Abstract
Although advanced surgical operation and chemotherapy have been under taken, pancreatic cancer remains one of the most aggressive and fatal human malignancies with a low 5-year survival rate of less than 5 %. Therefore, novel therapeutic strategies for prevention and remedy are urgently needed in pancreatic cancer. This present research aimed to investigate the anti-cancer effects of hyperoside in human pancreatic cancer cells. Our in vitro results showed that hyperoside suppressed the proliferation and promoted apoptosis of two different human pancreatic cancer cell lines, which correlated with up-regulation of the ratios of Bax/Bcl-2 and Bcl-xL and down-regulation of levels of nuclear factor-κB (NF-κB) and NF-κB’s downstream gene products. What’s more, using an orthotopic model of human pancreatic cancer, we found that hyperoside also inhibited the tumor growth significantly. Mechanically, these outcomes could also be associated with the up-regulation of the ratios of Bax/Bcl-2 and Bcl-xL and down-regulation of levels of NF-κB and NF-κB’s downstream gene products. Collectively, our experiments indicate that hyperoside may be a promising candidate agent for the treatment of pancreatic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer remains one of the most aggressive and fatal human malignancies, which has an astoundingly low 5-year survival rate of less than 5 % in spite of the superior therapy available today [1, 2]. Pancreatic cancer is now the eighth most deadly cancer in men and the ninth most deadly cancer in women in the world [3]. However, patients who have had multimodal therapy including surgery resection have a 5-year survival rate of more than 20 % [4]. The poor therapeutic effect of chemotherapy and radiotherapy, aggressive biological behavior of pancreatic tumors, and lack of diagnosis are the main reasons for the unfavorable prognosis of pancreatic cancer. Thus, novel therapeutic strategies are urgently needed for the effective treatment of this malignant disease.
Nuclear factor-κB (NF-κB), a nuclear transcription factor that plays an important role in regulating invasion, proliferation, growth, anti-apoptosis, and angiogenesis, is frequently activated in many cancer cells [5–7]. More importantly, many experimental results show that NF-κB plays a key role in the growth and proliferation of pancreatic cancer cells. First of all, NF-κB is frequently active in pancreatic cancer cells but poorly active in non-tumorigenic cell lines [6, 8]. Second, NF-κB can directly stimulate cellular proliferation by the induction of promoting growth genes [9, 10]. Third, NF-κB is capable of promoting pancreatic cancer growth through anti-apoptosis [11]. In summary, these lines of evidence elucidate the function of NF-κB in pancreatic cancer and imply that an agent that can down-regulate the activation of NF-κB may potentially suppress the growth of pancreatic cancer.
Numerous studies have shown that many natural herbal medicines have anti-tumor effects. Hyperoside (also known as quercetin 3-O-β-d-galactoside; Fig. 1c), a major active component from Hypericum perforatum [12], has many biological effects, including myocardial protection [13], antioxidant [14], antihyperglycemic [15], anti-inflammatory [16], analgesia [17], and anti-cancer [18–20]. However, no study has investigated the application of hyperoside in curing pancreatic cancer. Therefore, in this study, we attempted to investigate whether hyperoside could inhibit growth and induce apoptosis in pancreatic cancer.
Effect of hyperoside on cell viability. a Cell viability assay. Two pancreatic cancer cell lines (Bxpc-3, PANC-1) were treated with various concentrations of hyperoside for 24, 48, and 72 h, and cell viability was tested by CCK-8 assay. b Human umbilical vein endothelial cell line (HUVEC) and normal human live cell line (HL-7702) were treated with various concentrations of hyperoside for 24, 48, and 72 h, and cell viability was tested by CCK-8 assay. c Chemical structure of hyperoside. Data shown are representative of at least three independent experiments. *p < 0.05, compared with control; **p < 0.01, compared with control
In this study, we investigated whether hyperoside could inhibit the growth of pancreatic cancer cells in vivo and in vitro. We demonstrate that hyperoside could significantly suppress the growth and proliferation of two pancreatic cancer cell lines and pancreatic cancer tissues. Our results show that the anti-cancer effects of hyperoside are associated with molecular mechanisms related to the promotion of the Bax/Bcl-2 and Bax/Bcl-xL ratios and suppression of NF-κB activation.
Methods and materials
Materials
Hyperoside (Sigma-Aldrich, USA) was dissolved in dimethyl sulfoxide (DMSO; Sigma) as a 1000-mmol/L stock solution and stored at 4 °C. Annexin V-FITC and propidium iodide(PI) were purchased from Biosea Biotechnology (Biosea,China). Antibodies used in this study included antibodies against survivin, pro-caspase-3, c-Myc, Bcl-2, cyclin-D1, β-actin, PCNA, and Ki-67 (Santa Cruz Biotechnology, CA, USA), against COX-2 and CD31 (Abcam Inc, MA, USA) and against PARP Bcl-xL (Cell Signaling Technology, Inc, MA, USA). Nuclear Extract Kit and Trans-AM NF-κB p65 ELISA Kit were obtained from Active Motif (Carlsbad, CA, USA).
Cell culture
The human pancreatic cancer cell lines PANC-1 and BxPC-3 were obtained from the American Type Culture Collection (Rockville, USA) and were cultured in DMEM and RPMI 1640 medium, respectively, supplemented with fetal bovine serum (10 %), penicillin (100 U/ml), and streptomycin (100 mg/ml; Irvine Scientific, Irvine, CA). Normal human hepatic cells (HL-7702) were obtained from the Cell Collection of Chinese Academy of science (Shanghai, China) and were cultured in DMEM supplemented with fetal bovine serum (10 %), penicillin (100 U/ml), and streptomycin (100 mg/ml; Irvine Scientific, Irvine, CA). Human umbilical vein endothelial cell line (HUVEC) was obtained from all cells (Shanghai, China) and were cultured in complete medium (HUVEC-004, supplied by AllCells). All cells were maintained at 37 °C in humidified air with 5 % CO2. (All reagents were from HyClone China Ltd., China.) Mycoplasma contamination was tested using the Mycoplasma Stain Assay Kit (Beyotime Institute of Biotechnology, Beijing, China). None of the cell cultures were contaminated with mycoplasma.
Treatment of cells
Hyperoside dissolved in DMSO (final concentration, 0.1 % (v/v)] was used for the treatment of cells. The subconfluent cells (60–70 %) were treated with various concentrations of hyperoside in complete cell culture medium, and the cells treated with 0.1 % DMSO served as control. The following experiments were repeated thrice.
CCK-8 assay
Cell viability in the treated cells was determined by using Cell Counting Kit-8 (CCK-8) kit (Dojindo Laboratories, Kumamoto, Japan) following the instructions outlined by the manufacturer and as previously described by us [21]. Briefly, cells were plated at a density of 3–5 × 103 cells/well with 200 ml of medium in 96-well microtiter plates with increasing doses of hyperoside (0–600 μM, dissolved in DMSO). After treatment, CCK-8 solution (10 μl) was added to each well and the plates were incubated at 37 °C for 90 min. The absorbance of the cell suspension was measured with a microplate reader at a wavelength of 450 nm. The highest concentration of hyperoside does not interfere with the CCK-8 assay reagents in the absence of cells (data not shown). Medium containing 10 % CCK-8 served as a control.
Cell apoptosis assay
The percentage of cells actively undergoing apoptosis was determined by flow cytometry using an Annexin V assay kit following the instructions outlined by the manufacturer and as previously described by us [22]. Briefly, cells were incubated with increasing doses of hyperoside (control, 100, 300, and 500 μM) for 72 h, and then the cells were harvested with trypsin, washed in phosphate buffered saline (PBS), and counted. After counting, 1 × 105 cells were then resuspended in binding buffer at a concentration of 1 × 106 cells/ml. Next, 10 μl of Annexin V and 5 μl of PI were added, and the cells were incubated at room temperature for at least 15 minutes in the dark. After incubation, the percentage of apoptotic cells was analyzed by flow cytometry (Epics Altra II, Beckman Coulter, USA). Cells were also visualized under a laser scanning confocal microscope (LSM-510, Carl Zeiss Jena GmbH, Jena, Germany) to detect apoptosis.
Electrophoretic mobility shift assay
Following treatment, cells were collected and nuclear proteins were extracted according to a method described previously [23]. Electrophoretic mobility shift assay (EMSA) was conducted by preincubating 10 μg of nuclear extract with 1 μg of poly(deoxyinosinic-deoxycytidylic acid) in binding buffer for 30 min at 4 °C. DNA-binding activity was confirmed with biotin-labeled oligonucleotide bio-NF-κB probe (5′-AGTT-GAGGGGACTTTCCCA GGC-3′) using an EMSA kit according to the manufacturer’s instructions (Viagene, Beijing, China). The probe was resolved on a 4 % polyacrylamide gel containing 0.25× Tris/borate/EDTA (TBE) buffer and visualized with a Cool Imager imaging system (IMGR002).
Western blotting
Protein extracts and Western blotting were performed as described previously [21–23]. Cells treated with various concentrations of hyperoside (100, 300, or 500 μM) were harvested and washed twice in ice-cold PBS, sonicated in RIPA buffer (Beyotime Institute of Biotechnology, Beijing, China), and homogenized. Debris was removed by centrifugation at 12,000×g at 4 °C for 10 min, and protein concentration was determined using the BCA protein assay according to the manufacturer’s instructions. Samples containing equal amounts of protein (50 mg) were separated by electrophoresis on 10 or 15 % polyacrylamide SDS gels (100 V for 1 to 2 h) and transferred to polyvinylidene difluoride (PVDF) membranes by electroblotting (100 V for 1 h at 4 °C). The running time and voltage as well as transfer time and voltage, required some optimization depending on the circumstances. The membranes were then blocked by incubating with 5 % skim milk in TBS plus 0.1 % Tween 20 (TBST) buffer for 2 h appropriate primary antibody with gentle agitation overnight at 4 °C. The membranes were then washed several times and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotech, Santa Cruz, CA, USA) for 1 h at room temperature. The membranes were then washed and protein bands were visualized with the enhanced chemiluminescence (ECL) kit followed by exposure of the membrane to X-ray film. β-actin was simultaneously determined as a loading control.
Animal tumor model and treatments
We established animal orthotopic pancreatic tumor model according to methods described previously [24–29]. The female nude BALB/c mice (4–6 weeks old) were purchased from the Animal Research Center at The First Clinical Medical School of Harbin Medical University (Harbin, China). All surgical procedures and care administered to the animals were approved and reviewed by the Animal Care and Use Committee of The First Clinical Medical School of Harbin Medical University (Harbin, China). BxPC-3-luc cells were used for experiments in vivo. First, 5 × 106 cells were subcutaneously injected into the flanks of mice to establish tumors within 2 weeks. Then, the subcutaneous tumors were excised and cut into 1 mm3 fragments. The fragments were implanted into the tail of the pancreas of 6-week-old mice during surgery through a 1.5-cm vertical incision on the upper abdominal. The abdomen was closed after successful operation. After 2 weeks of tumor growth, the mice were randomly assigned to four groups (each group had seven mice): (a) 200 μl of PBS i.p. injection, (b) 10 mg/kg b.w. of hyperoside i.p. injection, (c) 20 mg/kg b.w. of hyperoside i.p. injection, and (d) 40 mg/kg b.w. of hyperoside i.p. injection. Intraperitoneal injections of various concentrations of hyperoside were given daily for 21 days. In vivo IVIS 200 biophotonic imaging system (Caliper Life Sciences, Hopkinton, MA) was used to capture images of pancreatic tumors every week during the course of treatment with hyperoside. Changes in tumor growth and sites of metastasis were evaluated in each treatment group. Body weights of mice were measured before the treatment.
PCNA and Ki-67 immunohistochemistry
The methodology has been described previously [21]. In brief, paraffin-embedded tissue sections (5 μm) were immunostained with an anti-PCNA or anti-Ki-67 Ab. The number of PCNA or Ki-67-positive cells was counted in ten randomly selected microscopic fields at ×400 magnification.
Quantification of apoptosis in tumor sections
The methodology has been described previously [21]. In brief, paraffin-embedded tissue sections (5 μm) were stained with the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) agent (Roche, Shanghai, China), and the number of TUNEL-positive cells was counted in ten randomly selected microscopic fields at ×400 magnification.
Tumor microvessel density
The methodology has been described previously [21]. In brief, tumor sections prepared from tumors 21 days after treatment were immunostained with anti-CD31. The number of microvessels was counted in randomly selected ten microscopic fields at ×200 magnification, and the microvessel density was recorded.
Statistical analysis
Results were expressed as the mean ± standard deviation (SD). The significance of differences between histopathologic scores was assessed by the Kruskal–Wallis test. Additionally, the continuous data were analyzed by ANOVA test and Student–Newman–Keuls test. The differences were considered statistically significant when p < 0.05.
Results
Effect of hyperoside on cell viability in pancreatic cancer cells
To test the effect of hyperoside on the viability of human pancreatic cancer cells, we used BxPC-3 and PANC-1 cells, all of which were exposed to increasing concentrations of hyperoside (0–600 μM) for 24, 48, and 72 h. As shown in Fig. 1a, hyperoside suppressed the proliferation of two pancreatic cancer cell lines in a dose- and time-dependent manner. These data demonstrate that hyperoside can effectively inhibit the proliferation of pancreatic cancer. With a simple linear regression analysis, inhibitory concentration (IC) 50 values were approximately 601.298, 453.586, and 348.493 μM (against BxPC-3) and 555.295, 451.904, and 374.37 μM (against PANC-1) for 24, 48, and 72 h treatment, respectively. However, hyperoside had a minimal effect on human umbilical vein endothelial cell (HUVEC) and normal human live cell (HL-7702) (Fig. 1b).
Hyperoside induces apoptosis in pancreatic cancer cells
In addition to the anti-proliferation effect, morphological detection of hyperoside-treated pancreatic cancer cells illustrated that hyperoside-induced growth suppression could also be associated with the induction of apoptosis. So we tested the apoptosis-inducing effect of hyperoside in pancreatic cancer cells. Cells were treated with various concentrations of hyperoside (100, 300, or 500 μM) for 72 h, stained with Annexin V/PI, subjected to flow cytometry to determine the apoptosis rate. As we can see in Fig. 2a, the treatment of pancreatic cancer cells with various concentrations of hyperoside led to an obvious dose-dependent improvement in both early and late stages of apoptosis. The apoptotic indices were 6.5, 16.6, 42.8, and 63.4 % in BxPC-3 and 3.1, 34.8, 53.7, and 65.2 % in PANC-1 at 0, 100, 300, and 500 μM concentrations of hyperoside, respectively (Fig. 2a). The stained cells were also tested by laser scanning confocal microscopy (Fig. 2b). The dose-dependent pro-apoptotic effects of hyperoside in pancreatic cancer cells are shown in photographs (Fig. 2a–c). Subsequently, the expression of pro-caspase-3 and cleaved PARP was examined by western blotting. Hyperoside treatment down-regulated the level of pro-caspase-3 and increased the expression of cleaved PARP in a dose-dependent manner (Fig. 2d, e).
Effect of hyperoside on apoptosis of pancreatic cancer cells in vitro. a The representative flow cytometry histograms of cell apoptosis for BxPC-3 and PANC-1 cells treated for 72 h with increasing doses of hyperoside (100, 300, 500 μM). b The above-treated cells were stained with Annexin V/PI to assess apoptotic cells under a laser scanning confocal microscopy. The early apoptotic cells show only green fluorescence (Annexin V staining), while the late apoptotic cells show red and green fluorescence together (Annexin V and PI double staining). c Histograms exhibit the apoptosis rates of the above-treated BxPC-3 and PANC-1 cells, as measured by flow cytometry. d The levels of pro-caspase-3 and cleaved PARP were analyzed by western blotting. β-actin was detected as a loading control. e The density of each band was measured and compared with that of the internal control, β-actin. *p < 0.05, compared with control; **p < 0.01, compared with control
Hyperoside regulates the levels of Bcl-2 family proteins in pancreatic cancer cells
Because Bcl-2 family proteins, which include promoters (e.g., Bax) and inhibitors (e.g., Bcl-2 or Bcl-xL) of cell death processes, play crucial roles in apoptosis, we tested the differences in the levels of Bax, Bcl-2, and Bcl-xL in BxPC-3 and PANC-1 cells. Western blot analysis revealed that the treatment of BxPC-3 and PANC-1 cells for 72 h with increasing concentrations of hyperoside led to a dose-dependent decrease in the expression of the anti-apoptotic proteins Bcl-2 and Bcl-XL (Fig. 3a). In contrast, the expression of the apoptotic protein Bax was increased dramatically in a dose-dependent manner by the treatment with hyperoside (Fig. 3a). Therefore, hyperoside treatment can regulate the expression of Bcl-2 family proteins. Treatment with hyperoside increases the ratios of Bax/Bcl-2 and Bcl-xL, which could lead to the observed effects on apoptosis (Fig. 3b).
Effect of hyperoside on the protein level of Bcl-xL, Bcl-2, and Bax. a As detailed in “Methods and materials,” pancreatic cancer cells (BxPC-3 and PANC-1) were treated with hyperoside (100, 300, or 500 μM) for 72 h, and the protein extracts were measured by western blotting using antibodies specific for Bcl-xL, Bcl-2, and Bax. β-actin was detected as a protein-loading control. b The density of each band was measured and compared with that of the internal control, β-actin. *p < 0.05, compared with control; **p < 0.01, compared with control
Hyperoside suppresses NF-κB activation and down-regulates NF-κb’s downstream gene products in pancreatic cancer cells
After treatment with various concentrations of hyperoside, nuclear extract was obtained for detecting NF-κB DNA-binding activity by EMSA and total protein was extracted to detect the gene levels downstream of NF-κB by western blot. As shown in Fig. 4a, hyperoside treatment markedly reduced the DNA-binding activity of NF-κB in a dose-dependent manner in BxPC-3 and PANC-1 cells. As shown in Fig. 4b, exposure to increasing concentrations of hyperoside suppressed NF-κB level and down-regulated the expression of survivin, c-Myc, cyclin D1, and COX-2 in a dose-dependent manner in pancreatic cancer cells (Fig. 4b, c).
Activation of NF-κB and the expression of NF-κB-regulated gene in vitro. a Nuclear extracts were extracted and subjected to EMSA to determine NF-κB DNA-binding activity. b Western blot analysis for NF-κB and the expression of NF-κB-regulated gene products: β-actin was detected as protein loading control. c The density of each band in (b) was measured, and the ratios of Bax/Bcl-2 and Bcl-xL were calculated compared with that of the internal control, β-actin. *p < 0.05, compared with control; **p < 0.01, compared with control
Hyperoside inhibits the growth of pancreatic tumors in mice
To assess the anti-tumor effect of hyperoside in vivo, we established a nude mice model, which was described in the previous section, and treated the mice with different concentration of hyperoside for 3 weeks (Fig. 5a). The tumor volume was measured by detecting the level of photonic fluxes, using the bioluminescence IVIS Imaging System (Fig. 5b). The bioluminescence imaging results demonstrated that compared with the treatment groups, the tumor volume of control groups gradually increased with the passage of time. The suppression of tumor volume by hyperoside was dose dependent (Fig. 5b). After the treatments, the mice were killed. We excised tumors and measured tumor volume using vernier calipers (Fig. 5c, d).
Effects of hyperoside on the growth of orthotopic pancreatic tumors. a Schematic representation of the experimental protocol described in the “Methods and materials.” b The representative bioluminescence images of nude mice treated with various concentrations of hyperoside at various time points: the flux was assessed every week. c The mice were killed, and the tumor was excised at the end of treatment. e The tumor volumes were measured with vernier calipers. *p < 0.05, compared with control; **p < 0.01, compared with control
Hyperoside inhibits cell proliferation and microvessel density and promotes apoptosis in pancreatic tumor tissues
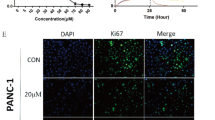
To determine the anti-proliferation and anti-angiogenesis effects of hyperoside in tumor tissues, the levels of Ki-67 (as a cell proliferation marker), PCNA (as a cell proliferation marker), and CD31 (as a microvessel density marker) were immunohistochemically examined. As shown in Fig. 6, compared with the control group, fewer PCNA and Ki-67-positive cells were examined in tumors that were treated by hyperoside. Similarly, as shown in Fig. 6, compared with the control group, hyperoside treatment also resulted in a significant reduction of microvessel density. Both cell proliferation and microvessel density were examined in a dose-dependent manner by immunohistochemistry.
Effects of hyperoside on cell proliferation, apoptosis, and angiogenesis in orthotopic pancreatic tumors. a Immunohistochemical analysis of Ki-67 and PCNA for cell proliferation and CD31 for microvessel density, and TUNEL analysis of apoptotic cells in orthotopic pancreatic tumor tissues. b Representative H&E-stained images obtained from section for orthotopic pancreatic tumor. c Ki-67-positive cells were counted to estimate the proliferation index. d PCNA-positive cells were counted to calculate the proliferation index. e CD31-stained microvessels were counted to estimate the microvessel density. f TUNEL-positive cells were counted to estimate the apoptosis index. *p < 0.05, compared with control; **p < 0.01, compared with control
To assess the apoptotic effect induced by hyperoside in tumor tissues, apoptosis index was evaluated with TUNEL staining. TUNEL staining showed that the percentage of TUNEL-positive cells was increased significantly in correlation with the concentration of hyperoside (Fig. 6). Furthermore, in tumor tissues, compared with the control group, the level of pro-caspase-3 tested by western blot was down-regulated and the expression of cleaved PARP tested by western blot was up-regulated in a dose-dependent manner (Fig. 7a, b).
Effects of hyperoside on the activation of Bcl-2 family and NF-κB pathways in orthotopic pancreatic tumors. a Western blot analysis was performed to measure the level of pro-caspase-3 and cleaved PARP in tumor tissues. b The density of each band in (a) was measured and compared with that of the internal control, β-actin. *p < 0.05, compared with control; **p < 0.01, compared with control. c Effect of hyperoside on the protein expression of Bcl-xL, Bcl-2, and Bax in tumor tissues, and the ratios of Bax/Bcl-2 and Bcl-xL were calculated compared with that of the internal control: β-actin. d EMSA analysis of NF-κB DNA-binding activity in tumor tissues. e The expressions of NF-κB and NF-κB-regulated gene products in pancreatic tumor tissue were also measured by western blot, with β-actin as protein internal control. f The density of each band in (e) was measured and compared with that of the internal control, β-actin. *p < 0.05, compared with control; **p < 0.01, compared with control
Hyperoside regulates the levels of Bcl-2 family proteins in pancreatic tumor tissues
To analyze the mechanism by which apoptosis is induced by hyperoside in tumor tissues, we next examined the expression of Bax, Bcl-2, and Bcl-XL in pancreatic tumor tissues. The western blot results revealed that treatment with increasing doses of hyperoside cause a dose-dependent decrease in the levels of Bcl-2 and Bcl-xL (Fig. 7c),which have anti-apoptotic function. In contrast, Bax, as an apoptotic protein, was significantly increased in a dose-dependently manner upon treatment with hyperoside (Fig. 7c). Thus, hyperoside treatment can modulate the expression of Bcl-2 family proteins and increases the ratios of Bax/Bcl-2 and Bcl-xL, which could contribute to apoptosis in tumor tissues (Fig. 7c).
Hyperoside inhibits NF-κB activation and down-regulates NF-κB’s downstream gene products in pancreatic tumor tissues
We also examined the effect of hyperoside on NF-κB levels in pancreatic tumor tissues. As shown in Fig. 7d, hyperoside, in a dose-dependent manner, significantly inhibits the DNA-binding activity of NF-κB tested by EMSA in pancreatic tumor tissues. We next measured NF-κB expression and downstream gene products of NF-κB. As shown in Fig. 7e, f, compared with the control group, the NF-κB-regulated gene products that were analyzed by western blot after hyperoside treatments decreased in a dose-dependent manner.
Discussion
The discovery of natural compounds might show novel approaches to treating pancreatic cancer, which remains one of the most malignant cancers in spite of great efforts [30]. In our present study, we investigated the remarkable effects of hyperoside on growth inhibition and the promotion of apoptosis in pancreatic cancer cells (PANC-1 and BxPC-3) and an orthotopic pancreatic cancer model. In addition, our results demonstrate that the anti-proliferative, anti-angiogenesis and pro-apoptotic functions on pancreatic cancer may be regulated through the inhibition of NF-κB and promotion of the ratios of Bax/Bcl-2 and Bax/Bcl-xL.
The proteins of the Bcl-2 family play a vital role in controlling the pathway of apoptosis. Some proteins, such as Bcl-2 and Bcl-XL, reduce apoptosis. In contrast, other Bcl-2 family proteins, such as Bax and Bak, induce apoptosis [31–33]. The Bcl-2 family proteins, including anti-apoptotic and pro-apoptotic proteins, can construct heterodimers, which define the boundary of pro- and anti-apoptosis. In our experiment, we found that treatment with hyperoside led to the up-regulation of Bax protein and down-regulation of Bcl-2 and Bcl-xL proteins, increases in the ratios of Bax/Bcl-2 and Bax/Bcl-xL, and apoptosis in vitro. Therefore, the hypothesis that treatment with hyperoside promotes apoptosis in pancreatic cancer cells by increasing Bax and decreasing Bcl-2 and Bcl-xL is reasonable and logical.
NF-κB is involved in different stages of the pancreatic cancer carcinogenetic process and is reported in many malignant tumors [34, 35]. NF-κB, which induces the genes involved anti-apoptosis, invasion, angiogenesis, metastasis, and proliferation, plays an important regulatory role in pancreatic cancer [5–7]. Plenty of natural bioactive compounds with roles in chemoprevention, such as shikonin, escin, dihydroartemisinin, curcumin and honokiol, suppress cell proliferation and induce apoptosis via mechanisms related to NF-κB [21, 23, 36–38]. We hypothesize that treatment with hyperoside may inhibit the activation of NF-κB in pancreatic cancer. In our study, our experiments confirm that treatment with hyperoside could inhibit the NF-κB DNA-binding activity and suppress the expression of total NF-κB p65.
NF-κB, which is active in the cytoplasm, can translocate into the nucleus and bind with specific response elements in DNA sequences and control several gene products implicated in anti-apoptosis (Bcl-2, Bcl-xL, survivin), proliferation (c-Myc, cyclin D1, COX-2), angiogenesis (VEGF), and invasion/metastasis (MMP-9) [6, 34, 39–41]. We next investigated the levels of NF-κB downstream gene products in pancreatic cancer cells. Our data indicated that treatment with hyperoside decreased the expression of NF-κB downstream gene products in a dose-dependent manner. Therefore, these results supported the hypothesis that hyperoside exerts anti-cancer effects through the suppression of the NF-κB signaling pathway in pancreatic cancer.
Most importantly, our findings showed that hyperoside was found to be effective at suppressing of tumor volume. We also found that hyperoside could suppress proliferation (demonstrated by less Ki-67 and PCNA immunostaining), inhibit angiogenesis (demonstrated by less CD31 immunostaining) and promote apoptosis (demonstrated by TUNEL staining) in tumor tissues. Additionally, our results indicated that hyperoside could up-regulate the expression of Bax protein, down-regulate the expression of Bcl-2 and Bcl-xL proteins, increase the ratios of Bax/Bcl-2 and Bax/Bcl-xL, and induce apoptosis in tumor tissues. Similarly to the pancreatic cell results, our results demonstrate that hyperoside could down-regulate NF-κB DNA-binding activity and the expression of total NF-κB p65 and NF-κB downstream gene products in an orthotopic model. These in vitro results are in agreement with results in vivo, demonstrating that the modulation of Bax/Bcl-2 and Bax/Bcl-xL ratios and regulation of NF-κB activity and NF-κB downstream gene products is, at least in part, the anti-cancer molecular mechanisms in pancreatic cancer.
In conclusion, we have shown that hyperoside can promote Bax/Bcl-2 and Bax/Bcl-xL ratios and inhibit NF-κB activation, resulting in the promotion of apoptosis and suppression of proliferation in pancreatic cancer. According to our results, hyperoside may be a good prospect for an anti-cancer drug that can prevent and treat pancreatic cancer. Further in-depth studies containing clinical trials are essential to support our viewpoint for the treatment of pancreatic cancer.
References
Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110(4):738–44.
Oettle H. Progress in the knowledge and treatment of advanced pancreatic cancer: from benchside to bedside. Cancer Treat Rev. 2014;40(9):1039–47.
Ryan DP, Hong TS, Bardeesy H. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–49.
Hartwig W, Werner J, Jäger D, et al. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14(11):e476–85.
Li L, Aggarwal BB, Shishodia S, et al. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101(10):2351–62.
Liptay S, Weber CK, Ludwig L, et al. Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J Cancer. 2003;105(6):735–46.
Maier HJ, Schmidt-Strassburger U, Huber MA, et al. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett. 2010;295(2):214–28.
Weichert W, Boehm M, Gekeler V, et al. High expression of RelA/p65 is associated with activation of nuclear factor-kappaB-dependent signaling in pancreatic cancer and marks a patient population with poor prognosis. Br J Cancer. 2007;97(4):523–30.
Holcomb B, Yip-Schneider M, Schmidt CM. The role of nuclear factor kappaB in pancreatic cancer and the clinical applications of targeted therapy. Pancreas. 2008;36(3):225–35.
Diehl JA, Benzeno S. Cyclin D1 and pancreatic carcinoma: a proliferative agonist and chemotherapeutic antagonist. Clin Cancer Res. 2005;11(16):5665–7.
Greten FR, Weber CK, Greten TF, et al. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123(6):2052–63.
Zou Y, Lu Y, Wei D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem. 2004;52(16):5032–9.
Wang WQ, Ma CG, Xu SY. Protective effect of hyperin against muocardial ischemia and reperfusion injury. Zhongguo Yao Li Xue Bao. 1997;17(4):341–4.
Zhou W, Oh J, Li W, et al. Phytochemical studies of Korean endangered plants: a new flavone from Rhododendron brachycarpum G. Don. Bull Kor Chem Soc. 2013;34(8):2535–38.
Verma N, Amresh G, Sahu PK, et al. Pharmacological evaluation of hyperin for antihyperglycemic activity and effect on lipid profile in diabetic rats. Indian J Exp Biol. 2013;51(1):65–72.
Kim SJ, Um JY, Lee JY. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-κB activation in mouse peritoneal macrophages. Am J Chin Med. 2011;39(1):171–81.
Rylski M, Duriasz-Rowińska H, Rewerski W. The analgesic action of some flavonoids in the hot plate test. Acta Physiol Pol. 1979;30(3):385–8.
Li FR, Yu FX, Yao ST, et al. Hyperin extracted from Manchurian rhododendron leaf induces apoptosis in human endometrial cancer cells through a mitochondrial pathway. Asian Pac J Cancer Prev. 2012;13(8):3653–6.
Yang FQ, Liu M, Li W, et al. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA-21. Mol Med Rep. 2015;11(2):1085–92.
Zhang N, Ying MD, Wu YP, et al. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of humanosteosarcoma cells. PLoS One. 2014;9(7):e98973.
Wang Y, Zhou Y, Jia G, Han B, Liu J, Teng Y, et al. Shikonin suppresses tumor growth and synergizes with gemcitabine in a pancreatic cancer xenograft model: involvement of NF-κB signaling pathway. Biochem Pharmacol. 2014;88(3):322–33.
Wang Y, Zhou Y, Zhou H, Jia G, Liu J, et al. Pristimerin aauses G1 arrest, induces apoptosis, and enhances the chemosensitivity to gemcitabine in pancreatic cancer cells. PLoS ONE. 2012;7(8):e43826.
Wang YW, Wang SJ, Zhou YN, Pan SH, Sun B. Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-κB and nuclear factor-κB-regulated gene products in pancreatic cancer both in vitro and in vivo. J Cancer Res Clin Oncol. 2012;138(5):785–97.
Metildi CA, Kaushal S, Luiken GA, Hoffman RM, Bouvet M. Advantages of fluorescence-guided laparoscopic surgery of pancreatic cancer labeled with fluorescent anti-carcinoembryonic antigen antibodies in an orthotopic mouse model. J Am Coll Surg. 2014;219:132–41.
Maawy AA, Hiroshima Y, Zhang Y, Heim R, Makings L, Garcia-Guzman M, et al. Near infrared photoimmunotherapy with anti-CEA-IR700 results in extensive tumor lysis and a significant decrease in tumor burden in orthotopic mouse models of pancreatic cancer. PLoS One. 2015;10:e0121989.
Maawy AA, Hiroshima Y, Zhang Y, Garcia-Guzman M, Luiken GA, Kobayashi H, et al. Photoimmunotherapy lowers recurrence after pancreatic cancer surgery in orthotopic nude mouse models. J Surg Res. 2015;197:5–11.
Hiroshima Y, Maawy A, Hassanein MK, Menen R, Momiyama M, Murakami T, et al. The tumor-educated-macrophage increase of malignancy of human pancreatic cancer is prevented by zoledronic acid. PLoS One. 2014;9:e103382.
Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89:5645–9.
Furukawa T, Kubota T, Watanabe M, Kitajima M, Hoffman RM. A novel “patient-like” treatment model of human pancreatic cancer constructed using orthotopic transplantation of histologically intact human tumor tissue in nude mice. Cancer Res. 1993;53:3070–2.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300.
Llambi F, Green DR. Apoptosis and oncogenesis: give and take in the BCL-2 family. Curr Opin Genet Dev. 2011;21(1):12–20.
Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122(Pt4):437–41.
Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27(50):6398–406.
Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–8.
Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–6.
Chen H, Sun B, Wang S, et al. Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: involvement of cell cycle arrest and inactivation of nuclear factor-kappaB. J Cancer Res Clin Oncol. 2010;136(6):897–903.
Kunnumakkara AB, Guha S, Krishnan S, et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67(8):3853–61.
Arora S, Bhardwaj A, Srivastava SK, et al. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS One. 2011;6(6):e21573.
Xiong HQ, Abbruzzese JL, Lin E, et al. NF-kappaB activity blockade impairs the angiogenic potential of human pancreatic cancer cells. Int J Cancer. 2004;108(2):181–8.
Pan X, Arumugam T, Yamamoto T, et al. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res. 2008;14(24):8143–51.
Sliva D. Signaling pathways responsible for cancer cell invasion as targets for cancer therapy. Curr Cancer Drug Targets. 2004;4(4):327–36.
Acknowledgments
This work was supported by the National Nature Scientific Foundation of China (No. 81372613, 81170431, 81302057), The National High Technology Research and Development Program of China (No. 2014AA020609), and Youth Science Foundation of Heilongjiang Province (No. QC2012C042).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Li, Y., Wang, Y., Li, L. et al. Hyperoside induces apoptosis and inhibits growth in pancreatic cancer via Bcl-2 family and NF-κB signaling pathway both in vitro and in vivo. Tumor Biol. 37, 7345–7355 (2016). https://doi.org/10.1007/s13277-015-4552-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4552-2