Abstract
The impact of coexistent Hashimoto’s thyroiditis (HT) on lymph node metastasis (LNM) and prognosis in papillary thyroid microcarcinoma (PTMC) remains controversial. We evaluated the association of coexistent HT with clinicopathologic parameters, LNM, and prognosis by retrospectively reviewing a series of consecutive patients treated for PTMC at Fudan University Cancer Center from January 2005 to December 2010. Of all 1,250 patients with complete data for analysis, 364 (29.1 %) had coexistent HT (HT group) and 886 patients (70.9 %) had no evidence of HT (control group). The HT group had higher proportion of female (87.9 vs 70.1 %) patients, higher mean level of thyroid-stimulating hormone (TSH) (2.39 vs 2.00 mIU/L), and lower incidence of extrathyroidal extension (7.4 vs 11.7 %) than those in the control group. However, the incidence of LNM and recurrence was similar between the two groups, and HT was not associated with LNM and recurrence. A series of clinicopathologic factors identified for predicting LNM and recurrence in the control group did not show any prediction in the HT group. In summary, this study suggested that coexistent HT had insignificant protective effect on LNM and prognosis in PTMC, which was inconsistent with prior studies. Further studies aiming to determine novel predictors are recommended in PTMC patients with coexistent HT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many regions of the world, the incidence of papillary thyroid cancer (PTC) is increasing more rapidly than that of any other malignancy [1]. This increase may be explained by the increased detection of papillary thyroid microcarcinoma (PTMC), which is defined as a lesion 10 mm or smaller in diameter. PTMC frequently presents lymph node metastasis (LNM) at the time of diagnosis, which implies a high probability of future recurrence [2–5].
Hashimoto’s thyroiditis (HT) is the most common inflammatory disorder of the thyroid gland, and the coexistence of PTC and HT has been reported to range from 10 to 58 % [6–8]. Current consensus on the explanation for the coexistence of these two disorders suggests that HT may represent the host immune response to preexisting PTC; PTC may be induced or triggered by preexisting HT [9, 10]. However, because of the autoimmune destruction of cancer cells, the coexitent HT is considered as a protective factor with regard to less invasiveness, lower LNM, and better prognosis in PTC [11, 12]. Few studies have reported the effect of coexistent HT on the lymph node and prognosis in PTMC. The aim of this study was to determine whether HT was associated with favorable clinicopathologic factors and prognosis in PTMC.
Materials and methods
Patients
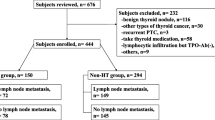
The study included the patients treated at the Department of Head and Neck Surgery, Fudan University Cancer Center, from January 2005 to December 2010. All patients provided written informed consent for their information to be stored in the hospital database and used for research, and this study was approved by the Ethical Committee of Fudan University Cancer Center. Among 5,820 patients treated for PTC during this period, 1,250 patients diagnosed with PTMC (21.5 %) were selected, while 102 patients were excluded because their microcarcinomas were incidentally identified during the thyroidectomy for benign diseases. The patients with PTMC were divided into two groups on the basis of the preoperative findings of autoantibodies or histopathologic confirmation of HT. The HT group was defined as those patients with any one of the following criteria: (1) positive for anti-thyroid peroxidase (TPO) antibody, (2) positive for antithyroglobulin antibody, and (3) pathologic confirmation of HT. In this group, the patients with a previous history of Grave’s disease or preoperative thyroid-stimulating hormone (TSH) receptor antibody (TRAb) were excluded. In addition, the patients with the following criteria were enrolled as a control group: (1) negative for autoantibodies (both anti-TPO antibody and antithyroglobulin antibody); (2) absence of HT pathology; (3) absence of previous history of a thyroid disorder, any thyroid-related prescription; and (4) the absence of the classic ultrasonographic findings of HT. For the final analysis, 364 patients were available for the HT group and 886 patients were available for the control group in this study.
Serum antithyroid antibody (anti-TPO, antithyroglobulin) analysis was performed by radioimmunoassay with a commercial kit (Anti-TPOn; Brahms, Berlin, Germany; anti-Tgn, Brahms). The TSH level was measured by a chemiluminescent microparticle immunoassays (CMIA) (Abbott Laboratories, Abbott Park, IL 60064, USA) with interassay coefficients of variation of less than 10 % over the ranges 0.35–4.94 μIU/mL. HT disease was defined as the presence of diffuse plasma and lymphocytic cell infiltration, oxyphilic cells, the formation of lymphoid follicles, and reactive germinal centers. However, a peritumor inflammatory response was not considered HT. The ultrasound findings of HT were the typical hypoechogenicity of the thyroid gland seen with this disorder.
Surgical treatment
Before surgery, each patient underwent an ultrasonography (US) examination. Lobectomy plus ipsilateral central lymph node dissection (CLND) was typically performed as the initial surgical treatment for the PTMC patients with malignant lesions that were limited to a single lobe [4]. The histology of the frozen sections (FS) guided the extent of the surgical procedures in these patients. Initially, the patients underwent a lobectomy according to the US results; then, if the nodule or nodules were identified as malignant by FS, an ipsilateral CLND was performed. When a benign or undetermined nodule was detected in the contralateral lobe by US, a subtotal lobectomy was performed in our hospital, including nodule enucleation; this procedure was performed on the suspicion of a lesion in the contralateral lobe following the preoperative US or partial lobe resection, in which approximately one fourth to two thirds of the contralateral lobe was resected on the suspicion of more than one lesion in the contralateral lobe following the preoperative US [4]. When malignant lesions were identified in both lobes of the thyroid by FS, a total thyroidectomy (TT) plus a bilateral CLND were performed [4].
Patients with lymphadenopathy in the central or lateral neck, as detected by palpation or US, underwent a computed tomography (CT) scan of the neck. Intraoperative inspection of lateral compartment lymph nodes was another technique. Clinically evident lateral neck lymph node metastasis (LLNM) was known to be nodal metastasis revealed by preoperative evaluation and/or intraoperative inspection. A modified lateral lymph node dissection (LLND), which included levels II–V, with the preservation of the sternocleidomastoid muscle, internal jugular vein, and spinal accessory nerve, was performed only in cases with clinically evident LLNM. The cervical compartment was defined using the Memorial Sloan-Kettering Cancer Center’s classification of cervical lymph node regions (levels II–VI).
Follow-up
All patients received TSH-suppressive hormonal therapy following surgery. Because of its strictly controlled use in China, radioactive iodine (RAI) therapy was not routinely prescribed for the PTMC patients following surgery unless the patients had distant metastasis [4]. The follow-up period for each patient was defined as the length of time from the initial therapy until the last known contact documented by a review of the medical record or a follow-up phone call to the patient. Postsurgical physical examinations were performed every 3–6 months. During the follow-up visits, all patients underwent US of the neck. The recurrence was defined as the appearance of the disease, with new biopsy-proven/secondary surgery-confirmed local disease or distant disease revealed by ultrasonography and/or imaging scans in any patient who had been free of disease (i.e., no palpable disease and negative radioactive iodine scan). The recurrence was classified as a “neck recurrence” if the contralateral lobe or lateral lymph nodes were involved or as a “distant recurrence” if the disease was located in other sites, including the lungs, bones, or brain.
Assessment of clinicopathological variables
The following variables were used to analyze the risk factors for LNM: gender, age at diagnosis, maximal tumor size, serum TSH level, extrathyroidal extension, and coexistent HT. Multifocality was defined as 2 or more tumor foci within the thyroid. According to the current staging system [13], the age of 45 years was used as the cutoff point to divide all patients into two groups: younger patients (≤45 years) and older patients (>45 years). For multifocal tumors, the diameter of the largest tumor focus was taken as the primary tumor. Bilaterality indicated that both lobes of the thyroid were affected by tumors, which was considered to be “bilateral multifocality” as opposed to “unilateral multifocality” indicating two or more tumor foci within unilateral lobe of the thyroid. The lymph node status was analyzed as a binary variable to indicate the detection of malignant lymph nodes. They were staged using the 2009 tumor-node-metastasis (TNM) classification of American Joint Committee on Cancer/International Union Against Cancer [13]. The recurrence was analyzed as a binary variable to indicate whether any recurrence had occurred. The tumor characteristics were assessed by the final pathologic findings.
Statistical analysis
The results are expressed as the mean ± SD. The statistical analyses were performed using Student’s t test, the χ 2 test, or the Mann–Whitney test as appropriate. The odds ratio (OR) and the 95 % confidence interval (CI) for the relationships between each variable and CLNM or LLNM (yes or no) were calculated using logistic regression. The testing of a correlation between TSH and other variables was conducted by Spearman approach. Recurrence-free survival (RFS) was defined as the time between the date of the initial surgery and the first recurrence or death from any cause. Patients who were alive and who did not relapse were censored at the date of their last follow-up visit. Overall survival (OS) was defined as the time between the date of the initial surgery and death (all causes or cause-specific). The survival rates were estimated using the Kaplan–Meier method and were compared with the log-rank test. The hazard ratio (HR) and the 95 % CI for the relationships between each variable and the recurrence were calculated using a Cox regression model. A p < 0.05 was considered significant. Statistical analyses were performed using SPSS for Windows 13.0 computer software (SPSS Inc., Chicago, IL).
Results
Patient characteristics and the comparison according to HT
There were a total of 1,250 patients who met the inclusion and exclusion criteria of the study. The age of these patients ranged from 15 to 78 years (mean 44.3 ± 10.6 years). Based on US and FS results, three different types of surgical procedures were performed: (1) lobectomy with ipsilateral CLND (909 patients); (2) lobectomy, ipsilateral CLND, and subtotal lobectomy of the contralateral thyroid lobe (218 patients); and (3) TT with a bilateral CLND (123 patients). Of the 188 patients who underwent LLND, 167 patients were confirmed as positive LNM in the lateral neck on the final pathological results. The numbers of patients staged from T1, T3, and T4 were 1,119, 101, and 30, respectively. According to the extent of nodal metastasis, 167 patients presented as N1b and 443 presented as N1a.
There were more women in the HT group; the mean level of TSH was higher; and the incidence of extrathyroidal extension was significantly lower in the HT group (p = 0.001 and 0.040, respectively). The other characteristics of the patients were similar regardless of the coexistence HT or not (Table 1).
Risk factors for CLNM according to HT
Zhang et al. [4] reported that male gender, younger age (<45 years of age), the presence of multifocal lesions, extrathyroidal extension, and larger sized primary tumor were risk factors for CLNM in PTMC; similar results were found in the control group in this study. HT was reported to be associated with a lesser incidence of CLNM in PTC by a series of studies; however, HT was not a negative predictor for CLNM in PTMC patients (p = 0.387). In the HT group, younger age (OR = 0.603, 95 % CI 0.392–0.928, p = 0.022) was the only indicator for CLNM, while other significant predictors identified in the control group did not show any prediction for CLNM in the HT group (Tables 2 and 3).
Risk factors for LLNM according to HT
HT was not associated with LLNM in PTMC patients in this study (p = 0.507). When investigating the risk factors for LLNM based on the coexistent HT, we found that CLNM predicted LLNM significantly in either the HT group or the control group; however, other independent indicators for LLNM identified in the control group, such as male gender (OR = 4.349, 95 % CI 1.797–10.524, p = 0.001), unilateral multifocality (OR = 2.955, 95 % CI 1.690–5.165, p = 0.001), extrathyroidal extension (OR = 3.623, 95 % CI 2.175–6.036, p = 0.001), and CLNM (OR = 6.138, 95 % CI 3.580–10.526, p = 0.001), did not show any prediction for LLNM in the HT group (Tables 4 and 5).
Risk factors for recurrence according to HT
The mean follow-up duration was 62.2 months (range 34–113 months). Neck recurrences occurred in 37 patients (3.0 %), which was divided based on the locations into (i) 22 (1.8 %) in contralateral lobe and central nodes, and (ii) 15 (1.2 %) in lateral nodes. Two distant recurrences (lung metastases) were also observed. During this time, no patients died of thyroid cancer. The incidence of neck recurrence was parallel between the HT and control group (Table 1), and HT was not a risk or protect factor for neck recurrence in PTMC patients (p = 0.240 by the log-rank test). Tables 6 and 7 showed the Cox regression analysis for neck recurrence based on the coexistent HT. Although extrathyroidal extension (HR = 2.340, 95 % CI 1.024–5.925, p = 0.044) and LLNM (HR = 3.782, 95 % CI 1.457–9.815, p = 0.006) were risk factors for neck recurrence in the control group, both of them did not show any significant prediction in the HT group.
The correlation between TSH and pathologic features of tumor
The level of TSH was associated with the presence of HT (p = 0.001) in PTMC; however, a correlation between TSH and pathologic features of tumor, such as size, unilateral multifocality, bilaterality, and extrathyroidal extension, was not significant by Spearman test.
Discussion
PTMC is considered as an indolent tumor presenting excellent prognosis; however, it is reported that as high as 40–60 % of the patients show regional LNM at diagnosis and recurrence is not uncommon during the follow-up period [4, 5, 14]. Recently, there has been a particular interest in the impact of HT in PTC; the current opinions consider that PTC in the presence of HT is associated with decreased tumor aggressiveness, better locoregional control, lesser rates of recurrence, and greater overall and disease-free survival [15–20]. However, few studies investigate the influence of HT in PTMC. This study firstly delineated the specific features of PTMC coexisted with HT and assessed the impact of HT on LNM and prognosis in PTMC. Although PTMC patients with coexistent HT had lower incidence of extrathyroidal extension, unlike previous findings in PTC, they did not exhibit less LNM and favorable prognosis. When exploring the differences in LNM and recurrence between subgroups with and without HT, a series of risk factors identified in PTMC without HT did not show any negative prediction in PTMC coexisted with HT, suggesting that HT might have an impact against aggressive behaviors and play a protective role in the natural history to some extent.
HT is an autoimmune disease characterized by widespread lymphocyte infiltration, fibrosis, and parenchymal atrophy of thyroid tissue [16, 18]. The autoimmune response to thyroid-specific antigens may be involved in the destruction of cancer cells expressing thyroid-specific antigens, thus preventing the progression and improving the prognosis in PTC. However, it remains unclear the actual rate and the effectiveness of coexisting HT in PTMC. According to current literatures, the frequency of coexistent HT in patients with PTC has been variously reported to range from 0.5 to 38 % [8, 19, 20]. Similarly, in the presently analyzed subjects, PTMC coexisted with HT was found in 364 (29.1 %) of the patients. Given the high frequency of the coexistence for these two diseases, we further determined the clinicopathologic differences based on the presence of HT and evaluated the effect of HT on the biologic behavior of PTMC.
The results of this study showed that PTMC coexisted with HT was associated with a higher likelihood of female patients and a lower frequency of extrathyroidal extension, which were consistent within the findings of previous studies in PTC [18, 21, 22]. However, there were no significant differences in the other clinicopathologic features and the distribution of T stage between the two groups. On the other hand, it is hypothesized that the inflammation caused by HT leads to atrophy and fibrosis of the thyroid gland [23]; this process would involve associated damage to the surrounding lymphatic vessels which impairs the lymphatic spread of PTC and ultimately leads to a reduced likelihood of LNM. Only a handful studies have reported on the association between HT and LNM [11, 21, 24] in PTC. Kim et al. [21] demonstrated that PTC with HT had a lesser incidence of LNM when adjusted for age, gender, tumor size, preoperative TSH levels, and mean number of dissected lymph nodes. They also showed that PTC with HT had a lesser N stage of disease at the time of initial treatment. However, in this study, no significant differences in the incidence of LNM or the distribution of N stage in PTMC regardless of the coexistent HT, suggesting that HT did not affect much on decreasing the nodal invassiveness in PTMC.
Since current guidelines have no specific recommendations for the nodal management of PTMC, numbers of studies attempt to investigate the risk factors for LNM, aiming to tailor the initial treatment for local nodes, especially for the central nodes [5, 14, 25–27]. A consensus indicates that CLND need to be considered in PTMC patients presenting with risk factors, such as male gender, younger age, multifocal lesions, extrathyroidal extension, and larger size of the primary tumor. However, none of the mentioned studies distinguished the subjects by the coexistence of HT. The comparison of predictors for LNM between the two groups in this study indicated that a series of risk factors identified in the control group lost their predictions in the HT group, which challenged the current predictive systems for LNM in PTMC.
HT is the most common cause of hypothyroidism, and our data showed that the preoperative TSH levels were significantly higher in the HT group compared with those in the control group. It is reported that a high TSH level is associated with an increased tumorigenesis of thyroid cancer and advanced stage disease [8, 28], which is the foundation for the effectiveness of TSH suppression by decreasing the risk of tumorigenesis and cancer recurrence after lobectomy. Although TSH level had no relations with LNM or stage of disease in PTMC according to our data, the impact on the tumorigenesis in PTMC needed to be verified in all patients with micronodular thyroid disease, and further studies on the gene mutation (i.e., BRAF, RET/PTC) were expected to study for the relationship to LNM in PTMC with HT [29–31].
The presence of HT in patients with PTC has been reported to be associated with a favorable prognosis, because of the destroying for cancer cells from autoimmunity stimulated by the thyroid-specific antigen of cancer itself. Although PTMC with HT was associated with a lower frequency of extrathyroidal extension, which was considered as one of the risk factors for poor prognosis, over a mean follow-up period of 62.2 months, the protective impact on prognosis was found to be insignificant in PTMC. However, risk factors such as extrathyroidal extension and LLNM in PTMC without HT did not predict recurrence in PTMC with HT; moreover, a high TSH level was not associated with the increased incidence of recurrence, either. These reflected that HT might influence the progression of PTMC to some extent, but given the excellent prognosis of PTMC itself, HT was not powerful enough to improve the outcome significantly in PTMC.
In conclusion, we found a relatively common occurrence of HT in patients with PTMC. The coexistent HT was associated with a lower frequency of extrathyroidal extension and a higher level TSH in PTMC. However, unlike most of prior reports clarifying the coexistent HT might protect against LNM and improve prognosis in PTC, our finding suggested that HT had insignificant protective effect on LNM and prognosis in PTMC. Given the lost ability of prediction for LNM and prognosis by some traditional clinicopathologic factors identified in PTMC without HT, further studies aiming to determine novel predictors, such as molecular markers, were recommended in PTMC patients with the coexistent HT.
References
McLeod DS, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet. 2013;381:1046–57.
Malandrino PPG, Attard M. Papillary thyroid microcarcinomas: a comparative study of the characteristics and risk factors at presentation in two cancer registries. J Clin Endocrinol Metab. 2013;2013(98):1427–34.
Lee J, Park JH, Lee CR, Chung WY, Park CS. Long-term outcomes of total thyroidectomy versus thyroid lobectomy for papillary thyroid microcarcinoma: comparative analysis after propensity score matching. Thyroid. 2013;23:1408–15.
Zhang L, Wei WJ, Ji QH, Zhu YX, Wang ZY, Wang Y, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab. 2012;97:1250–7.
Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237:399–407.
Tamimi DM. The association between chronic lymphocytic thyroiditis and thyroid tumors. Int J Surg Pathol. 2002;10:141–6.
Kebebew E, Treseler PA, Ituarte PH, Clark OH. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg. 2001;25:632–7.
Zhang L, Li H, Ji QH, Zhu YX, Wang ZY, Wang Y, et al. The clinical features of papillary thyroid cancer in Hashimoto’s thyroiditis patients from an area with a high prevalence of Hashimoto’s disease. BMC Cancer. 2012;12:610.
Zeppa P, Cozzolino I, Peluso AL, Troncone G, Lucariello A, Picardi M, et al. Cytologic, flow cytometry, and molecular assessment of lymphoid infiltrate in fine-needle cytology samples of hashimoto thyroiditis. Cancer. 2009;117:174–84.
Muzza M, Degl’Innocenti D, Colombo C, Perrino M, Ravasi E, Rossi S, et al. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol (Oxf). 2010;72:702–8.
Jara SM, Carson KA, Pai SI, Agrawal N, Richmon JD, Prescott JD, et al. The relationship between chronic lymphocytic thyroiditis and central neck lymph node metastasis in North American patients with papillary thyroid carcinoma. Surgery. 2013;154:1272–80. discussion 1280–1272.
Sciuto R, Romano L, Rea S, Marandino F, Sperduti I, Maini CL. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann Oncol. 2009;20:1728–35.
Sobin LHGM, Wittekind CH. UICC: TNM classification of malignant tumors. 7th ed. New York: Wiley-Liss; 2009.
Lee J, Park JH, Lee CR, Chung WY, Park CS. Long-term outcomes of total thyroidectomy versus thyroid lobectomy for papillary thyroid microcarcinoma: comparative analysis after propensity score matching. Thyroid. 2013;1408–15. doi:10.1089/thy.2012.0463.
Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:458–63.
Kashima K, Yokoyama S, Noguchi S, Murakami N, Yamashita H, Watanabe S, et al. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid. 1998;8:197–202.
Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, et al. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab. 1995;80:3421–4.
Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009;71:581–6.
Yoon YH, Kim HJ, Lee JW, Kim JM, Koo BS. The clinicopathologic differences in papillary thyroid carcinoma with or without co-existing chronic lymphocytic thyroiditis. Eur Arch Otorhinolaryngol. 2012;269:1013–7.
Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999;126:1070–6. discussion 1076–1077.
Kim SS, Lee BJ, Lee JC, Kim SJ, Jeon YK, Kim MR, et al. Coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma: the influence of lymph node metastasis. Head Neck. 2011;33:1272–7.
Schaffler A, Palitzsch KD, Seiffarth C, Hohne HM, Riedhammer FJ, Hofstadter F, et al. Coexistent thyroiditis is associated with lower tumour stage in thyroid carcinoma. Eur J Clin Investig. 1998;28:838–44.
Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335:99–107.
Kim YS, Choi HJ, Kim ES. Papillary thyroid carcinoma with thyroiditis: lymph node metastasis, complications. J Korean Surg Soc. 2013;85:20–4.
Zheng X, Wei S, Han Y, Li Y, Yu Y, Yun X, et al. Papillary microcarcinoma of the thyroid: Clinical characteristics and BRAF(V600E) mutational status of 977 cases. Ann Surg Oncol. 2013;20:2266–73.
Hyun SM, Song HY, Kim SY, Nam SY, Roh JL, Han MW, et al. Impact of combined prophylactic unilateral central neck dissection and hemithyroidectomy in patients with papillary thyroid microcarcinoma. Ann Surg Oncol. 2012;19:591–6.
Vayisoglu Y, Ozcan C. Involvement of level IIb lymph node metastasis and dissection in thyroid cancer. Gland Surg. 2013;2:180–5.
Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–14.
Kim SK, Song KH, Lim SD, Lim YC, Yoo YB, Kim JS, et al. Clinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent hashimoto thyroiditis. Thyroid. 2009;19:137–41.
Crawford S, Belajic D, Wei J, Riley JP, Dunford PJ, Bembenek S, et al. A novel B-RAF inhibitor blocks interleukin-8 (IL-8) synthesis in human melanoma xenografts, revealing IL-8 as a potential pharmacodynamic biomarker. Mol Cancer Ther. 2008;7:492–9.
Puxeddu E, Moretti S, Elisei R, Romei C, Pascucci R, Martinelli M, et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:2414–20.
Acknowledgments
This research is supported by grants from the Key Project of Science and Technology Commission of Shanghai Municipality (11DJ1400200).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Ning Qu, Ling Zhang, and Dao-zhe Lin contributed equally to the paper.
Rights and permissions
About this article
Cite this article
Qu, N., Zhang, L., Lin, Dz. et al. The impact of coexistent Hashimoto’s thyroiditis on lymph node metastasis and prognosis in papillary thyroid microcarcinoma. Tumor Biol. 37, 7685–7692 (2016). https://doi.org/10.1007/s13277-015-4534-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4534-4