Abstract
The present study aims to evaluate whether repression of the Numb/Notch signaling pathway affects the radiosensitivity of human pancreatic cancer cell lines. Different doses of X-rays (0, 2, 3, 4, and 5 Gy) were applied to the PANC-1, SW1990, and MIA PaCa-2 human pancreatic cancer cell lines, and the Numb/Notch pathway inhibitor DAPT was added at different doses (0, 1, 3, and 5 μmol/l). MTT assay, colony formation assay, flow cytometry, scratch assay, and Transwell experiments were performed, and qRT-PCR and Western blot were conducted for the detection of Numb expression. Tumorigenicity assay in nude mice was carried out to verify the influence of blocker of the Numb/Notch signaling pathway on the radiosensitivity of xenograft tumors. The MTT assay, colony formation assay and flow cytometry experiments revealed that proliferation decreased as radiation dose increased. The viability of PANC-1 cells at 5 Gy, SW 1990 cells at 4 Gy and 5 Gy, and MIA PaCa-2 cells at 2–5 Gy was significantly lower than that of non-irradiated cells (all P < 0.05). The migration and invasion assays indicated that the PANC-1 cell line was least radiosensitive, while the MIA PaCa-2 cell line was the most radiosensitive. Numb expression significantly increased with increasing radiation dose, whereas the expression of Hes1, Notch1, and Hes5 significantly decreased compared to non-irradiated cells (P < 0.05). Compared to untreated control cells, DAPT dose dependently increased Numb expression and inhibited Notch1, Hes1, and Hes5 expressions at 2 Gy (P < 0.05). Subcutaneous tumorigenicity assay in nude mice demonstrated that DAPT increased the radiosensitivity of PANC-1, SW 1990, and MIA PaCa-2 cells. These findings suggest that Numb/Notch signaling in pancreatic cancer cells is associated with X-ray radiation and that inhibition of the Numb/Notch signaling pathway can enhance radiosensitivity, suggesting that inhibition of the Numb/Notch signaling pathway may serve as a potential target for clinical improvement of the radiosensitivity of pancreatic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is a malignant tumor with a poor prognosis and a relatively high mortality rate among digestive tract tumors, partly due to difficulties in its treatment and diagnosis [1, 2]. The causes of pancreatic cancer are not yet clear, though its occurrence has been related to many risk factors, such as smoking, obesity, chronic pancreatitis, family history, and diabetes [3]. As for treatment method, radiotherapy (primarily ionizing radiation) is an effective treatment widely used in the clinic for treatment of pancreatic cancer [4]. Radiotherapy delivers a high dose of radiation to the tumor mass while avoiding damage to normal tissues [5]. However, large doses of ionizing radiation are harmful to human health. Additionally, due to the deep anatomical location of the pancreas and the poor tolerance of the liver and surrounding bone marrow to radiation as well as insensitivity to radiotherapy, the efficacies of conventional radiotherapy are poor [6]. Therefore, the identification of a scientific and feasible approach to enhance the effect of radiotherapy and improve the prognosis has become an exciting area of research or pancreatic cancer treatment.
Notch and Numb have been identified as asymmetric cell division signaling proteins in multicellular organisms. The release of Numb inhibition on the Notch signaling pathway is closely related to tumorigenesis [7]. A recent study has found that the Notch signaling pathway is involved in cell proliferation and apoptosis and affects organ development and function [8]. High active Notch-1 expression in pancreatic cancer cells is closely related to their malignant proliferation [9, 10]. The asymmetric cell division determinant Numb plays a decisive role in multicellular organisms [11]. Numb expression is primarily dependent on pathway activation and radiation dose. Meanwhile, a previous study has shown that the primary function of Numb in tumors is to prevent tumor suppressor protein degradation [12]. To date, there has been little research on the effects of the Numb/Notch signaling pathway on human pancreatic cancer cell radiosensitivity. Therefore, we examined the expression of the Numb/Notch signaling pathway in pancreatic cancer and suggest a theoretical basis for further examination of the molecular mechanisms of pancreatic cancer and the identification of new molecular-targeted therapeutic targets.
Materials and methods
Cell culture
The PANC-1, SW 1990, and MIA PaCa-2 human pancreatic cancer cell lines (purchased from the Shanghai Institute of Cell Bank) were cultured as monolayers in DMEM medium (Gibco, USA) containing 10 % fetal bovine serum (HyClone Company, USA) in 5 % CO2 (Thermo, USA; model #: Thermo Scientific 8000) at 37 °C with 95 % humidity. Cells were allowed to reach 90 % confluence; then, they were washed twice with PBS and digested with 0.25 % trypsin (Gibco, USA). Trypsin was decanted after cell detachment was observed. A DMEM medium containing 10 % fetal bovine serum was added to the cells to make a single-cell suspension.
Ionizing radiation
Cells in the logarithmic growth phase were digested with trypsin and counted. Suspensions of the appropriate cell density were prepared and seeded into a suitable dish or culture plate for incubation for 12 h. A Varian 21 EX linear accelerator with high-energy radiation X-ray (6 MV) at a dose rate of 3 Gy/min was applied with different doses of radiation (0, 2, 3, 4, 5 Gy) to vertically irradiate cells. Cells were covered by a 1-cm plexiglass to allow for dose build-up. The irradiation distance was 100 cm, and the irradiation range was 10 cm × 10 cm. Cells were allowed to recover for 48 h in the incubator prior to subsequent experiments.
DAPT treatment
After the cells were cultured for 12 h using the above procedure, the Numb/Notch signaling pathway inhibitor DAPT was added to the cells at a final concentration of 0, 1, 3, or 5 μmol/l [13–15]. Cells were treated with 2 Gy radiation as described above. The cells were allowed to recover in the incubator and cultured for an additional 48 h for subsequent experiments.
Colony formation assay
Cells in the logarithmic growth phase were digested to create a cell suspension. Cells were diluted in a gradient dilution and were seeded in 10-cm cell culture dishes at a density of 1000 cells per dish. After incubation for 12 h, cells were irradiated or treated with DAPT. Experiments were performed in triplicate. Cells were cultured for 10 to 14 days. Once clones were visible, the cells were washed twice with PBS and fixed in 95 % methanol (5 ml) for 5 min. Cells were stained with a crystal violet solution for 10 min, followed by gently washing with flowing water. Cells were allowed to air dry, grid lines were made, and clones containing more than 50 cells were counted using the naked eye. The number of clones was calculated within each grid. The plating efficiency (PE) was calculated as follows: plating efficiency (PE) = (number of cloned cells/number of cells seeded) × 100 %; survival fraction (SF) = (PE in radiation group/PE in control group) × 100 %. The SF under a dose of 2 Gy (SF2) is an international and important parameter to measure radiosensitivity. Pancreatic cancer cell radiosensitivity differences were compared to the calculated SF2. Experiments were repeated three times and an average value was calculated.
MTT assay
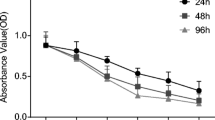
Cells were seeded into 96-well plates at a density of 3 × 105 cells/well in 200 μl of medium. After incubation for 12 h, cells were irradiated or treated with DAPT in triplicate. After 48 h, 5 mg/ml MTT solution (20 μl, Sigma, USA) were added to each well for 4 h. A volume of 150 μl DMSO (Amresco Company, US) was added to each well, which was gently shaken for 10 min to promote crystal dissolution. The absorbance (OD) value of each well was measured with an ELISA reader (OD490). The MTT curve was generated by setting the absorbance value as the vertical axisand the interval as the horizontal axis. The procedure was repeated three times.
Apoptosis analysis
Cells were seeded into 6-well plates at a density of 3 × 103 cells/well. After incubation for 12 h, cells were irradiated or treated with DAPT in triplicate. After 48 h, cells were collected by centrifugation and washed twice with PBS. Cells were added to 200 μl Binding Buffer and shaken. Then 2.5 μl of Annexin V-FITC were added to the cell suspension. The reaction was performed in the dark at room temperature for 10 to 15 min. A volume of 5 μl PI was added to the cells, and flow cytometry (MACSQuant TM Analyzer, Miltenyi Biotec) was immediately performed to detect apoptosis. The procedure was repeated three times.
Scratch assay
Horizontal lines were evenly drawn at an interval of 0.5–1 cm on the back of 6-well plates using markers. Cells (3 × 103) were seeded into 6-well plates and incubated overnight. When the cell density was between 80 and 90 % the next day, pipette tips perpendicular to the horizontal lines were used to scratch the plates. Cells were subsequently irradiated or treated with DAPT. After 48 h, eight fields with a scratch were randomly selected in each well. Cell migration across the scratch was analyzed, and samples and photos were acquired. Motic Images Advanced (version 3.2) was used to calculate the relative scratch width (the percentage relative to the 0-h scratch width), which reflected the cell migration. Each experiment was repeated at least three times.
Transwell experiments
Matrigel was thawed overnight at 4 °C and diluted 1:3 in serum-free DMEM medium. Matrigel (30 μl) was aliquoted three times (15, 7.5, and 7.5 μl) onto the upper chamber of a transwell membrane. Each aliquot was spread evenly onto the bottom surface of the upper chamber to cover the micropores and incubated for 10 min before the next layer was added. The cell suspension was inoculated onto the upper chamber at a density of 3 × 104 cells per pore. A DMEM medium (0.5 ml) containing 10 % fetal bovine serum was added into the lower chamber of a 24-well plate. Cells were irradiated or treated with DAPT after 12 h. After 48 h, the number of cells that had migrated through the Matrigel was quantified as a measure of their invasiveness.
Xenograft tumor in nude mice
Four- to six-week-old Balb/c male nude mice (weighing 18–22 g) were purchased from Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China. Tumor cell suspension at concentration of 4 × 106 were prepared with human pancreatic cancer cell lines PANC-1, SW 1990, and MIA PaCa-2, and a 0.2 ml tumor cell suspension was injected into the dorsum of nude mice to establish a xenograft model. Tumor growth was observed each week and tumor volume was measured by a vernier caliper and calculated following the formula: V = π/6 × L × W × H, where L, W and H, respectively, stand for the length, width, and height of the xenograft tumor. One week after the inoculation of tumor cells, tumors initiated to grow in the dorsum of nude mice. When tumors grew to about 5 mm after 2 weeks, all mice were randomly divided into two groups: DAPT treatment group and control group, with five mice in each group. The DAPT treatment group was treated with an intraperitoneal injection of DAPT, while the control group was given an intraperitoneal injection of normal saline. Each 5 mg of DAPT was fully dissolved with 1 ml DMSO + 19 ml dd H2O, and nude mice were intraperitoneally injected with 100 mg/kg DAPT each time [16]. The two groups were injected once every other day. The tumors in the dorsum of nude mice in each group were locally irradiated with electron beam at 16 Gy once every 7 days and a total of four times. After 4 weeks of intervention, the mice were sacrificed and the xenografts were taken out, weighed, and photographed.
Real-time quantitative polymerase chain reaction assay for Numb, Notch1, Hes1, and Hes5 mRNA levels
Total RNA was extracted from cells by the Trizol method (Invitrogen, USA). Ultrapure diethylpyrocarbonate (DEPC) water was used to dissolve RNA. The OD260/280 value of each RNA extraction was examined using an ND-1000 UV/VIS spectrophotometer (Nanodrop Company, USA), and the amount of total RNA was calculated. RNA concentration was adjusted for qRT-PCR. Reverse transcription was performed according to the kit manufacturer’s instructions (Fermentas, USA). The primer sequences are listed in Table 1. PCR reaction conditions were as follows: 70 °C (10 min), ice bath (2 min), 42 °C (60 min), and 70 °C (10 min). The complementary DNA (cDNA) obtained by reverse transcription was stored at −80 °C. The TaqMan probe method was used for qRT-PCR, and the reaction system was performed according to the manufacturer’s instructions (Fermentas, USA). The PCR reaction conditions were as follows: initial denaturation at 95 °C for 30 s followed by denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s, and extension at 70 °C for 10 s for a total of 40 cycles. A real-time quantitative PCR instrument (Bio-Rad, USA, Bio-Rad iQ5) was used to detect the reaction. β-actin served as an internal control, and relative quantification was used. The relative expression of target genes was calculated using 2-ΔΔCt and each experiment was repeated three times.
Western blot for Numb, Notch1, Hes1 and Hes5 protein expressions
Cultured cells were rinsed in cold PBS buffer three times, and the protein extraction buffer was added (100 μl/50 ml culture flasks) to lyse the cells. The cells were placed on ice for 30 min and centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant was aliquoted into 0.5-ml centrifuge tubes and stored at −20 °C. A 2 μg/μl BSA protein standard was serially diluted in PBS to a final concentration of 20, 15, 10, 5, 2.5, and 0 μg/μl. After the amount of BCA reagents (Thermo, USA) was calculated according to the number of samples, BCA reagents were prepared (volume ratio of liquid A to B solution = 50: 1). A total of 2 μl lysed protein sample was diluted in 18 μl double distilled water. Each sample was added to two wells. After 200 μl detection liquid was added to each well of a 96-well plate, the sample and the standard were added into each well for detection. The dose of each well was 10 μl. The mixture was gently shaken, incubated at 37 °C for 30 min, then cooled to room temperature. OD values of samples at 490 nm were tested using a microplate reader. A standard curve was generated to calculate the protein concentration of each sample. Proteins were separated at 60 V at the beginning of electrophoresis and 120 V through the resolving gel for 1–2 h at 4 °C. After electrophoresis, PVDF transfer membrane (wet transfer method) was used. The transfer time was 2 h, and the transfer was performed in a cold room at 4 °C. After transfer, the PVDF membrane was blocked with 5 % nonfat milk in TBST and incubated at room temperature for 1–2 h. Primary antibodies against Numb, Notch1, Hes1, and Hes5 (CST, USA) were added and the membrane was incubated at 4 °C overnight. The membrane was washed three times with TBST for 10 min each. Mouse secondary antibodies were added (Abcam, UK), and the membrane was incubated for 1 h at a room temperature. The membrane was washed three times with TBST for 10 min each. Blots were developed using chemiluminescence.
Statistical analysis
All data analysis was performed using SPSS (version 20.0, IBM) and measurement data were presented as the mean ± standard deviation (SD). Multiple comparisons among groups were examined using variance analysis, while comparisons between two groups were examined using an LSD-t test. A P value less than 0.05 was considered statistically significant.
Results
Effects of ionizing radiation on cell proliferation and apoptosis
The MTT assays (Fig. 1) revealed that the OD values of PANC-1 cells did not significantly change after exposure to 2, 3, or 4 Gy for 48 h compared to non-irradiated cells (0 Gy dose) (P > 0.05). However, there was a statistically significant difference in OD values after treatment with 5 Gy (P < 0.05). The OD values of SW1990 cells at 2 and 3 Gy were not significantly different than non-irradiated cells (P > 0.05), while the differences in OD values under 4 and 5 Gy were statistically significant (P < 0.05). The OD values of MIA PaCa-2 cells under 2, 3, 4, and 5 Gy were significantly lower than non-irradiated cells (P < 0.05).
Colony formation assay (Fig. 2) showed that the colony-forming efficiencies of PANC-1 cells treated with 2, 3, or 4 Gy for 48 h were not significantly different than that of non-irradiated cells (P > 0.05), but there was a significant difference in colony-forming efficiency when the cells were treated with 5 Gy (P < 0.05). The colony-forming efficiencies of SW1990 cells at 2 and 3 Gy were not significantly different than that of non-irradiated cells (P > 0.05), while they were significant after treatment with 4 or 5 Gy irradiation (P < 0.05). Colony-forming efficiencies of MIA PaCa-2 cells at 2, 3, 4, and 5 Gy were significantly lower than those of non-irradiated cells (P < 0.05). The survival fractions (SF2) of PANC-1, SW 1990 and MIA PaCa-2 cells were 43.63 %, 39.89 % and 31.01 %, respectively. Our results further confirmed that the PANC-1 line was least radiosensitive, while the MIA PaCa-2 line was the most radiosensitive.
Colony formation of pancreatic cancer cell lines PANC-1, SW 1990, and MIA PaCa-2 after different doses of X-ray irradiation. Survival fractions after different doses of X-ray irradiation detected by colony formation assay. a Pictorial diagrams of colony-forming of PANC-1, SW 1990, and MIA PaCa-2 cells after different doses of X-ray irradiation. b Histograms of survival fractions of PANC-1, SW 1990, and MIA PaCa-2 cells after different doses of X-ray irradiation; *P < 0.05 compared to non-irradiated cells
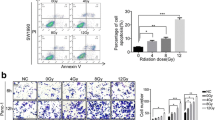
Apoptosis analysis (Fig. 3) revealed that PANC-1 cell apoptosis was not significantly different than non-irradiated cells after exposure to 2, 3, or 4 Gy for 48 h (P > 0.05), although the apoptosis rates were significantly different after 5 Gy irradiation (P < 0.05). The overall apoptosis rates of SW1990 cells under 2 and 3 Gy were not significantly different than non-irradiated cells (P > 0.05), while the apoptosis rates at 4 and 5 Gy were significantly different (P < 0.05). The overall apoptosis rates of MIA PaCa-2 cells after 2, 3, 4, and 5 Gy treatment were significantly higher than non-irradiated cells (P < 0.05). These results were consistent with the colony formation assay results, which suggested that the PANC-1 was the least radiosensitive, while the MIA PaCa-2 was the most radiosensitive.
Effects of different doses of X-ray on pancreatic cancer PANC-1, SW 1990, and MIA PaCa-2 cell apoptosis. a Apoptosis maps of PANC-1, SW 1990, and MIA PaCa-2 cells after different doses of X-ray irradiation detected by flow cytometry. b A bar graph of the overall apoptosis rates of PANC-1, SW 1990, and MIA PaCa-2 cells at different doses; *P < 0.05 compared to non-irradiated cells
Effects of ionizing radiation on cell invasion and migration
Scratch assays (Fig. 4) revealed that the relative scratch widths (percentage relative to the 0-h scratch width) of PANC-1 cells after 48 h 0–5 Gy were 29.58 ± 2.34, 30.37 ± 3.65, 31.28 ± 3.63, 43.54 ± 5.64, and 54.86 ± 5.36, respectively, and after 4 and 5 Gy exposure, cell migration significantly decreased compared to non-irradiated cells (all P < 0.05). The relative scratch widths of SW1990 cells were 28.56 ± 2.51, 30.01 ± 3.43, 35.26 ± 1.63, 43.53 ± 5.64, and 55.28 ± 6.35, respectively, and after 3, 4, and 5 Gy exposure, cell migration significantly decreased compared to non-irradiated cells (all P < 0.05). The relative scratch widths of MIA PaCa-2 cells were 26.58 ± 3.36, 37.37 ± 4.62, 47.26 ± 4.63, 53.53 ± 5.45, and 62.86 ± 6.36, respectively, and after 2, 3, 4, and 5 Gy exposure, cell migration significantly decreased compared to non-irradiated cells (all P < 0.05).
Effects of ionizing radiation on cell migration of PANC-1, SW 1990, and MIA PaCa-2 cells detected by the scratch assay. a Scratch maps of PANC-1, SW 1990, and MIA PaCa-2 cells at different radiation doses. b A bar graph of the relative scratch widths of PANC-1, SW 1990, and MIA PaCa-2 cells at different radiation doses; *P < 0.05 compared to non-irradiated cells
Transwell experiments (Fig. 5) showed that exposure to 0–5 Gy radiation for 48 h decreased the number of invaded PANC-1 cells compared to non-irradiated cells and that the differences after 4 and 5 Gy exposure were statistically significant (P < 0.05). The number of invaded SW 1990 cells after 3, 4, and 5 Gy exposure significantly decreased compared to non-irradiated cells (P < 0.05). The number of invaded MIA PaCa-2 cells after 2, 3, 4, and 5 Gy exposure significantly decreased compared to non-irradiated cells (all P < 0.05).
Effects of ionizing radiation on cell invasion detected by transwell experiments. a Diagrams of PANC-1, SW 1990, and MIA PaCa-2 cells at different doses passing through chambers. b A bar graph of the number of PANC-1, SW 1990, and MIA PaCa-2 cells at different doses passing through the chambers; *P < 0.05 compared to non-irradiated cells
Effects of ionizing radiation and DAPT on the Numb/Notch signaling pathway
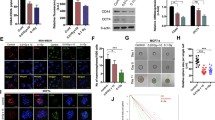
We performed Western blotting and qRT-PCR to evaluate the expression of the Numb/Notch signaling pathway members Numb, Notch1, Hes1, and Hes5 after ionizing radiation and treatment with the Numb/Notch inhibitor DAPT. Compared to non-irradiated cells, Numb expression significantly increased in irradiated cells, while Notch1, Hes1, and Hes5 expression significantly decreased. The changes were more obvious with increasing radiation dose (P < 0.05) (Fig. 6). The expression of Notch1, Hes1, and Hes5 was the highest in PANC-1 cells, while Numb expression was the lowest. In contrast, the expression of Notch1, Hes1, and Hes5 was the lowest and Numb expression was the highest in MIA PaCa-2 cells. These results suggest that Notch signaling pathway activation in pancreatic cancer cells and radiation dose was correlated with sensitivity of pancreatic cancer cells to radiation. We treated cells with various doses of DAPT after 2 Gy radiation treatment. Compared to irradiated cells not treated with DAPT (0 μmol/l DAPT), DAPT-treated cells had evidently increased Numb expression and obviously decreased expression of Notch1, Hes1, and Hes5 in a dose-dependent manner (Fig. 7).
Effects of DAPT on cell proliferation and apoptosis under radiotherapy
MTT assays (Fig. 8a) showed that the OD values of the cells decreased as the concentration of DAPT increased, and the differences in OD values between treated and untreated PANC-1 and SW 1990 cells were statistically significant (P < 0.05).
Effects of different doses of DAPT on pancreatic cancer cell proliferation and apoptosis. a line chart of pancreatic cancer PANC-1, SW 1990, and MIA PaCa-2 cell growth after DAPT treatment detected by MTT assay. b Survival fractions of pancreatic cancer cells after DAPT treatment at different doses detected by clonogenic assay at the radiation dose of 2 Gy. c The overall apoptosis rates of pancreatic cancer cells after DAPT treatment at different doses etected by flow cytometry at the radiation dose of 2 Gy; *P < 0.05 compared to cells not treated with DAPT
Colony formation assay (Fig. 8b) showed that at the same radiation dose (2 Gy), the colony-forming efficiencies of PANC-1, MIA PaCa-2, and SW 1990 cells decreased as DAPT concentration increased. PANC-1 and SW 1990 cells showed significant differences in colony-forming efficiencies between treated and untreated cells (P < 0.05). At a DAPT concentration of 5 μmol/l, the SF2 of PANC-1, SW 1990, and MIA PaCa-2 cells was (23.66 ± 4.38)%, (13.57 ± 2.80)%, and (25.25 ± 2.45)%, respectively. Compared to cells not treated with DAPT, the SF2 of these cells was significantly different (P < 0.05).
Apoptosis analysis (Fig. 8c) revealed that at the same radiation dose (2 Gy), the overall apoptosis rates of the three cell lines increased as DAPT concentration increased. Relatively low radiosensitive PANC-1 and SW 1990 cells showed significant differences in overall apoptosis rates between treated and untreated cells (P < 0.05). The above results strongly suggest that the Numb/Notch signal inhibitor DAPT can improve pancreatic cancer cell sensitivity to radiation.
Effects of DAPT on cell migration and invasion under radiotherapy
Scratch experiments (Fig. 9a) showed that the relative scratch widths increased as DAPT concentration increased. The less adiosensitive PANC-1 and SW 1990 cell lines showed significant differences in relative scratch widths between treated and untreated cells (P < 0.05).
Effects of DAPT on the migration and invasion of pancreatic cancer cells. a Relative scratch widths of pancreatic cancer PANC-1, SW 1990, and MIA PaCa-2 cells after DAPT treatment assessed by scratch assay at the radiation dose of 2 Gy. b The numbers of cells passing through chambers after DAPT treatment detected by Transwell experiments at the radiation dose of 2 Gy; *P < 0.05 compared to cells not treated with DAPT
We used Transwell chambers plated with Matrigel to detect the invasiveness of the pancreatic cancer cell lines treated or untreated with different concentrations of DAPT after 2 Gy irradiation. The results (Fig. 9b) showed that compared to the DAPT-untreated group, 1, 3, or 5 μmol/l DAPT effectively inhibited the invasion of the cells. The less radiosensitive PANC-1 and SW 1990 cell lines showed significant differences in the numbers of cells passing through chambers between treated and untreated cells (P < 0.05).
Effects of inhibition of the Numb/Notch signaling pathway on radiosensitivity of xenografts in nude mice
Tumors grew in all nude mice and the tumorigenic rate was 100 %. After DAPT treatment, subcutaneous xenografts of negative control group grew rapidly, and with the tumor growth, the activity of nude mice decreased, weight slightly dropped, appetite became bad, and reaction delayed. Compared to the control group, subcutaneous xenografts of the DAPT treatment group significantly slowed down, the nude mice moved freely and reacted quickly, and all nude mice survived in the process of experiment. The tumor growth curve of nude mice injected with PANC-1, SW 1990, and MIA PaCa-2 cells showed that the subcutaneous xenograft growth of the DAPT treatment group was remarkably slower than the control group at the same radiation dose (P < 0.05) (Fig. 10). After the experiment finished, all mice were sacrificed and the xenografts were completely separated and then weighed and photographed. The results demonstrated that tumor weight of the control group injected with PANC-1, SW 1990, and MIA PaCa-2 cells was higher than the DAPT treatment group (P < 0.05).
Effects of DAPT on the radiosensitivity of xenografts in nude mice. a The comparison of subcutaneous tumor growth curve and tumor weight in pancreatic cancer PANC-1 cell among all groups. b The comparison of subcutaneous tumor growth curve and tumor weight in pancreatic cancer SW 1990 cell among all groups. c The comparison of subcutaneous tumor growth curve and tumor weight in pancreatic cancer MIA PaCa cell among all groups
Discussion
In the present study, we found that ionizing radiation is inversely related to proliferation activity, migration, and invasion of the PANC-1, SW 1990, and MIA PaCa-2 human pancreatic cancer cell lines. Ionizing radiation, which is widely used in radiation treatment, induces tumor cell death through several signaling pathways [17]. Radiation ionizes atoms, which is an important method for the diagnosis and treatment of diseases [18]. A previous study has shown that the survival fraction of PANC-1 and SW1990 cells irradiated with 125I seeds decreased as dose increased [19]. DNA breakage occurs in the vast majority of cancer cells after exposure to a lethal dose of ionizing radiation, and the proliferation of damaged cells accordingly decreased [20], which is consistent with the results of this study. A study has shown that different human pancreatic cancer cell lines, such as PANC-1, SUIT-2, and MIA PaCa-2, differentially influenced pancreatic cancer radiosensitivity [21]. The study suggests that PANC-1 cells were less radiosensitive than MIA PaCa-2 cells.
This study also found that the Numb/Notch signaling pathway inhibitor (DAPT) increased Numb expression; decreased the expression of Notch1, Hes1, and Hes5; and increased pancreatic cell radiosensitivity. The Notch signaling pathway is composed of three parts—the Notch receptors (Notch1–4), Notch ligands (Jagged-1, 2 and Delta-1, 3, 4) and CSL DNA binding protein [22]. Recent studies have found that the Notch-1 signaling pathway promotes the proliferation and migration of some tumor cells [23, 24]. Therefore, modulation of Notch-1 activity may be a potential cancer therapy target. At the same time, a study has reported that the Notch-1 signaling pathway is highly expressed in pancreatic cancer cells and is closely related to the malignant proliferation of pancreatic cancer cells [25], which is consistent with the results of our study. DAPT, a γ-secretase inhibitor, blocks Notch signaling by inhibiting Notch receptor dissociation [26]. The Notch1 receptor is an important component of the Notch signaling pathway and is abnormally highly expressed in tumors [27]. Hes1 and Hes5 are downstream Notch signaling pathway target genes, and their expression can affect cell proliferation and differentiation [28]. A study of the SHG-44 human glioma cell line revealed that DAPT treatment significantly decreased Hes1 and Notch-1 gene expression compared to the simple induction group and decreased the number of cancer cells [29], suggesting that DAPT inhibits radiotherapy. This is consistent with the results of this study. Therefore, it is plausible to conclude that DAPT can inhibit the Numb/Notch signaling pathway by upregulating Numb expression and downregulating Notch1 expression through inhibiting Notch receptor dissociation, and subsequently downregulating Hes1 and Hes5 which are downstream Notch signaling pathway target genes, resulting in a decrease in cell proliferation and differentiation and a corresponding increase in radiosensitivity.
Furthermore, we constructed a xenograft tumor model in nude mice to verify our results, which demonstrated that compared to the control group, subcutaneous xenografts of the DAPT treatment group significantly slowed down, the nude mice moved freely and reacted quickly, and all nude mice survived in the process of experiment. The tumor growth curve of nude mice injected with PANC-1, SW 1990, and MIA PaCa-2 cells showed that the subcutaneous xenograft growth of the DAPT treatment group was remarkably slower than the control group at the same radiation dose. Additionally, tumor weight of the control group injected with PANC-1, SW 1990, and MIA PaCa-2 cells was higher than the DAPT treatment group. The above results suggest that DAPT treatment enhances the radiosensitivity of PANC-1, SW 1990, and MIA PaCa-2 cells in nude mice.
In summary, Numb/Notch signaling pathway activation and the dose of X-ray radiation are related to pancreatic cancer cell sensitivity to radiation. Suppression of the Numb/Notch signaling pathway increases the radiosensitivity of pancreatic cancer cell lines, suggesting that inhibition of the Numb/Notch signaling pathway may serve as a potential target for clinical improvement of the radiosensitivity of pancreatic cancer. However, there are still some limitations to this study. We only discussed the relevance of the Numb/Notch signaling pathway and radiotherapy in the context of pancreatic cancer. Future studies should explore the relevant mechanisms to more fully understand the molecular mechanisms underlying pancreatic cancer to identify novel targets for the treatment of pancreatic cancer.
References
Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7(3):163–72.
Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 2013;6(4):321–37.
Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378(9791):607–20.
Loehrer Sr PJ, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29(31):4105–12.
Torres-Roca JF, Stevens CW. Predicting response to clinical radiotherapy: past, present, and future directions. Cancer Control. 2008;15(2):151–6.
Guan HT, Xue XH, Dai ZJ, et al. Down-regulation of survivin expression by small interfering RNA induces pancreatic cancer cell apoptosis and enhances its radiosensitivity. World J Gastroenterol. 2006;12(18):2901–7.
Rebeiz M, Miller SW, Posakony JW. Notch regulates numb: integration of conditional and autonomous cell fate specification. Development. 2011;138(2):215–25.
Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci. 2009;66(10):1631–46.
Ma J et al. Notch signaling pathway in pancreatic cancer progression. Pancreat Disord Ther. 2013;3(114).
Bao B, Wang Z, Ali S, et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307(1):26–36.
Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132(4):583–97.
Ding X, Zhu F, Li T, et al. Numb protects renal proximal tubular cells from puromycin aminonucleoside-induced apoptosis through inhibiting Notch signaling pathway. Int J Biol Sci. 2011;7(3):269–78.
Guo Z, Jin X, Jia H. Inhibition of ADAM-17 more effectively down-regulates the Notch pathway than that of gamma-secretase in renal carcinoma. J Exp Clin Cancer Res. 2013;32:26.
Su F, Zhu S, Ruan J, et al. Combination therapy of RY10-4 with the gamma-secretase inhibitor DAPT shows promise in treating HER2-amplified breast cancer. Oncotarget. 2016;7(4):4142–54.
Huang Y, Yang X, Wu Y, et al. Gamma-secretase inhibitor induces adipogenesis of adipose-derived stem cells by regulation of Notch and PPAR-gamma. Cell Prolif. 2010;43(2):147–56.
Hallahan AR, Pritchard JI, Hansen S, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64(21):7794–800.
Baskar R, Lee KA, Yeo R, et al. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193–9.
Connell PP, Hellman S. Advances in radiotherapy and implications for the next century: a historical perspective. Cancer Res. 2009;69(2):383–92.
Wang ZM, Lu J, Zhang LY, et al. Biological effects of low-dose-rate irradiation of pancreatic carcinoma cells in vitro using 125I seeds. World J Gastroenterol. 2015;21(8):2336–42.
Vignard J, Mirey G, Salles B. Ionizing-radiation induced DNA double-strand breaks: a direct and indirect lighting up. Radiother Oncol. 2013;108(3):362–9.
Kozono S, Ohuchida K, Ohtsuka T, et al. S100A4 mRNA expression level is a predictor of radioresistance of pancreatic cancer cells. Oncol Rep. 2013;30(4):1601–8.
Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–6.
Wang Z, Li Y, Banerjee S, et al. Emerging role of Notch in stem cells and cancer. Cancer Lett. 2009;279(1):8–12.
Reedijk M. Notch signaling and breast cancer. Adv Exp Med Biol. 2012;727:241–57.
Ma YC, Shi C, Zhang YN, et al. The tyrosine kinase c-Src directly mediates growth factor-induced Notch-1 and Furin interaction and Notch-1 activation in pancreatic cancer cells. PLoS One. 2012;7(3):e33414.
Crawford TQ, Roelink H. The notch response inhibitor DAPT enhances neuronal differentiation in embryonic stem cell-derived embryoid bodies independently of sonic hedgehog signaling. Dev Dyn. 2007;236(3):886–92.
Purow BW, Haque RM, Noel MW, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65(6):2353–63.
Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134(7):1243–51.
Liu X, Xu QR, Xie WF, et al. DAPT suppresses the proliferation of human glioma cell line SHG-44. Asian Pac J Trop Med. 2014;7(7):552–6.
Acknowledgments
This study was supported by a grant from the Beijing Municipal Science and Technology Commission (NO. Z151100004015213). We acknowledge the helpful comments on this paper received from our reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Yi-Liang Bi and Min Min contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bi, YL., Min, M., Shen, W. et al. Numb/Notch signaling pathway modulation enhances human pancreatic cancer cell radiosensitivity. Tumor Biol. 37, 15145–15155 (2016). https://doi.org/10.1007/s13277-016-5311-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5311-8