Abstract
Long non-coding RNA TUG1 is involved in the development and progression of a variety of tumors. Little is known about TUG1 function in high-grade muscle-invasive bladder cancer (MIBC). The aims of our study were to determine expression levels of long non-coding RNA TUG1 in tumor tissue, to evaluate its relationship with clinico-pathological features of high-grade MIBC, and to describe its function in MIBC cells in vitro. TUG1 expression levels were determined in paired tumor and adjacent non-tumor bladder tissues of 47 patients with high-grade MIBC using real-time PCR. Cell line T-24 and siRNA silencing were used to study the TUG1 function in vitro. We observed significantly increased levels of TUG1 in tumor tissue in comparison to adjacent non-tumor bladder tissue (P < 0.0001). TUG1 levels were significantly increased in metastatic tumors (P = 0.0147) and were associated with shorter overall survival of MIBC patients (P = 0.0241). TUG1 silencing in vitro led to 34 % decrease in cancer cell proliferation (P = 0.0004) and 23 % reduction in migration capacity of cancer cells (P < 0.0001). We did not observe any significant effects of TUG1 silencing on cell cycle distribution and number of apoptotic cells. Our study confirmed overexpression of TUG1 in MIBC tumor tissue and described its association with worse overall survival in high-grade MIBC patients. Together with in vitro observations, these data suggest an oncogenic role of TUG1 and its potential usage as biomarker or therapeutic target in MIBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is one of the most common urologic malignancies, with approximately 429,800 new cases and 165,100 deaths in 2012 worldwide [1, 2]. The incidence of bladder cancer rises with age, and prognosis of muscle-invasive bladder cancer (MIBC) remains poor despite therapeutic improvements in its clinical management [3, 4]. Therefore, uncovering specific molecular mechanisms of MIBC and development of novel therapeutic targets and molecular biomarkers, that can predict progression, metastasis, and prognosis of the disease, remain a challenge.
Long non-coding RNAs (lncRNAs) refer to a class of endogenous RNAs that range from 200 nucleotides to ~100 kilobases (kb) and lack significant open reading frames. Expression levels of lncRNAs appear to be lower than protein-coding genes, and some lncRNAs are preferentially expressed in specific tissues [5]. Increasing evidence has confirmed that lncRNAs are closely related to development and progression of human cancers [5, 6]. Alterations in the expression or structure of lncRNAs may promote tumor formation, progression, and metastasis [7], whereas some lncRNAs act as tumor suppressors (such as GAS5 [8], MEG3 [9]), while others serve as oncogenes, such as MALAT1 [10] and HOTAIR [11]. Over the past several years, lncRNAs have been implicated as promising therapeutic targets and biomarkers for early detection of tumor or prognosis prediction. As an example, PCA3 is an approved diagnostic biomarker for prostate cancer prior to biopsy [12].
LncRNA taurine upregulated gene 1 (TUG1; also known as TI-227H; Linc00080; ncRNA00080) was initially detected in a genomic screen for genes upregulated in response to taurine treatment in developing mouse retinal cells [13]. However, more recent findings suggest that TUG1 could also participate in the development and progression of a variety of tumors [13–21]. Expression levels of TUG1 were found to be deregulated in non-small cell lung cancer [14], colorectal cancer [15], esophageal squamous cell carcinoma [16], gastric cancer [17], hepatocellular carcinoma [18], osteosarcoma [19], and bladder cancer [20, 21]. Little is known about TUG1 which acts as a prognostic biomarker in high-grade MIBC and its influence on migration capacity of bladder cancer cells.
In the present study, we determined the expression levels of TUG1 in tumor and paired normal bladder tissue and evaluated their association with metastasis and overall survival in high-grade MIBC patients. In addition, we investigated how TUG1 silencing affects proliferation, migration, cell cycle, and apoptosis of bladder cancer cells in vitro.
Methods
Study population
Forty-seven patients with high-grade (G3) MIBC who underwent partial or radical cystectomy at the Masaryk Memorial Cancer Institute (Brno, Czech Republic) between 2008 and 2012 were included in the study. Histological diagnosis was established according to the guidelines of the World Health Organization. Informed consent, approved by the local Ethics Board (Masaryk Memorial Cancer Institute, Czech Republic), was obtained from each patient. All subjects were of the same ethnicity (Caucasian). Cases were selected according to tissue availability and were not stratified for any known preoperative or pathological prognostic factor. Clinical follow-up data in the form of annually assessed survival time were available for all patients. Other baseline characteristics of the patients are presented in Table 1.
RNA isolation and real-time PCR
RNA was isolated using mirVana™ miRNA Isolation Kit (Ambion, TX, USA) according to manufacturer’s recommendations. Concentration and purity of RNA were determined spectrophotometrically by measuring its optical density (A260/280 > 2.0; A260/230 > 1.8) using a NanoDrop ND-1000 (Thermo Scientific, Wilmington, DE, USA). Reverse transcription of 1 μg of total RNA was carried out in total volume of 20 μl using the High-Capacity cDNA Reverse Transcription Kit (Life technologies, Carlsbad, USA) according to manufacturer’s recommendations. Real-time PCR was performed in total volume of 20 μl (1 μl of cDNA) using TaqMan® Gene Expression Master Mix and TaqMan assay for TUG1 (Hs00215501_m1). RNU48 was used as reference gene for relative quantification of TUG1. We used TaqMan® MicroRNA Reverse Transcription (Life technologies, Carlsbad, USA) for cDNA synthesis of RNU48 from 6.7 ng of total RNA, and TaqMan Universal Master Mix II, no UNG (Life technologies, Carlsbad, USA), 1.33 μl of cDNA and TaqMan Assay for RNA, C/D Box 48 (RNU48) (ID:001,006) (Life technologies, Carlsbad, USA) for real-time PCR.
Cell line cultivation and TUG1 silencing
For in vitro experiments, bladder cancer cell line T-24 (ATCC, USA) was used. Cells were grown in McCoy’s 5A medium supplemented with 10 % of fetal bovine serum, 1 % streptomycin/penicillin, and GlutaMAX™ (ThermoFischer Scientific, MA, USA). Cells were cultivated at 37 °C in humidified atmosphere containing 5 % CO2. For TUG1 and control silencing, cells was transfected with specific siRNA (siRNA ID: 4390771) or Silencer® Negative Control No. 1 siRNA (AM4611) (both Life technologies, Carlsbad, USA) using Lipofectamine RNAiMAX transfection reagent according to manufacturer’s instructions (Invitrogen, Carlsbad, USA).
MTT proliferation assay
Proliferation of T-24 cell line was analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, USA) proliferation assay. Cells were seeded on 24-well plates and incubated overnight, then transfected with TUG1 siRNA and negative control Silencer® Negative Control, respectively. After 24 h of incubation, the medium was changed with 500 μl of fresh medium supplemented with 10 % FBS and antibiotics. Proliferation of cells was evaluated 24, 48, 72, and 96 h after transfection. Plates were read on Fluostar Omega (BMG LABTECH, Ortenberg, Germany) reader at a wavelength of 570 nm.
Each measurement was performed in three replicates, and all experiments were repeated three times.
Scratch wound assay
The cell monolayer was wounded with a sterile pipette tip 24 h after transfection and then rinsed twice with PBS to remove cellular debris. Subsequently, warm fresh medium was added. Migration was measured at time 0 and 24 h after wounding using microscope Nikon Diaphod 300 INV (109) and camera Canon Power shot A95. Images were analyzed by the Tscratch software (CSE, Switzerland). All measurements were repeated three times.
Cell cycle distribution and analysis of apoptosis
Cells were cultivated on 60-mm dishes. The distribution of cells in specific cell cycle stages was evaluated by assessment of DNA content by flow cytometry 48 h post-transfection/radiation. Cells were stained with propidium iodide (PI) and analyzed by flow cytometer FACSVerse™ (BD, NJ, USA) based on DNA content. To evaluate the number of cells undergoing apoptosis, cells were seeded on 60-mm plates; 48 h post-transfection, they were stained by Annexin V: PE Apoptosis Detection Kit I (BD Biosciences, USA) according to manufacturer’s protocol. Measurement was performed on FACSVerse™ flow cytometer (BD, NJ, USA).
Data analysis
In tumor samples, relative TUG1 expression levels were determined by 2−ΔC T method, where ΔCTs were calculated as follows: ΔCT = CT (TUG1) − CT (RNU48). To detect how many times is the level of TUG1 elevated in tumors in comparison to surrounding tissue, 2−ΔΔC T method was used. Statistical significance was evaluated by Mann-Whitney U test, Kruskal-Wallis test, and long-rank test for Kaplan-Meier survival analysis. A ROC (receiver operating characteristic) curve was generated to identify optimal cut-off value of TUG1 expression for discrimination between good and poor prognosis cases. Data were statistically analyzed with software GraphPad Prism v.5 (GraphPad Inc., USA). P values below 0.05 were considered as statistically significant.
Results
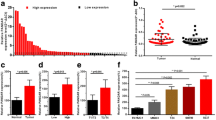
We observed significantly higher expression levels of TUG1 (P < 0.0001) in MIBC tumor tissues samples compared to control non-tumor bladder tissues (Fig. 1a, fold-change=6.68 ± 1.43, mean ± SEM) and higher levels of TUG1 in metastatic tumors (pN+ and/or pM+) (P = 0.0147, Fig. 1b). Survival analysis showed that higher levels of TUG1 are associated also with shorter overall survival of MIBC patients (P = 0.0241, HR, 2.54; 95 % CI, 1.13–5.74, cut-off value=0.1232) (Fig. 1c). We did not observe any other associations of TUG1 expression with clinico-pathological features of MIBC patients (summarized in Table 1).
TUG1 expression levels in tumor tissue and its association with clinico-pathological features. We observed significantly higher levels of TUG1 in tumor tissue in comparison to adjacent non-tumor bladder tissue (a). Increased expression levels of TUG1 were described in the tumors of patients with positive lymph node status (N1, N2, N3) and/or presence of distant metastasis (b). Kaplan-Meier analysis revealed the association of TUG1 higher expression with worse overall survival in MIBC patients (c)
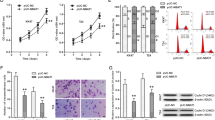
To analyze the effects of TUG1 silencing on cell viability, migration, cell cycle, and apoptosis, we transfected T-24 cells with TUG1 and control siRNAs and determined the efficiency of silencing by qRT-PCR 24, 48, and 72 h after transfection. We observed significant reduction of TUG1 expression levels characterized by 66 % (P < 0.001), 74 % (P < 0.001), and 78 % (P < 0.001) decrease after 24, 48, and 72 h, respectively (Fig. 2a). To determine whether TUG1 affects cell proliferation, T-24 cells were transfected with TUG1 siRNA or control siRNA and subsequently MTT assay was carried out and absorbance was measured 48, 72, and 96 h post-transfection. We observed that TUG1 silencing decreased viability of T-24 cells by 22 % (P = 0.004), 27 % (P = 0.004), and 34 % (P = 0.0004) after 48, 72, and 96 h, respectively (Fig. 2b). Further, we evaluated effects of TUG1 silencing on migration capacity of T-24 cells by the use of scratch wound migration assay and observed that migration of T-24 cells was reduced by 23 % when compared to cells transfected with control siRNA (p < 0.0001) (Fig. 2c, d). There were no significant effects of TUG1 silencing on cell cycle distribution or number of apoptotic cells (P > 0.05).
Effects of TUG1 silencing on proliferation and migration of bladder cancer cells. Transfection with TUG1 siRNA reduced its expression levels in comparison to cells transfected with control siRNA (a). TUG1 silencing significantly decreased proliferation (b) and migration (c) of bladder cancer cells in vitro
Discussion
To date, the newly discovered lncRNAs have emerged as important factors in cellular development and human diseases. One of these lncRNAs is cancer-associated TUG1. Previous studies found that TUG1 is upregulated in colorectal cancer [15], esophageal squamous cell carcinoma [16], gastric cancer [17], hepatocellular carcinoma [18], osteosarcoma [19], and bladder cancer [20, 21]. On the other hand, it was found that TUG1 is downregulated in non-small-cell lung carcinoma [14]. LncRNAs exhibit remarkably tissue-specific expression patterns compared to protein-coding genes indicating that TUG1 may have a tissue-specific expression pattern. However, TUG1 mostly acts as oncogene promoting cancer cell proliferation [16–20] and migration/invasion [15, 16, 21].
Our data are in agreement with this general mode of TUG1 functioning in cancer. Consistently with Han et al. [20] and Tan et al. [21], we observed significantly higher levels of TUG1 in bladder cancer tumor tissue. Moreover, we described significantly higher levels of TUG1 in metastatic tumors, and in agreement with Tan et al. [21], also association of increased TUG1 levels with worse overall survival. However, our patient cohort is the most homogenous one published so far with only high-grade MIBC patients included. Worse overall survival was significantly linked with higher TUG1 levels also in gastric cancer [17] and colorectal cancer [15].
In our study, we observed significant decrease in bladder cancer cells proliferation after TUG1 silencing, which was described by Han et al. [20] in the bladder cancer, and also by others in different cancer types [16–19]. There were several mechanisms suggested in the literature to explain how TUG1 enhances cancer cell proliferation. TUG1 associates with PRC2 (polycomb repressive complex 2) and represses a number of cell cycle genes through promoter methylation mediated by methyltransferase EZH2. KLF2 (Kruppel-like factor) is an example of a gene regulated by this mechanism, which is a negative regulator of β-catenin/TCF signaling, responsible for activation of cell cycle promoters like MYC or cyclin D1 [18]. Through binding to PRC2 complex, TUG1 was also described to epigenetically repress a wide range of cell cycle inhibitors, including p15, p16, p21, p27, and p57 [17].
Further, we observed significant decrease in migration of the bladder cancer cells after TUG1 silencing. Similarly, Tan et al. described involvement of TUG1 in invasion of bladder cancer by promoting the process of epithelial-mesenchymal transition (EMT). Authors suggested that TUG1 promotes EMT by direct negative regulation of miR-145, which is known to suppress EMT-inducer ZEB2 [21]. Promoting functioning of TUG1 in cancer cell invasion was described also in colorectal [15] and esophageal cancer [16].
In conclusion, we confirmed that TUG1 is significantly overexpressed in bladder cancer tissues and described its association with metastatic MIBC and shorter overall survival of high-grade MIBC patients. Our in vitro data support general mode of TUG1 oncogenic functioning based on its promoting effects on cancer cell proliferation and migration/invasion. Our results suggested that TUG1 acts as an oncogene and is a potential prognostic biomarker or therapeutic target in MIBC.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Chavan S, Bray F, Lortet-Tieulent J, Goodman M, Jemal A. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66:59–73.
Apolo AB, Kim JW, Bochner BH, Steinberg SM, Bajorin DF, Kelly WK, et al. Examining the management of muscle-invasive bladder cancer by medical oncologists in the United States. Urol Oncol. 2014;32:637–44.
Burgess EF. Individualized management of advanced bladder cancer: where do we stand? Urol Oncol. 2015;33:187–95.
Sana J, Faltejskova P, Svoboda M, Slaby O. Novel classes of non-coding RNAs and cancer. J Transl Med. 2012;10:103.
Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–19.
Malek E, Jagannathan S, Driscoll JJ. Correlation of long non-coding RNA expression with metastasis, drug resistance and clinical outcome in cancer. Oncotarget. 2014;5:8027–38.
Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208.
Yin DD, Liu ZJ, Zhang E, Kong R, Zhang ZH, Guo RH. Decreased expression of long noncoding RNA MEG3 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Tumour Biol. 2015;36:4851–9.
Gutschner T, Hammerle M, Eissmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–9.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6.
Sidaway P. Prostate cancer: urinary PCA3 and TMPRSS2:ERG reduce the need for repeat biopsy. Nat Rev Urol. 2015;12:536.
Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol. 2005;15:501–12.
Zhang EB, Yin DD, Sun M, Kong R, Liu XH, You LH, et al. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5:e1243.
Sun J, Ding C, Yang Z, Liu T, Zhang X, Zhao C, et al. The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial-mesenchymal transition. J Transl Med. 2016;14:42.
Xu Y, Wang J, Qiu M, Xu L, Li M, Jiang F, et al. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumour Biol. 2015;36:1643–51.
Zhang E, He X, Yin D, Han L, Qiu M, Xu T, et al. Increased expression of long noncoding RNA TUG1 predicts a poor prognosis of gastric cancer and regulates cell proliferation by epigenetically silencing of p57. Cell Death Dis. 2016;7:e2109.
Huang MD, Chen WM, Qi FZ, Sun M, TP X, Ma P, et al. Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer. 2015;14:165.
Zhang Q, Geng PL, Yin P, Wang XL, Jia JP, Yao J. Down-regulation of long non-coding RNA TUG1 inhibits osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J Cancer Prev. 2013;14:2311–5.
Han Y, Liu Y, Gui Y, Cai Z. Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J Surg Oncol. 2013;107:555–9.
Tan J, Qiu K, Li M, Liang Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015;589:3175–81.
Acknowledgments
This work has been financially supported by the Czech Ministry of Health, grant no. 31071A, project MZ CR-RVO (MOU, 00209805) and the Ministry of Education, Youth and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601). We would like to thank Jon B. Smith for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Iliev, R., Kleinova, R., Juracek, J. et al. Overexpression of long non-coding RNA TUG1 predicts poor prognosis and promotes cancer cell proliferation and migration in high-grade muscle-invasive bladder cancer. Tumor Biol. 37, 13385–13390 (2016). https://doi.org/10.1007/s13277-016-5177-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5177-9