Abstract
Glycometabolism is a distinctive aspect of energy metabolism in breast cancer, and key glycometabolism enzymes/pathways (glycolysis, hexosamine biosynthetic pathway, and pentose phosphate pathway) may directly or indirectly affect the clinical features. In this study, we analyzed the particular correlation between the altered glycometabolism and clinical features of breast cancer to instruct research and clinical treatment. Tissue microarrays containing 189 hollow needle aspiration samples and 295 triple-negative breast cancer tissues were used to test the expression of M2 isoform of pyruvate kinase (PKM2), glutamine-fructose-6-phosphate transaminase 1 (GFPT1), glucose-6-phosphate dehydrogenase (G6PD), and p53 by immunohistochemistry and the intensity of these glycometabolism-related protein was evaluated. Chi-square test, Kaplan-Meier estimates, and Cox proportional hazards model were used to analyze the relationship between the expression of these factors and major clinical features. PKM2, GFPT1, and G6PD affect the pathologic complete response rate of neoadjuvant chemotherapy patients in different ways; pyruvate kinase muscle isozyme 2 (PKM2) and G6PD are closely associated with the molecular subtypes, whereas GFPT1 is correlated with cancer size. All these three factors as well as p53 have impacts on the progression-free survival and overall survival of triple-negative breast cancer patients. Cancer size shows significant association with PKM2 and GFPT1 expression, while the pN stage and grade are associated with PKM2 and G6PD expression. Our study support that clinical characteristics are reflections of specific glycometabolism pathways, so their relationships may shed light on the orientation of research or clinical treatment. The expression of PKM2, GFPT1, and G6PD are hazardous factors for prognosis: high expression of these proteins predict worse progression-free survival and overall survival in triple-negative breast cancer, as well as worse pathologic complete response rate in neoadjuvant chemotherapy breast cancer. However, p53 appears as a protective factor only in the patients receiving neoadjuvant chemotherapy. All the four proteins, PKM2, GFPT1, G6PD and p53, are prognostic markers of breast cancer. The correlation among them suggests that there may be crosstalk of the four proteins in breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a common cancer globally. It is the highest occurring cancer among women and one of the leading causes of death [1]. Breast cancer is an incurable disease, though the application of multi-modality cancer treatments, including chemotherapy, radiation therapy, target therapy, and endocrine therapy, has improved the prognosis. For inoperable or locally inflammatory advanced cancers, chemotherapy prior to surgery (neoadjuvant) is the standard treatment. According to the guidelines from National Comprehensive Cancer Network (NCCN), neoadjuvant chemotherapy (NACT) should be considered primarily for the treatment of patients with stage IIIA (T0-3, N2, M0), stage IIIB (T4, N0-2, M0), and stage IIIC (any T, N3, M0) cancer, as well as patients who have breast preservation desires and have stage IIA (T2, N0, M0), stage IIB (T2, N1, M0 T3, N0, M0), and stage IIIA (T3, N1, M0) cancer. Pathologic complete response (pCR) is referred to a status without invasive carcinoma both in the breast and lymph nodes after treatment and is commonly used as a curative effect criterion of NACT. The association between pCR and long-term outcomes was the strongest in patients with triple-negative breast cancer (TNBC) as well as in patients who have human epidermal growth factor receptor 2 (HER2) positive, hormone receptor-negative tumors and received trastuzumab [2, 3]. HER2, estrogen receptor (ER), and progesterone receptor (PR) are three molecular markers that categorize breast cancer into different molecular subgroups: luminal A, luminal B, TNBC, or HER2 overexpression breast cancer. Breast cancer that is negative for the three markers is generally termed as TNBC. TNBC is often aggressive and associated with a higher mortality than the other subtypes of breast cancer (p < 0.01) [4]. TNBC is characterized by high cell proliferation, poor cellular differentiation, increased recurrent copy number imbalances of p53, and, in most cases, mutations in the p53 tumor suppressor gene [5–7].
Cancer growth is frequently associated with a deviation of a set of energy generation mechanisms. This phenomenon is termed as the Warburg effect, which describes that cancer cells primarily utilize glycolysis and lactic acid fermentation for energy production rather than oxidation of pyruvate through the tricarboxylic acid cycle. Altered hexosamine biosynthetic pathway (HBP) and pentose phosphate pathway (PPP) are also involved in improper energy metabolism[8, 9].
Pyruvate kinase, which converts phosphoenolpyruvate (PEP) into pyruvate and generates one molecule of ATP, is the key enzyme of the glycolytic pathway. The M2 isoform of pyruvate kinase (PKM2) is preferentially expressed in cancer, and the complex regulation of its activity is important to control cell metabolism [10]. Hence, as a new chemotherapeutic strategy, pyruvate kinase muscle isozyme 2 (PKM2) becomes an attractive target. PKM2, an embryonic isoform that is also abundant in tumor cells, is less active than PKM1, allowing the accumulation of glycolytic intermediates and the energy metabolism diverting into biosynthetic pathways. The PKM2 accumulation is demanded by rapid-proliferating cells in the MCF-7 cells lines [11]. Small interfering RNAs (siRNAs) specifically targeting PKM2, instead of PKM1, can effectively suppress oncogenic pathways and induce growth arrest, re-establishment of basally polarized acini of human malignant breast epithelial cells in 3D cell culture. Furthermore, the elevation of pyruvate and ATP in glucose-starved malignant cells after PEP addition is also blocked by PKM2 knockdown [12]. Multiple unique non-metabolic functions of PKM2 have been proposed to play a vital role in cancer cell proliferation and tumor growth [13–15].
Hexosamine biosynthetic pathway is also involved in the reciprocal regulation of glycolytic pathway and oncogenic events [12]. The main product of HBP is uridine diphosphate-N-acetyl-d-glucosamine (GlcNAc), a primary donor molecule for post-translational modifications, such as N- and O-glycosylation. Glycosylation plays critical roles in varieties of biological processes. It is established that addition of GlcNAc to glucose-depleted cells completely prevents the death of transformed cells. This phenomenon stresses the important role of glucose in HBP fuelling to increase cell survival [16]. Glutamine-fructose-6-phosphate transaminase 1 (GFPT1) is the first and rate-limiting enzyme of HBP [12]. Yasuhito Onodera and Mina J. Bissell confirmed by Western blot that GFPT1 level was high in malignant human breast epithelial cell colonies. The two different inhibitors of GFPT, azaserine, and 6-diazo-5-oxonorleucine, induced growth arrest, re-established tissue polarity, suppressed oncogenic signaling, and reduced glycolytic activity. The same phenotypes could also be observed after silencing GFPT1 by siRNA in malignant cells [12].
The pentose phosphate pathway is a biochemical pathway parallel to glycolysis. Pentose phosphate pathway (PPP) generates NADPH and ribose-5-phosphate (R5P) which is the stock for de novo synthesis of RNA and DNA [17]. Numerous intermediary glucose metabolites are used for diverse biosynthetic processes [18], and NADPH, a reducing equivalent generated by glucose metabolism, sequesters ROS to make cells resist cell death [19, 20]. Thus, the PPP plays an essential role in protecting cells from oxidative stress-induced apoptosis [21] and promoting cell proliferation. Glucose-6-phosphate dehydrogenase (G6PD) is the first and rate-controlling enzyme of PPP oxidative branch. It catalyzes the conversion of glucose-6-phosphate to 6-phosphate-gluconolactone and generates NADPH. C13-glucose tracers show the activation of pentose phosphate cycle leads to fast growth of MDA-MB-231 cells lines [22]. The fact that estradiol-treated cells have increased transcript levels of G6PD compared to control cells explains the phenomenon that G6PD-deficient women have reduced breast cancer risk [23].
p53, a well-studied tumor suppressor, plays a critical role in controlling numerous cellular processes, including apoptosis, cell cycle arrest, and genomic stability. p53 is also known to reduce the glycolysis rate by increasing the activity of a fructose-2,6-bisphosphatase which is involved in the regulatory pathways of apoptosis. Therefore, a reduced expression of protein p53 in cancer cells was linked to the Warburg effect in regulating cell apoptosis [19, 24]. Recent studies have revealed a number of new functions of p53 in the regulation of glucose and energy metabolic pathways, including glucose transport [25], glycolysis [19], tricarboxylic acid cycle [26], mitochondrial respiratory chain/oxidative phosphorylation [27], and PPP [28, 29].
The close association of the three primary glucose metabolic pathways with breast cancer has been elaborated (Fig. 1). However, the interaction among them remains to be explored in breast cancer. The aims of this study are to investigate the effect of these factors of glucose metabolism on NACT breast cancer and the prognosis of TNBC, as well as to explore their potential interactions, so that their potential as predictors of tumor progression and treatment targets can be revealed.
Materials and methods
Patients and tissue samples
Formalin-fixed paraffin-embedded breast cancer sections were obtained from The Third Affiliated Hospital of Harbin Medical University. The patients were firstly diagnosed as breast cancer without any severe systemic diseases or combined tumor, and did not receive any treatment before diagnosis. The patients with NACT were diagnosed between December 2009 and January 2014, while the TNBC patients were diagnosed between January 2003 and January 2009. Patients were excluded from this study if they could not bear first-line chemotherapy due to pregnancy, breast feeding, or poor compliance, or if they were less than 18 years of age.
Totally, 189 hollow needle aspiration breast cancer samples were collected. The median age of these patients was 48 (ranged from 25 to 76). Among these cases, 44 (23.28 %) patients achieved pathologic complete response. The median age of the 295 TNBC patients enrolled in this project was 50 (ranged from 24 to 76), the progression-free survival (PFS) ranged from 1 to 134 months (the median was 61.59) and the overall survival (OS) ranged from 5 to 134 months (the median was 72.21). Patients who did not relapse were censored at the time of the last follow-up or death. Thirty-six relative healthy control samples were included in this study. This study was conducted with approval from the Ethics Committee of the Third Affiliated Hospital of Harbin Medical University.
Immunohistochemistry (IHC)
Four micrometers of paraffin sections were used for immunohistochemical staining. They were dewaxed, incubated in saline sodium citrate (pH = 7.0) for 1 min (2 min for G6PD IHC) in pressure heating environment for antigen retrieval, then soaked in 3 % H2O2 solution for 25 min. Mouse monoclonal PKM2 antibody (1:10,000, OriGene, USA), rabbit monoclonal GFPT1 antibody (1:600 Abcam, UK), and G6PD (1:300, Millipore, USA) were applied at 4 °C overnight, and the sections were incubated with secondary antibody at room temperature for 25 min, then visualized by DAB.
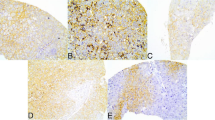
The stained specimens were reviewed by two pathologists independently. At least five visual fields were observed for each section under high power lens (×400) to calculate the percentage of positive cells. The sections were then scored semi-quantitatively for PKM2 and GFPT1 as the following criterions: 0 (<10 %), 1 (10 to 50 %), and 2 (>50 %); intensities were scored as 0 (no staining), 1 (weak staining), and 2 (strong staining). Specimen with a total score (percentage score multiplied intensity score) > 1 were classified as PKM2-positive (+), otherwise were PKM2-negtive (−). G6PD was scored as the same criterion of PKM2, but the percentage scores were determined as the following: 0 (< 10 %), 1 (10 to 30 %), and 2 (>30 %) (Fig. 2).
Statistical analysis
All the data were analyzed with statistical package SPSS (version 20.0 for Windows, IBM SPSS statistics) software and chi-square tests were used to compare the differences of the associated factor expressions in breast cancer tissues. The correlations of the PKM2, GFPT1, and G6PD expression levels were also analyzed using chi-square tests. Survival curves were generated with the Kaplan-Meier method, and Cox’s proportional hazards regression model was used to evaluate the hazards. Differences with p< 0.05 were considered statistically significant.
Result
Patients and clinical characteristics
Forty-four patients achieved pCR according to the criterion of calculation script (www.mdanderson.org/breastcancer_RCB) [25] among the 189 patients treated with NACT. Clinicopathological and treatment characteristics in relation to pCR are shown in Supplementary Table 1. Molecular subtypes and tumor size show significant differences (p < 0.001; p = 0.024) between the pCR group and non-pCR group (Supplementary Table 1). In TNBC patients, several factors have impacts on PFS and OS, such as the number of chemotherapy cycle (p < 0.001, p = 0.050, respectively), operation (p = 0.001, p < 0.001, respectively), and radiotherapy (p = 0.013, p = 0.005, respectively) (Supplementary Table 2). Compared with the 484 breast cancer tissues, the 36 control breast tissues show markedly low expressions of PKM2, GFPT1, and G6PD (p = 0.026, p = 0.041, and p = 0.029, respectively) (Fig. 2, Supplementary Figure).
PKM2 expression is associated with pCR and prognosis
PKM2 displayed molecular subtype-specific expression (p < 0.001) in the patients receiving neoadjuvant chemotherapy. The miscellaneous PKM2 expression also affects the rate of pCR (p = 0.001) (Table 1). In TNBC, the expression of PKM2 is significantly associated with tumor size (p = 0.045), lymph node positivity (p = 0.008), grade (p = 0.005), and histology subtype (p = 0.013) (Table 2). Kaplan-Meier analyses reveal that the expression of PKM2 has a significant influence on the PFS (p < 0.001) and OS (p < 0.001) of TNBC (Fig. 3a and b).
Expression of PKM2, GFPT1, and G6PD as well as p53 mutation associate with PFS and OS in TNBC. Positive expression of PKM2 leads to worse PFS and OS (a, b); GFPT1 expression is related to worse PFS and OS (c, d); G6PD expression is associated with PFS and OS (e, f); p53 mutations results in worse PFS and OS (g, h)
GFPT1 expression is associated with pCR and prognosis
In neoadjuvant chemotherapy breast cancer, the expression of GFPT1 has notable difference in the patients with different tumor size and is associated with the rate of pCR (p = 0.023, p < 0.001, respectively) (Table 1). The larger the tumor size is the stronger the expression of GFPT1. The same phenomenon was also observed in the TNBC (p = 0.005) (Table 2). Patients with low expression of GFPT1 may achieve pCR more easily than the patients with high expression of GFPT1 (p < 0.001). Patients with lower GFPT1 expression also have longer PFS (p = 0.002) and OS (p = 0.004) (Fig. 3c and d).
G6PD expression is associated with pCR and prognosis
The cohorts of luminal A and luminal B show stronger expression of G6PD than the TNBC and HER2 overexpression cohorts (p = 0.036). In addition, there are more G6PD-high expression patients in non-pCR cohort than the pCR cohort (p = 0.015) (Table 1). In TNBC, the expression of G6PD shows significant difference among patients of different pN stage (p = 0.007) and grade (p = 0.009) (Table 2). Furthermore, it is also notably associated with the PFS (p = 0.001) and OS (p < 0.001) (Fig. 3e and f).
p53 mutation is associated with TNBC prognosis
In the TNBC group, it is observed that p53 mutation is closely associated with the PFS (p < 0.001) and OS (p < 0.001) (Fig. 3g, h), which means the patients with p53 mutations have worse prognosis.
The relationship of PKM2, GFPT1, G6PD expressions, and p53 mutation
In order to reveal the potential connections of these factors, we analyze the relationship among PKM2, GFPT1, G6PD expressions, and p53 mutation. PKM2 and G6PD expression show correlation both in NACT patients and TNBC patients (p = 0.013, p = 0.021, respectively), so do G6PD expression and p53 mutation (p = 0.025, p = 0.027, respectively). Moreover, GFPT1 expression is associated with G6PD expression and p53 mutation (p < 0.001, p = 0.024, respectively) in NACT breast cancer patients, but the association between PKM2 expression and p53 mutation is only observed in TNBC patients (p = 0.020).
Discussion
Although pCR (including node-negative) status has consistently signified an excellent prognosis in published studies, precise report of residual disease has become a meaningful tool for NACT breast cancer patients because of its prognosis prediction [25]. Therefore, the pCR in this study includes the status with minimal residual disease.
The tumor cells preferentially use glycolysis over mitochondrial oxidative phosphorylation for glucose-dependent ATP production [30], which is consistent with the fact that the cancer cells use an alternative energy-producing mechanism to maintain a higher ATP level since their mitochondria are significantly less capable of producing ATP [31]. Taken together, these data suggest that intracellular ATP plays a central role in the cancer process [31] and stress the importance of altered glucose metabolism, including HBP and PPP in cancer research.
Breast cancer is classified into various metabolic phenotypes according to its heterogeneous metabolic status. The Warburg effect and mixed metabolic alteration had been identified, and they are closely associated with the triple-negative breast cancer phenotype, whereas the reverse Warburg effect was frequently identified within the luminal type of breast tumors, suggesting a correlation between metabolic phenotype and the biology of breast cancer [9, 32].
The metabolic profiles of cancer cells are distinct from those of normal cells due to the Warburg effect, so SR9243, which can significantly inhibit the Warburg effect by reducing glycolytic activity, induces apoptosis in tumors without inducing weight loss, hepatotoxicity, or inflammation. Therefore, by targeting glycolysis, a broad range of cancers can be treated [33].
There are also some reports that high pyruvate kinase activity induced by exogenous PKM1 expression or pharmacological activation of PKM2 can impair tumor growth and decrease levels of metabolites critical for biosynthesis in vivo, along with the statements that high level of PKM2 promotes cell rapid proliferation [34]. There has been controversy on whether PKM2 should be activated or inhibited for cancer therapy [34, 35]. In our study, the expression of PKM2 in cancer patient samples is higher compared with the 36 control tissues, which is a powerful argument for supporting the theory that we should reduce PKM2 level to treat cancer.
Although PKM2, GFPT1, and G6PD affect the pCR rate of NACT breast cancer patients, their working mechanisms may be diverse as they have different effect on specific clinical items. In details, the expressions of PKM2 and G6PD are closely associated with the molecular subtypes, whereas GFPT1 is correlated with tumor size. All these three factors and p53 have impacts on the PFS and OS of TNBC patients, while in the NACT patients, their functions are expressed in distinct molecular subtypes. Tumor size showed significant correlation with PKM2 and GFPT1 expression, while the pN stage and grade are associated with PKM2 and G6PD expression.
The previous findings stated that the activation of HBP pathway partially deregulated tissue polarity in nonmalignant cells and disorganized polarized tumor cells and might be sufficient to induce the malignant phenotype [12]. Therefore, glycosylation and glycolysis may play an important role in cell proliferation in order to achieve larger tumor size in TNBC, even though currently the crosstalk mechanism has not been understood clearly. Though the results of cell culture suggest that the activation of both glycolysis and HBP is essential for the induction and/or maintenance of canonical oncogenic pathways [36], the synergistic effect between GFPT1 and PKM2 has not been observed in our experiment. This might be due to the miscellaneous molecular subtypes of the cohort. In conclusion, suppression of both HBP and glycolysis may be a new direction of research or treatment in TNBC with large tumor size to achieve preferential PFS and/or OS.
The synergistic effect of PKM2 and G6PD was observed both in NACT and TNBC groups, which is a reflection on ER/PR expression situation, pN stage and grade, respectively. This result was in line with the theory that PKM2 overexpression results in the accumulation of glycolytic intermediates for alternative pathways including the PPP [37]. Considering the reciprocal promotion of the two pathways, we speculate that reducing their common substrates may bring better outcome in cancer treatment.
The PPP possesses an oxidative and non-oxidative branch, which generate NADPH and R5P, respectively. Both the oxidative and non-oxidative branches have been demonstrated to be activated in human cancer. G6PD is the first and rate-limiting enzyme of the oxidative branch, irreversibly oxidizes G6P to 6-Pgluconolactone, and generates NADPH. The non-oxidative branch of the PPP generate R5P reversibly for nucleotide synthesis during proliferation, and the excess amount of pentose phosphate can enter to glycolysis [38]. Furthermore, previously research has demonstrated that oxidative stress causes oxidation of PKM2 that results in reversible enzyme inactivation and enhanced channeling of glucose metabolites through PPP [39, 40]. That may indicate that we should disrupt both PPP and PKM2 activity if we want to deplete glycolysis completely. Taking our results and previous research together, we should repress both PPP and glycolysis for breast cancer patients with the advanced pN stage and/or grade, which may be a new thought of research or cure direction in TNBC to achieve preferential prognosis. The expression of G6PD is higher in luminal A patients probably due to ER expression, and this result is consistent with previous finding that the estrogen receptor antagonist reduced estrogen-induced G6PD protein in patient-derived cells [23]. G6PD inhibitors may enhance the effect of endocrine therapy and show positive effect in endocrinotherapy-resistant patients.
Our study shows that wild p53 inhibits the PPP through G6PD both in NACT patients and TNBC patients, which may indicate that the G6PD inhibitors could exhibit anti-cancer effect in p53 mutation patients [28]. The previous conclusion that p53 regulates biosynthesis through direct inactivation of G6PD [28] has been confirmed in the patient tissues. We expect the combination of p53 and G6PD might shed light on a new direction of research and treatment.
In summary, our result supports the standpoint that PKM2 should be inhibited for cancer therapy. Patients with positive hormone receptor will have favorable prognosis from the suppression of PKM2 and G6PD due to their effect on tumor size, whereas the TNBC patients will prolong their survival period by downregulating the expression of PKM2 and GFPT1 due to their effect on tumor size. Patients who have positive lymph node will benefit from the suppression of PKM2 and G6PD. The synergetic effect of PKM2, GFPT1, G6PD, and p53 on clinical characteristics may shed light on a new direction of research and treatment. Additionally, they might be promising predictors of tumor progression.
References
Ishikawa M, Inoue T, Shirai T, Takamatsu K, Kunihiro S, Ishii H, et al. Simultaneous expression of cancer stem cell-like properties and cancer-associated fibroblast-like properties in a primary culture of breast cancer cells. Cancers. 2014;6(3):1570–8. doi:10.3390/cancers6031570.
Rampurwala MM, Rocque GB, Burkard ME. Update on adjuvant chemotherapy for early breast cancer. Breast Cancer Basic Clin Res. 2014;8:125–33. doi:10.4137/BCBCR.S9454.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (Lond, Engl). 2014;384(9938):164–72. doi:10.1016/s0140-6736(13)62422-8.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74. doi:10.1073/pnas.191367098.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi:10.1038/35021093.
Thompson PA, Brewster AM, Kim-Anh D, Baladandayuthapani V, Broom BM, Edgerton ME, et al. Selective genomic copy number imbalances and probability of recurrence in early-stage breast cancer. PLoS ONE. 2011;6(8):e23543. doi:10.1371/journal.pone.0023543.
Comprehensive molecular portraits of human breast tumours. Nature. 2012;490 Suppl 7418:61–70. doi:10.1038/nature11412.
Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14.
Gonzalez CD, Alvarez S, Ropolo A, Rosenzvit C, Bagnes MF, Vaccaro MI. Autophagy, Warburg, and Warburg reverse effects in human cancer. BioMed Res Int. 2014;2014:926729. doi:10.1155/2014/926729.
Chaneton B, Gottlieb E. Rocking cell metabolism: revised functions of the key glycolytic regulator PKM2 in cancer. Trends Biochem Sci. 2012;37(8):309–16. doi:10.1016/j.tibs.2012.04.003.
Wang HJ, Hsieh YJ, Cheng WC, Lin CP, Lin YS, Yang SF, et al. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1alpha-mediated glucose metabolism. Proc Natl Acad Sci U S A. 2014;111(1):279–84. doi:10.1073/pnas.1311249111.
Onodera Y, Nam JM, Bissell MJ. Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J Clin Invest. 2014;124(1):367–84. doi:10.1172/JCI63146.
Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45(5):598–609. doi:10.1016/j.molcel.2012.01.001.
Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012;150(4):685–96. doi:10.1016/j.cell.2012.07.018.
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. 2012;14(12):1295–304. doi:10.1038/ncb2629.
Palorini R, Cammarata FP, Balestrieri C, Monestiroli A, Vasso M, Gelfi C, et al. Glucose starvation induces cell death in K-ras-transformed cells by interfering with the hexosamine biosynthesis pathway and activating the unfolded protein response. Cell Death Dis. 2013;4, e732. doi:10.1038/cddis.2013.257.
Du W, Jiang P, Mancuso A, Stonestrom A, Brewer MD, Minn AJ, et al. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat Cell Biol. 2013;15(8):991–1000. doi:10.1038/ncb2789.
Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330(6009):1340–4. doi:10.1126/science.1193494.
Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–20. doi:10.1016/j.cell.2006.05.036.
Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10(12):1477–83. doi:10.1038/ncb1807.
Fico A, Paglialunga F, Cigliano L, Abrescia P, Verde P, Martini G, et al. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ. 2004;11(8):823–31. doi:10.1038/sj.cdd.4401420.
Laderoute KR, Calaoagan JM, Chao WR, Dinh D, Denko N, Duellman S, et al. 5′-AMP-activated protein kinase (AMPK) supports the growth of aggressive experimental human breast cancer tumors. J Biol Chem. 2014;289(33):22850–64. doi:10.1074/jbc.M114.576371.
Sun Y, Gu X, Zhang E, Park MA, Pereira AM, Wang S, et al. Estradiol promotes pentose phosphate pathway addiction and cell survival via reactivation of Akt in mTORC1 hyperactive cells. Cell Death Dis. 2014;5, e1231. doi:10.1038/cddis.2014.204.
Bensinger SJ, Christofk HR. New aspects of the Warburg effect in cancer cell biology. Semin Cell Dev Biol. 2012;23(4):352–61. doi:10.1016/j.semcdb.2012.02.003.
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(28):4414–22. doi:10.1200/JCO.2007.10.6823.
Contractor T, Harris CR. p53 negatively regulates transcription of the pyruvate dehydrogenase kinase Pdk2. Cancer Res. 2012;72(2):560–7. doi:10.1158/0008-5472.can-11-1215.
Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, et al. p53 regulates mitochondrial respiration. Science. 2006;312(5780):1650–3. doi:10.1126/science.1126863.
Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 2011;13(3):310–6. doi:10.1038/ncb2172.
Gottlieb E. p53 guards the metabolic pathway less travelled. Nat Cell Biol. 2011;13(3):195–7. doi:10.1038/ncb2177.
Garber K. Energy deregulation: licensing tumors to grow. Science. 2006;312(5777):1158–9. doi:10.1126/science.312.5777.1158.
Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72(1):304–14. doi:10.1158/0008-5472.can-11-1674.
Smith-Vikos T. A report of the James Watson lecture at Yale University. Yale J Biol Med. 2012;85(3):417–9.
Flaveny CA, Griffett K, El-Gendy Bel D, Kazantzis M, Sengupta M, Amelio AL, et al. Broad anti-tumor activity of a small molecule that selectively targets the Warburg effect and lipogenesis. Cancer Cell. 2015;28(1):42–56. doi:10.1016/j.ccell.2015.05.007.
Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8(10):839–47. doi:10.1038/nchembio.1060.
Lim JY, Yoon SO, Seol SY, Hong SW, Kim JW, Choi SH, et al. Overexpression of the M2 isoform of pyruvate kinase is an adverse prognostic factor for signet ring cell gastric cancer. World J Gastroenterol WJG. 2012;18(30):4037–43. doi:10.3748/wjg.v18.i30.4037.
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi:10.1056/NEJMoa021967.
Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452(7184):181–6. doi:10.1038/nature06667.
Lamonte G, Tang X, Chen JL, Wu J, Ding CK, Keenan MM, et al. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab. 2013;1(1):23. doi:10.1186/2049-3002-1-23.
Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334(6060):1278–83. doi:10.1126/science.1211485.
Harris I, McCracken S, Mak TW. PKM2: a gatekeeper between growth and survival. Cell Res. 2012;22(3):447–9. doi:10.1038/cr.2011.203.
Acknowledgments
This study was supported by grant YJSCX2014-49HYD from Harbin Medical University, Harbin, China, The Department of Medical Oncology of the Third Affiliated Hospital of Harbin Medical University, Harbin, China, and the Tumor Research Institute of Heilongjiang, Harbin, China.
Authors’ contributions
Tieying Dong performed IHC experiments, wrote the manuscript, prepared the figures, participated in study design, and interpretation of data. Qijia Xuan prepared the figures and tables. Wenjie Ma collected the information of patients enrolled in this study. Xinmei Kang developed microscopy tools, analyzed specimens, and made a significant contribution to the study design and interpretation of data. Zhaoliang Liu revised the manuscript and polished the language. Shu Zhao and Hang Liu made substantial contributions to review the IHC specimens independently. Zhipeng Wang was involved in critical revisions to the manuscript for important intellectual content. Dr. Qingyuan Zhang participated in the experiment design and interpretation of data. Dr. Qingyuan Zhang gave final approval of the manuscript version to be published and agree to be accountable for questions related to any part of the work. All authors have critically read, edited, and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was conducted with approval from the Ethics Committee of the Third Affiliated Hospital of Harbin Medical University.
Conflicts of interest
None.
Additional information
This experiment was performed in the Oncology Key Lab of the Department of Higher Education of Heilongjiang Province Institution of Higher Education
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 14 kb)
ESM 2
(DOCX 14 kb)
Supplementary Figure
The percentages of positive expression of PKM2, GFPT1, and G6PD in breast cancer group is significantly different from those in relative healthy control group. (PNG 176 kb)
Rights and permissions
About this article
Cite this article
Dong, T., Kang, X., Liu, Z. et al. Altered glycometabolism affects both clinical features and prognosis of triple-negative and neoadjuvant chemotherapy-treated breast cancer. Tumor Biol. 37, 8159–8168 (2016). https://doi.org/10.1007/s13277-015-4729-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4729-8