Abstract
Breast cancer is the most common cancer in women worldwide, identification of new biomarkers for early diagnosis and detection will improve the clinical outcome of breast cancer patients. In the present study, we determined serum levels of vitronectin (VN) in 93 breast cancer patients, 30 benign breast lesions, 9 precancerous lesions, and 30 healthy individuals by enzyme-linked immunosorbent assays. Serum VN level was significantly higher in patients with stage 0–I primary breast cancer than in healthy individuals, patients with benign breast lesion or precancerous lesions, as well as those with breast cancer of higher stages. Serum VN level was significantly and negatively correlated with tumor size, lymph node status, and clinical stage (p < 0.05 in all cases). In addition, VN displayed higher area under curve (AUC) value (0.73, 95 % confidence interval (CI) [0.62–0.84]) than carcinoembryonic antigen (CEA) (0.64, 95 % CI [0.52–0.77]) and cancer antigen 15-3 (CA 15-3) (0.69, 95 % CI [0.58–0.81]) when used to distinguish stage 0–I cancer and normal control. Importantly, the combined use of three biomarkers yielded an improvement in receiver operating characteristic curve with an AUC of 0.83, 95 % CI [0.74–0.92]. Taken together, our current study showed for the first time that serum VN is a promising biomarker for early diagnosis of breast cancer when combined with CEA and CA15-3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer in females worldwide and a main cause of cancer-related mortality [1, 2]. Improved early detection and diagnosis is contributed to the reduced breast cancer mortality. Although mammographic screening is currently the best available approach for early detection of breast cancer in women over the age of 50 years old, it has several limitations that include poor diagnostic accuracy in dense breast tissue and an insufficient number of screening facilities [3, 4]. Serum biomarkers are more appealing diagnostic tools due to their test simplicity, noninvasiveness, and capability of continuous patient monitoring. However, most of the breast cancer biomarkers currently used in clinical settings, including carcinoembryonic antigen (CEA) and cancer antigen 15-3 (CA15-3), are frequently elevated in patients with late stage and metastatic disease and lack sensitivity in detecting primary breast cancer at an early stage [5–8]. None of them is used for early diagnosis of breast cancer [9]. Therefore, it is very urgent to find serum biomarkers with high sensitivity and specificity for effective early diagnosis of breast cancer.
The human serum component vitronectin (VN), also termed serum spreading factor and complement S-protein, is a 75-kDa adhesive glycoprotein, which plays important roles in inflammation, cell adhesion, cell necrosis, and blood coagulation [10]. As a major component or master organizer of extracellular matrix (ECM), VN has been found to regulate vascular endothelial growth factor (VEGF)-induced angiogenesis, a key step in tumor formation [11]. Importantly, VN was reported to play a key role in the development and progression of solid tumors including breast cancer [12–15]. VN has been shown to promote breast cancer migration and invasion [16, 17] and participate in differentiation of breast cancer stem cells [18]. Moreover, several biologically active complexes of VN are known to exist in the plasma and stabilize its partner proteins, such as PAI-1 and fibrinogen [19, 20]. In the blood, VN circulates in a monomeric form. It consists of a mixture of a 75-kDa polypeptide and its nicked product of 65 plus 10 kDa [21]. Notably, VN level was found to be higher in blood samples of breast cancer patients than in healthy women and may serve as a useful biomarker for the early detection of breast cancer [22–24]. However, a better understanding of the performance of VN alone or in combination with other clinically used biomarkers in early detection and diagnosis of breast cancer is still necessary.
In this study, we investigated the relationship of serum VN level with various clinicopathologic parameters of breast cancer and assessed the diagnostic value of VN and the other two biomarkers, CEA and CA15-3. Our aim was to explore whether VN alone or in combination with the conventional breast cancer biomarkers CEA and CA15-3 could be used as new biomarker panels to recognize cancer cells with high sensitivity and specificity for early detection of breast cancer.
Materials and methods
Patients’ data
This study obtained approval from the ethics review boards in Affiliated 307 Hospital and Institute of Basic Medical Sciences. All patients and healthy volunteers provided written informed consent. Ninety-three breast carcinoma patients aged between 32 and 79 years old were enrolled in the study. The control groups included 30 patients with benign breast tumor, 9 with precancerous lesions and 30 healthy women. Characteristics of cancer patients and control subjects were shown in Table 1. The diagnosis of breast cancer and benign and precancerous lesions was established by histopathological analysis of biopsy or surgical mammary tissue specimens. The breast carcinoma and benign and precancerous lesions serum samples were obtained prior to surgery or prior to any treatment.
The cancer was staged according to tumor-node-metastasis (TNM) by the American Joint Committee on Cancer (AJCC) staging system.
Serum collection and storage
All blood samples were collected from preoperative patients and healthy individuals. The samples were allowed to clot at room temperature for at least 30 min, and then centrifuged at 4 °C for 10 min at 3000 rpm. The serum aliquots were stored at 80 °C until assayed.
VN enzyme-linked immunosorbent assay
Crude sera were diluted 1:2000 and the diluted samples were analyzed using human vitronectin enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource, San Diego, USA) according to the manufacturer’s instructions. Natural forms of VN (75 kDa) were detected in this study. Protein concentrations were determined as absorbance with the SM-3 Microplate Reader (Tianshi, Beijing, China).
CEA and CA15-3 serum analysis
Both the CEA and CA15-3 serum testing were performed with sandwich ELISA kits (CanAg Diagnostics AB, Gothenburg, Sweden) according to the manufacturer’s instructions. The reaction was stopped by addition of stop solution, and the resulting absorbance was measured at 405 nm with the SM-3 Microplate Reader (Tianshi, Beijing, China). All the samples were assessed in duplicate.
Statistical analysis
The relationships between biomarkers and tumor characteristics were examined with the Kruskal-Wallis test and t test. Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as number and percentage. Spearman’s rank correlation coefficient was used to assess the correlations among biomarkers. Nonparametric receiver operating characteristic (ROC) curve analysis was used to obtain the area under curve (AUC) with 95 % confidence intervals of individual as well as combined biomarkers and calculate their sensitivity and specificity for distinguishing stage 0–I patients and normal control groups. Logistic regression was used to calculate the sensitivity and specificity of the optimal combination of VN, CEA, and CA 15-3. p values less than 0.05 were considered statistically significant for all statistical analyses. All statistical analysis was performed using the SPSS software package version 17.0 (SPSS, Chicago, IL, USA).
Results
Clinical characteristics of the study cohort
Characteristics of the total study population are shown in Table 1. Among the 93 breast cancer patients investigated, the most frequently occurring cancer was invasive ductal carcinoma (80 %) followed by ductal carcinoma in situ (16 %). The most frequent clinical stage was stage I (38 %). Thirty-two percent of breast cancer patients were diagnosed in stage II, while only one tumor was diagnosed in stage IV. The median age of breast cancer patients was 48 years. In benign lesions, 40 % of the patients were fibroadenoma followed by adenopathy (33 %). The median age of patients with benign lesion was 37 years. Seven of nine precancerous lesions (78 %) were diagnosed with intraductal papillomatosis. The median age of patients with precancerous lesion was 48 years.
Measurement of serum VN level in breast cancer patients and control subjects
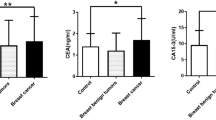
The serum levels of VN from healthy individuals and patients with breast cancer, benign lesions, and precancerous lesions were analyzed by ELISA. The expression levels of VN in breast cancer patients at different stages and in non-cancer groups are shown in Fig. 1. The data showed that the mean value of serum VN level was higher in stage 0–I breast cancer patients than in normal controls, benign lesions, precancerous lesions, and patients with breast cancers of higher stages. VN level showed significant difference between stage 0–I patients (310.98 ± 36.78 μg/ml) and the other groups, including normal controls (277.23 ± 45.87 μg/ml) (p = 0.0014), stage II breast cancer patients (274.67 ± 38.12 μg/ml) (p = 0.0001), stage III–IV breast cancer patients (270.78 ± 48.08 μg/ml) (p = 0.0365), patients with precancerous lesions (282.06 ± 31.25 μg/ml) (p = 0.0299), and patients with benign breast lesions (292.57 ± 35.28 μg/ml) (p = 0.0344) (Fig. 1). There is no significant difference between normal control and benign breast lesion groups (p = 0.1547). No significant difference was found among stage II, stage III–IV, and benign breast lesion patients (p > 0.05 in all cases).
Scatter-plot analysis of serum VN expression. Data are presented as mean values (solid lines). NF normal female, PL precancerous lesions, BL benign lesions, 0–I stage 0–I breast cancer, II stage II breast cancer, III–IV stage III–IV breast cancer. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant (p > 0.05)
Relationship of serum VN level with clinicopathological parameters of breast cancer patients
The association of VN, CA15-3, and CEA with patient and clinicopathological parameters such as age, tumor size, menopausal status, lymph node status, histologic grade, estrogen receptor (ER) and progesterone receptor (PgR) status, clinical stage, HER2 status, and Ki67 expression were examined. As shown in Table 2, serum VN level was not associated with age, menstrual status, histological grade, ER expression, PR expression, HER2 overexpression, and Ki67 expression (p > 0.05 in all cases). However, the decreased VN level correlated with increased tumor size (p = 0.0175) and clinical stage (p = 0.0004). Furthermore, serum VN level in lymph node-negative group was higher than in the positive group (p = 0.0024) (Table 2). Serum VN level was significantly and negatively associated with tumor size, lymph node status, and clinical stage. As for CEA and CA15-3, both biomarkers’ serum concentrations were significantly higher in patients with tumor size >2 cm than in patients with tumor size <2 cm. In addition, serum CEA and CA15-3 levels were significantly correlated with patient clinical stage and lymph nodal status (p < 0.05 in all cases).
Correlation and diagnostic values of the three biomarkers
To assess the correlation of serum levels of VN, CEA, and CA15-3, Spearman’s rank correlation coefficients were used in the analysis of the three biomarkers in normal control and stage 0–I patients. As shown in Table 3, there was no significant correlation between VN and CEA in both stage 0–I patients (Spearman r = −0.205, p = 0.17168) and the normal control group (Spearman r = −0.104, p = 0.58263). Similarly, VN had no significant correlation with CA15-3 in both stage 0–I patients (Spearman r = 0.159, p = 0.29162) and the normal control group (Spearman r = 0.219, p = 0.24416).
In order to quantify the diagnostic values of the three biomarkers for distinguishing stage 0–I patients and normal control, ROC curve analysis was performed on the biomarkers individually (Fig. 2) and in combinations (Fig. 3). As shown in Fig. 2a–c, all three biomarkers had AUC significantly higher than 0.5; the AUC value for VN (0.73) was higher than the AUC for CEA (0.64) and CA15-3 (0.69).VN has the highest value with 95 % confidence interval (CI) [0.62, 0.84] (Table 4). Likewise, the superiority of VN over the other two markers was evident with higher sensitivities when specificity was fixed at 90 and 80 %, respectively (Table 4). As shown in Fig. 3a–d and Table 4, combining biomarkers resulted in increased AUC value as compared to individual biomarkers. Combining CEA and CA15-3 yielded a ROC curve with the same AUC value as VN alone (0.73). Combining VN and CEA yielded a ROC curve with higher AUC value (0.81) than the combination of VN and CA15-3 (0.78). More importantly, combining VN, CEA, and CA15-3 resulted in an improvement in ROC curve with the highest AUC value (0.83) among all individual biomarkers and combinations. At a fixed specificity of 90 %, the sensitivity of the three biomarker combination reached 63 % (95 % confidence interval, CI 0.47–0.76).
ROC curves for serum levels of individual biomarkers analyzed (VN, CEA, and CA 15-3) for distinguishing stage 0–I patients and normal controls. The diagnostic potentials were assessed by logistic regression and ROC curves. The area under the ROC curve (AUC) of the three panels is indicated. a VN = 0.73, b CEA = 0.64, and c CA 15-3 = 0.69
ROC curves for serum levels of combinations of the biomarkers for distinguishing stage 0–I patients and normal controls. The diagnostic potentials were assessed by logistic regression and ROC curve. The area under the ROC curve (AUC) of the four panels is indicated. a CA15-3 and CEA = 0.73; b VN and CA15-3 = 0.78; c VN and CEA = 0.81; and d VN, CA15-3, and CEA = 0.83
Taken together, these data indicates that VN could be used as a breast cancer biomarker capable of distinguishing stage 0–I breast cancer patients from normal control with higher sensitivity and specificity than existing breast cancer biomarkers CEA and CA15-3. More importantly, combining VN, CEA, and CA15-3 increased the diagnostic value with superior sensitivity and specificity for early stage breast cancer diagnosis.
Discussion
Previous studies indicated that VN may be a promising biomarker for breast cancer diagnosis [22–24]. In the present study, we examined serum level of VN in patients with breast cancer in comparison with normal individuals and patients with pre-malignant cancer or cancer at later stages. Our study is the first to thoroughly investigate and compare the serum VN levels at various breast cancer stages and with various non-cancer groups. Our results demonstrated that VN may serve as an early stage breast cancer diagnostic biomarker by distinguishing stage 0–I breast cancer and breast cancer patients at higher stage than stage I, non-cancers including normal, benign, and precancerous lesions. Another key finding of this study is that combination of VN with two existing breast cancer markers CEA and CA15-3 could improve sensitivity and specificity of early stage breast cancer diagnosis.
In this study, we observed and firstly reported statistically significant negative correlation between VN level and tumor size, lymph node metastatic status, and clinical stage. However, there was no correlation between VN level and patient menstrual status, histological grade, ER expression, PR expression, HER2 overexpression, and Ki67 expression. Moreover, our results showed that serum CEA and CA15-3 levels increased with clinical stage and correlated with breast cancer progression, which is similar to observations in other studies [25, 26].
Immunohistochemical analysis of breast carcinomas showed particularly prominent accumulation of VN in ECM structures [14]. VN was reported to be present as extracellular deposits in close proximity to vessels and constituents of a major component of the vascularized breast cancer microenvironment [14]. The urokinase-type plasminogen activator (uPA) and its cellular receptor (uPAR) has been reported to participate in multiple physiological and pathologic processes including cell migration, angiogenesis, inflammation, embryogenesis, tumor growth, and metastasis [27–29]. VN was found to play important roles in the tumor cell adhesion, migration, invasion, and growth of solid tumors via interaction with uPAR [30–33]. Angiogenesis is a key step in tumor formation. VN was reported to regulate angiogenesis through VEGF [11] and found to regulate tumor cell invasion and migration through ECM surrounding the tumor epithelium [15, 17]. Furthermore, the high level of VN in the serum could promote circulating tumor cells to disseminate and adhere to the distal organ [12]. Therefore, VN participates in tumor angiogenesis, invasion, migration, and adhesion, which are major steps of cancer metastasis [11, 12, 16, 17].
Previous study showed serum VN level (including 65- and 75-kDa polypeptides) was found to be higher in breast cancer patients of all stages as compared to normal control [22]. While the highest serum VN level in stage 0–I patients was found compared to the breast cancer in higher stage in our study, that might be the natural form of VN (75 kDa) which was detected in the present study. Moreover, matrix metalloproteinase (MMP)-2 secreted by tumor cells has been reported to degrade VN [34]. With the tumor progression, more proteinases such as MMP2 were secreted by tumor cells, leading to natural form of VN (75 kDa) degradation in ECM. That might be a good explanation for the lower VN expression in breast cancer serum of higher stages than that of stage 0–I.
Currently, very few serum biomarkers were clinically used for breast cancer diagnosis. CEA and CA15-3 are among the few serum markers presently used for monitoring advanced breast cancer progression and treatment response in clinic. The most important characteristic of a tumor marker is to detect the disease in early stages. However, the levels of CEA and CA15-3 are mostly elevated in stage III–IV breast cancer patients, and they have low sensitivity and specificity when used to distinguish stage 0–I and non-cancer subjects; thus, they are not recommended to be used as biomarkers for diagnosis of breast cancer [9]. Therefore, there is a strong need for developing new serum biomarkers with higher diagnostic performance than currently used biomarkers for early stage breast cancer diagnosis. Our ROC curve analysis clearly showed that VN displayed higher diagnostic sensitivity and specificity than CA15-3 and CEA in distinguishing stage 0–I breast cancer patients from normal control subjects. Diagnostic accuracy may be improved considerably by combining multiple biomarkers [35]. Therefore, there is a need for combining VN with other clinically used markers to increase the diagnostic performance. Notably, we are the first to identify that combining VN, CEA, and CA15-3 resulted in an improvement in ROC curve AUC values and test sensitivity and specificity as compared to individual biomarker or combinations of two of the three biomarkers. The AUC value of VN, CEA, and CA15-3 combination (0.83) is higher than the other groups, suggesting that the VN, CEA, and CA15-3 panel has good diagnostic performance. In the present study, we evaluated 162 samples in total; however, the number of samples with precancerous lesions was only 9. Thus, the proposed results will require replication in large cohorts, particularly with more precancerous lesions, for the findings could be implemented in clinical laboratories for individual patients.
In conclusion, we demonstrated that VN is a promising biomarker alone or in combination with CEA and CA15-3 for diagnosis of early stage breast cancer. To our knowledge, this is the first study to use combination measurement of serum levels of VN, CEA, and CA15-3 in the screening and diagnosis of early stage breast cancer. Further studies are needed to reveal the mechanisms responsible for altered VN expression levels in breast cancer patients.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208.
Allemani C, Sant M, Weir HK, Richardson LC, Baili P, Storm H, et al. Breast cancer survival in the US and Europe: a CONCORD high-resolution study. Int J Cancer. 2013;132(5):1170–81. doi:10.1002/ijc.27725.
Bohm D, Keller K, Wehrwein N, Lebrecht A, Schmidt M, Kolbl H, et al. Serum proteome profiling of primary breast cancer indicates a specific biomarker profile. Oncol Rep. 2011;26(5):1051–6. doi:10.3892/or.2011.1420.
Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA. 2005;293(10):1245–56.
Duffy MJ, Evoy D, McDermott EW. CA 15-3: uses and limitation as a biomarker for breast cancer. Clinica Chimica Acta. 2010;411:1869–74. doi:10.1016/j.cca.2010.08.039.
Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006;52(3):345–51.
Lumachi F, Basso SMM. Serum tumor markers in patients with breast cancer. Expert Rev Anticancer Ther. 2004;4(5):921–31.
Mirabelli P, Incoronato M. Usefulness of traditional serum biomarkers for management of breast cancer patients. Biomed Res Int. 2013;2013:685641. doi:10.1155/2013/685641.
Maric P, Ozretic P, Levanat SS, Oreskovic S, Antunac K, Beketic-Oreskovic L. Tumor markers in breast cancer—evaluation of their clinical usefulness. Coll Antropol. 2011;35(1):241–7.
Preissner K, Reuning U. Vitronectin in vascular context: facets of a multitalented matricellular protein. Semin Thromb Hemost. 2011;37(4):408–24. doi:10.1055/s-0031-1276590.
Li R, Luo M, Ren M, Chen N, Xia J, Deng X, et al. Vitronectin regulation of vascular endothelial growth factor-mediated angiogenesis. J Vasc Res. 2014;51(2):110–7. doi:10.1159/000360085.
Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991;88(6):1924–32.
Leavesley DI, Kashyap AS, Croll T, Sivaramakrishnan M, Shokoohmand A, Hollier BG, et al. Vitronectin-master controller or micromanager? IUBMB Life. 2013;65(10):807–18. doi:10.1002/iub.1203.
Aaboe M, Offersen BV, Christensen A, Andreasen PA. Vitronectin in human breast carcinomas. Biochim Biophys Acta. 2003;1638(1):72–82.
Zhu W, Li W, Yang G, Fu C, Jiang G, Hu Q. Vitronectin silencing inhibits hepatocellular carcinoma in vitro and in vivo. Future Oncol. 2015;11(2):251–8. doi:10.2217/fon.14.202.
Pola C, Formenti SC, Schneider RJ. Vitronectin-alphavbeta3 integrin engagement directs hypoxia-resistant mTOR activity and sustained protein synthesis linked to invasion by breast cancer cells. Cancer Res. 2013;73(14):4571–8. doi:10.1158/0008-5472.
Kashyap AS, Hollier BG, Manton KJ, Satyamoorthy K, Leavesley DI, Upton Z. Insulin-like growth factor-I: vitronectin complex-induced changes in gene expression effect breast cell survival and migration. Endocrinology. 2011;152(4):1388–01. doi:10.1210/en.2010-0897.
Hurt EM, Chan K, Serrat MA, Thomas SB, Veenstra TD, Farrar WL. Identification of vitronectin as an extrinsic inducer of cancer stem cell differentiation and tumor formation. Stem Cells. 2010;28(3):390–8. doi:10.1002/stem.271.
Ehrlich HJ, Gebbink RK, Keijer J, Linders M, Preissner KT, et al. Alteration of serpin specificity by a protein cofactor. Vitronectin endows plasminogen activator inhibitor 1 with thrombin inhibitory properties. J Biol Chem. 1990;265(22):13029–35.
Fay WP, Parker AC, Ansari MN, Zheng X, Ginsburg D. Vitronectin inhibits the thrombotic response to arterial injury in mice. Blood. 1999;93(6):1825–30.
Kubota K, Katayama S, Matsuda M, Hayashi M. Three types of vitronectin in human blood. Cell Struct Funct. 1988;13(2):123–8.
Kadowaki M, Sangai T, Nagashima T, Sakakibara M, Yoshitomi H, Takano S, et al. Identification of vitronectin as a novel serum marker for early breast cancer detection using a new proteomic approach. J Cancer Res Clin Oncol. 2011;137(7):1105–15. doi:10.1007/s00432-010-0974-9.
Kim BK, Lee JW, Park PJ, Shin YS, Lee WY, Lee KA, et al. The multiplex bead array approach to identifying serum biomarkers associated with breast cancer. Breast Cancer Res. 2009;11(2):R22. doi:10.1186/bcr2247.
Cho W, Jung K, Regnier FE. Sialylated Lewis x antigenbearing glycoproteins in human plasma. J Proteome Res. 2010;9(11):5960–8. doi:10.1021/pr100747p.
Ruibal A, GarridoPumar M, Arias JI. Preoperative serum CA15.3 and CEA levels and clinical-biological parameters in breast tumors. Rev Esp Med Nucl. 2006;25(3):180–3.
Samy N, Ragab HM, El Maksoud NA, Shaalan M. Prognostic significance of serum Her2/neu, BCL2, CA15-3 and CEA in breast cancer patients: a short follow-up. Cancer Biomark. 2010;6(2):63–72. doi:10.3233/CBM-2009-0119.
Halamkova J, Kiss I, Tomasek J, Pavlovsky Z, Tucek S, Penka M. Significance of urokinase and its inhibitors in the invasiveness and metastasing of malignant tumors. Vnitr Lek. 2012;58(2):129–34.
Tang L, Han X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed Pharmacother. 2013;67(2):179–82. doi:10.1016/j.biopha.2012.10.003.
Halamkova J, Kiss I, Tomasek J, Pavlovsky Z, Cech Z, Tutek S, et al. Plasminogen activator system and its clinical significance in patients with a malignant disease. Klin Onkol. 2011;24(6):418–23.
Pirazzoli V, Ferraris GM, Sidenius N. Direct evidence of the importance of vitronectin and its interaction with the urokinase receptor in tumor growth. Blood. 2013;121(12):2316–23. doi:10.1182/blood-2012-08-451187.
Rea VE, Lavecchia A, Di Giovanni C, Rossi FW, Gorrasi A, Pesapane A, et al. Discovery of new small molecules targeting the vitronectin-binding site of the urokinase receptor that block cancer cell invasion. Mol Cancer Ther. 2013;12(8):1402–16. doi:10.1158/1535-7163.
Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol. 2007;177(5):927–39.
Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994;269(51):32380–8.
Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118(4):1367–79. doi:10.1172/JCI33775.
Liu C, Liu A, Halabi S. A min-max combination of biomarkers to improve diagnostic accuracy. Stat Med. 2011;30(16):2005–14. doi:10.1002/sim.4238.
Acknowledgments
We are grateful to the patients for allowing the use of their sera for this research project. This work was supported by the National High-tech Research and Development Program (2011AA02A120, 2011–2015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interests
None
Additional information
Wende Hao, Xuhui Zhang and Bingshui Xiu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hao, W., Zhang, X., Xiu, B. et al. Vitronectin: a promising breast cancer serum biomarker for early diagnosis of breast cancer in patients. Tumor Biol. 37, 8909–8916 (2016). https://doi.org/10.1007/s13277-015-4750-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4750-y