly bind to both sites at the ELK1 3′-UTR. Furthermore, Western blot data revealed that overexpression of miR-150-5p significantly inhibited expression of the ELK1 protein, whereas knockdown of miR-150-5p expression induced ELK1 expression (Fig. 5c, d).
Discussion
Although CCA is a relatively rare malignancy globally, CCA incidence has been increasing in the world for the past several decades [18] and advanced CCA has limited treatment options to effectively control the relapsed disease. Thus, early detection and novel treatment are spectacularly needed [19]. On the other hand, miRNA is a desirable tool and biomarker because of its high stability in many types of body fluids. Several blood-circulating miRNAs have been shown to be associated with cancer development and progression [20], and detection of serum and bile miRNAs from PSC and CCA patients could be novel biomarkers and possessed a potential diagnostic value for these diseases [21, 22], while up to date, there are a very few studies in Chinese patients. In our current study, we found that blood-circulating miRNA levels differed significantly in PSCs and CCAs compared to healthy controls after microarray profiling. However, we detected a different profile of differentially expressed miRNAs from previous studies [23], and the discrepancy could be due to different population and race of the samples. Our current study identified nine differentially expressed miRNAs in both serum and bile of CCAs, and three of these nine miRNAs were dysregulated in both PSC and CCA samples and miR-150-5p was the only downregulated one. Thus, we speculated that miR-150-5p could take part in the development of PSC and CCA; thus, our further experiments were focused on this particular miRNA.
PSC, a chronic fibro-inflammatory syndrome involving the biliary tract, is the most significant risk factor in CCA development [24]. In our current study, qRT-PCR data further demonstrated the dynamic change in miR-150-5p expression from healthy controls to PSCs and CCAs, indicating that miR-150-5p could contribute to transition of chronic inflammation to CCA. Another previous study supported that miR-150 was downregulated in the systemic sclerosis and contributes to the constitutive type I collagen overexpression in scleroderma dermal fibroblasts [25]. Moreover, detection of increased CA19-9 level is clinically used in CCA diagnosis [26]. Our current data showed an inverse association of miR-150-5p with CA19-9 and CCA pathological grades. Our current data indicate that miR-150-5p could be further evaluated as a biomarker for CCA early detection and combination of miR-150-5p with CA19-9 could detect CCA earlier.
Thus, our in vitro experiments further investigated the effect of miR-150-5p on regulation of CCA cell proliferation, migration, and invasion capacity. We found that miR-150-5p overexpression inhibited tumor cell proliferation, wound healing, and Transwell invasion capacity, whereas knockdown of miR-150-5p expression induced tumor cell proliferation, migration, and invasion in vitro. However, previous studies showed that miR-150-5p could be an oncogene and tumor suppressor gene in different human cells [27, 28], whereas other studies demonstrated that miR-150-5p expression suppressed pancreatic, colorectal, lymphoma, and glioma cancer cell invasion and metastasis [29–32]. The high-throughput miRNA sequencing data showed that miR-150 was downregulated in gastric cancer and regulated epigenetically [33]. However, it remains to be determined how miR-150-5p functions in cells and what its targeting genes are. We therefore, searched TargetScan and Sanger miRNA databases [11] to predict the potential miR-150-5p-targeting genes and found that ELK1 is one of them. Our luciferase reporter assay confirmed that miR-150-5p binds to both sites of the ELK1 3′-UTR, and Western blot data confirmed miR-150-5p downregulation of the ELK1 protein level in CCA cells. As we know, ELK1 is a transcription activator and has important roles in various diseases, including Alzheimer’s disease and breast cancer [34]. Thus, lost miR-150-5p expression in CCA could upregulate ELK1 expression (lost function of miR-150-5p) and contribute to CCA development. However, further study is needed to confirm this speculation.
In addition, our current study performed KEGG and GO analyses to predict the gene pathways that are regulated by the three overlapping dysregulated miRNAs, and these gene pathways included proteoglycans in cancer, miRNAs in cancer, pathways in cancer, apoptosis, Wnt signaling pathway, and MAPK signaling pathway. However, it needs further investigation on how miR-150-5p and the other two dysregulated miRNAs regulate these gene and gene pathways in CCA cells. Moreover, among these dysregulated miRNAs, miR-150-5p was displayed as the only downregulated miRNA in both PSC and CCA; we further speculate that miR-150-5p was a key tumor suppressor in CCA by inhibition of cancer cell growth, invasion, and metastasis by targeting oncogene ELK1. This hypothesis is supported by a previous study showing that induced ELK1 expression decreased cell proliferation, migration, and invasion of hepatocellular carcinoma [35].
In conclusion, our current study profiled and identified differentially expressed miRNAs in serum or bile samples of PSC and CCA patients. miR-150-5p expression was downregulated in sera, bile, and CCA tissue specimens and reduced miR-150-5p was inversely associated with CA19-9 and pathological grades ex vivo. Overexpression of miR-150-5p suppressed CCA cell proliferation, migration, and invasion in vitro, whereas knockdown of miR-150 expression upregulated CCA cell proliferation, migration, and invasion. At the gene level, miR-150-5p binds to both sites of the ELK1 3′-UTR and inhibited CCA cells to express ELK1 protein. However, our current study is just a proof of principle and lots of more works are needed to fully disclose the function and role of miRNA alterations in CCA development and progression and then utilization of miR-150-5p as a novel target and predictor for CCA prevention and treatment.
References
Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–25. doi:10.1055/s-2004-828889.
Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366(9493):1303–14. doi:10.1016/S0140-6736(05)67530-7.
Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45(6):856–67. doi:10.1016/j.jhep.2006.09.001.
Rizvi S, Eaton JE, Gores GJ. Primary sclerosing cholangitis as a premalignant biliary tract disease: surveillance and management. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2015;13(12):2152–65. doi:10.1016/j.cgh.2015.05.035.
Charatcharoenwitthaya P, Lindor KD. Primary sclerosing cholangitis: diagnosis and management. Curr Gastroenterol Rep. 2006;8(1):75–82.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153(3):516–9. doi:10.1016/j.cell.2013.04.003.
Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev. 2014. doi:10.1016/j.addr.2014.09.001.
Silakit R, Loilome W, Yongvanit P, Chusorn P, Techasen A, Boonmars T, et al. Circulating miR-192 in liver fluke-associated cholangiocarcinoma patients: a prospective prognostic indicator. J Hepato-Biliary-Pancreat Sci. 2014;21(12):864–72. doi:10.1002/jhbp.145.
Li L, Masica D, Ishida M, Tomuleasa C, Umegaki S, Kalloo AN, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology. 2014;60(3):896–907. doi:10.1002/hep.27050.
Joung JG, Hwang KB, Nam JW, Kim SJ, Zhang BT. Discovery of microRNA-mRNA modules via population-based probabilistic learning. Bioinformatics. 2007;23(9):1141–7. doi:10.1093/bioinformatics/btm045.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. Gene Ontol Consortium Nat Genet. 2000;25(1):25–9. doi:10.1038/75556.
Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, et al. A systems biology approach for pathway level analysis. Genome Res. 2007;17(10):1537–45. doi:10.1101/gr.6202607.
An F, Gong B, Wang H, Yu D, Zhao G, Lin L, et al. miR-15b and miR-16 regulate TNF mediated hepatocyte apoptosis via BCL2 in acute liver failure. Apoptosis: Int J Program Cell Death. 2012;17(7):702–16. doi:10.1007/s10495-012-0704-7.
An F, Zhan Q, Xia M, Jiang L, Lu G, Huang M, et al. From moderately severe to severe hypertriglyceridemia induced acute pancreatitis: circulating MiRNAs play role as potential biomarkers. PLoS One. 2014;9(11):e111058. doi:10.1371/journal.pone.0111058.
Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145(3):521–36. doi:10.1053/j.gastro.2013.06.052.
Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29. doi:10.1053/j.gastro.2013.10.013.
Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10.
Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57(1):69–76. doi:10.1016/j.jhep.2012.02.022.
Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol. 2014;11(3):145–56. doi:10.1038/nrclinonc.2014.5.
Voigtlander T, Gupta SK, Thum S, Fendrich J, Manns MP, Lankisch TO, et al. MicroRNAs in serum and bile of patients with primary sclerosing cholangitis and/or cholangiocarcinoma. PLoS One. 2015;10(10):e0139305. doi:10.1371/journal.pone.0139305.
Bernuzzi F, Marabita F, Lleo A, Carbone M, Mirolo M, Marzioni M, et al. Serum micrornas as novel biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Clin Exp Immunol. 2016. doi:10.1111/cei.12776.
Letelier P, Riquelme I, Hernandez AH, Guzman N, Farias JG, Roa JC. Circulating microRNAs as biomarkers in biliary tract cancers. Int J Mol Sci. 2016;17(5):E791. doi:10.3390/ijms17050791.
Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54(5):1842–52. doi:10.1002/hep.24570.
Honda N, Jinnin M, Kira-Etoh T, Makino K, Kajihara I, Makino T, et al. miR-150 down-regulation contributes to the constitutive type I collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin beta3. Am J Pathol. 2013;182(1):206–16. doi:10.1016/j.ajpath.2012.09.023.
Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95(1):204–7. doi:10.1111/j.1572-0241.2000.01685.x.
Vasilatou D, Papageorgiou S, Pappa V, Papageorgiou E, Dervenoulas J. The role of microRNAs in normal and malignant hematopoiesis. Eur J Haematol. 2010;84(1):1–16. doi:10.1111/j.1600-0609.2009.01348.x.
Feng J, Yang Y, Zhang P, Wang F, Ma Y, Qin H, et al. miR-150 functions as a tumour suppressor in human colorectal cancer by targeting c-Myb. J Cell Mol Med. 2014;18(10):2125–34. doi:10.1111/jcmm.12398.
Srivastava SK, Bhardwaj A, Singh S, Arora S, Wang B, Grizzle WE, et al. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32(12):1832–9. doi:10.1093/carcin/bgr223.
Wang WH, Chen J, Zhao F, Zhang BR, Yu HS, Jin HY, et al. MiR-150-5p suppresses colorectal cancer cell migration and invasion through targeting MUC4. Asian Pac J Cancer Prev: APJCP. 2014;15(15):6269–73.
Ito M, Teshima K, Ikeda S, Kitadate A, Watanabe A, Nara M, et al. MicroRNA-150 inhibits tumor invasion and metastasis by targeting the chemokine receptor CCR6, in advanced cutaneous T-cell lymphoma. Blood. 2014;123(10):1499–511. doi:10.1182/blood-2013-09-527739.
Sakr M, Takino T, Sabit H, Nakada M, Li Z, Sato H. miR-150-5p and miR-133a suppress glioma cell proliferation and migration through targeting membrane-type-1 matrix metalloproteinase. Gene. 2016;587(2):155–62. doi:10.1016/j.gene.2016.04.058.
Assumpcao MB, Moreira FC, Hamoy IG, Magalhaes L, Vidal A, Pereira A, et al. High-throughput miRNA sequencing reveals a field effect in gastric cancer and suggests an epigenetic network mechanism. Bioinforma Biol Insights. 2015;9:111–7. doi:10.4137/BBI.S24066.
Chai Y, Chipitsyna G, Cui J, Liao B, Liu S, Aysola K, et al. c-Fos oncogene regulator Elk-1 interacts with BRCA1 splice variants BRCA1a/1b and enhances BRCA1a/1b-mediated growth suppression in breast cancer cells. Oncogene. 2001;20(11):1357–67. doi:10.1038/sj.onc.1204256.
Hsieh YH, TT W, Tsai JH, Huang CY, Hsieh YS, Liu JY. PKCalpha expression regulated by Elk-1 and MZF-1 in human HCC cells. Biochem Biophys Res Commun. 2006;339(1):217–25. doi:10.1016/j.bbrc.2005.11.015.
Acknowledgments
The authors would like to thank our staff in both Departments for their contributions to this study and Genminix Company for their technical assistance. This study was supported in part by grants from the National Natural Science Foundation of China (no. 81502038 to FA, no. 81302382 to YJ, and no. 31400720 to WW), the Natural Science Foundation of Jiangsu Province (no. BK2012098 to FA), and the Wuxi Municipal Science and Technology Development Planning Funds (no. CSZ00N1304 to FA).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study was approved by the Institutional Review Board (IRB) of Nanjing Medical University. All patients provided written informed consent before participation of this study.
Conflicts of interest
None
Additional information
Xiongbo Wu and Min Xia are authors contributed equally to this work.
Electronic supplementary material
Table S1
(Clinical characteristics of CCA patients. The table shows the relevant clinical information of CCA patients. The gender, age, tumor location, CA-19-9 and pathological grade of 13 CCA patients with tumor tissues and matched adjacent-tumor tissues were listed. DOCX 13 kb)
Fig. S1
(The Gene Ontology (GO) analyze of 3 differentially expressed miRNAs-regulated genes in body fluids of CCAs. The GO term predicted functions of genes regulated by these 3 miRNAs (P < 0.001). The vertical axis represented GOs and the horizontal axis is enrichment degree. Enrichment degree means the contribution of one miRNA to the surrounding GOs or the contribution one GO to the surrounding miRNAs. The key functions always have the higher enrichment degrees. The top 50 enrichment of GOs were showed. JPEG 434 kb)
Fig. S2
(The Kyoto Encyclopedia of Genes and Genomes (KEGG) analyze of 3 differentially expressed miRNAs-regulated pathways in body fluids of CCAs. KEGG predicted 29 pathways related to tumors regulated by these 3 miRNAs, (P < 0.001). The vertical axis is the pathway category and the horizontal axis is the P value of each pathway. The lower P value was, the more miRNAs regulated the pathways and the pathways had more important roles in CCA. JPEG 195 kb)
Fig. S3
(miRNA-mRNA network. The miRNAs target genes were predicted by TargetScan and Sanger and then miRNA–mRNA-network was generated. The blue circles represent genes, and the blue squares represent miRNAs. The lines indicate the relationship between genes and miRNA. The degree (size) of the blue square indicates the regulatory functionality of miRNA, i.e., the greater the degree, the more functions of the miRNA possess. The figure shows the relationship among these 3 overlapping miRNAs and their targeted genes, including tumor development and miR-150-5p targeted ELK1, Notch3, and MMP14. PNG 1689 kb)
.
Fig. S4
(Assay for the transfection efficiency. 150-5pM, NSM, 150-5pI, NSI were transiently transfected into HCCC-9810 and RBE cells respectively for 24 h and then subjected to qRT-PCR analysis of miR-150-5p expression. qRT-PCR was in triplicate and repeated three times. JPEG 590 kb)
Rights and permissions
About this article
Cite this article
Wu, X., Xia, M., Chen, D. et al. Profiling of downregulated blood-circulating miR-150-5p as a novel tumor marker for cholangiocarcinoma. Tumor Biol. 37, 15019–15029 (2016). https://doi.org/10.1007/s13277-016-5313-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5313-6