Abstract
Betaine homocysteine methyltransferase (BHMT) catalyzes the synthesis of methionine using betaine and homocysteine (Hcy), which is restricted to the liver and kidney. Impaired BHMT pathway has been associated with hepatocellular carcinogenesis in Bhmt−/− mice model, and decreased BHMT was observed in a small sample of human hepatocellular carcinoma (HCC) patients. However, the prognostic significance of BHMT in HCC has not been elucidated. This study aimed to examine the expression of BHMT in HCC and investigate the relationship between its expression and prognosis of HCC patients. BHMT expression was analyzed in 68 paired HCC samples (HCC tissues vs matched adjacent non-cancerous liver tissues), 115 paraffin-embedded HCC sections (primary cohort), and 65 paraffin-embedded HCC sections (validation cohort) using immunohistochemistry (IHC). The results of IHC analysis showed that BHMT was decreased in tumorous tissues in 85.2 % (58/68) of cases compared to the corresponding adjacent non-tumorous liver tissues. Further correlation analyses indicated that the decreased BHMT expression was closely correlated with serum α-fetoprotein (AFP) (p = 0.011), tumor size (p = 0.039), and vascular invasion (p = 0.017). Moreover, HCC patients with low BHMT expression had shorter overall survival (OS) and time to recurrence (TTR) than those with high BHMT expression in both primary cohort (p < 0.0001) and validation cohort (p < 0.05) assessed by the Kaplan–Meier method. In addition, multivariate analysis showed that BHMT was an independent prognostic factor for OS and TTR in the two cohorts (all p < 0.005). Collectively, our study demonstrated that BHMT could be served as a potential prognostic marker for HCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third major cause of cancer-related death globally [1]. Despite many improvements have been achieved in clinical treatments such as surgery, chemotherapy, and liver transplantation, the prognosis of HCC patients is still poor largely due to the high recurrence and metastasis rates even after resection [2, 3]. The 5-year survival rate of HCC patients is as low as 25–39 %, and its recurrence rate remains about 80 % [4, 5]. Evaluating risk factors that can predict the prognosis of HCC patients affect the selection of an appropriate treatment regimen for each HCC patient. Although intensive efforts have been made to identify prognostic markers of HCC, and many prognostic markers have been assessed in HCC, such as Smad4 [6], AP-4 [7], CD133 [8], and KMO [9], the accuracy and reproducibility of these markers currently used to predict prognosis after surgical resection are still unsatisfactory. For example, AP-4 was only highly expressed in 53.6 % of the HCC patients and just served as an independent prognostic parameter for the overall survival (OS) rate of HCC patients [7]. In a meta-analysis nominally involved 21 studies, only ten of the included studies reported data on the association between CD133 expression and OS, while only five (24 %) reported data on the association between CD133 expression and disease-free survival (DFS) [8].
Betaine homocysteine methyltransferase (BHMT) is a Zn2+-dependent thiolmethyltransferase and catalyzes the synthesis of methionine using homocysteine (Hcy) and the methyl donor betaine [10]. BHMT catalyzes up to 50 % of homocysteine metabolism in the liver and regulates tonicity in the liver and kidney, as its substrate (betaine) acts as a cellular osmolyte to maintain cellular volume [11, 12]. In most species, BHMT is very abundant in the liver, representing 0.6–1.6 % of the total protein, whereas had low activity in the kidney of humans, pigs, and rat [13–15]. It has been reported that upregulation of BHMT could prevent hyperhomocysteinemia, endoplasmic reticulum (ER) stress, and liver injury in the rat but not mouse against alcohol-induced liver injury [16]. Recently, BHMT, as well as the folate cycle, was also found to contribute to a methyl pool required for development of normal inner cell mass (ICM) of the mouse blastocyst and establish initial embryonic DNA methylation [17]. In addition, the expression of BHMT has shown a significant reduction in HepG2 cells and in liver tumoral tissues vs healthy liver tissues, which was caused by a splicing variant of BHMT gene [18]. However, the prognostic significance of BHMT in HCC remains unclear. In this study, we verified the expression of BHMT and evaluated its prognostic significance in HCC patients. Our data indicated that BHMT was remarkably decreased in HCC and could be served as a promising biomarker of HCC prognosis.
Materials and methods
Patients and specimens
Paraffin-embedded pathological specimens (n = 248) were randomly recruited from the archives of the Qilu Hospital of Shandong University, Qingzhou People’s Hospital, Affiliated Hospital of Guilin Medical University, from January 2008 to December 2011. Among those, 68 HCC cases with paired peritumoral tissue were used as expression pattern cohort and 180 HCC cases without paired peritumoral tissue were used as prognostic cohort. Prognostic cohort was randomly divided into primary cohort (n = 115) and validation cohort (n = 65). Institutional review board approval and written informed consent from each patient were obtained.
For suspected cases with elevated serum AFP level (>20 ng/ml as positive), computed tomography (CT) and/or magnetic resonance imaging (MRI) were used to verify tumor recurrence. All patients in this study did not receive sorafenib or any other neoplastic and immunomodulating agents treatment. Interval between surgery and death or between surgery and the last observation point was defined as overall survival (OS). Interval between date of surgery and date of any diagnosed relapse was defined as time to recurrence (TTR). Tumor stage was defined according to the American Joint Committee on Cancer (AJCC 2010, 7th edition) tumor-node-metastasis (TNM) staging system [18]. The grade of tumor differentiation was assigned by the Edmondson-Steiner grading system. Micrometastases were defined as tumors adjacent to the border of the main tumor that were only observed under the microscope [19].
Immunohistochemistry and scoring
Tissue microarrays (TMAs) were constructed according to the method described previously [19]. In this study, TMAs contained 115 HCC tissues, 65 HCC tissues, and 68 paired HCC tissues and peritumoral tissues, respectively. IHC analysis for BHMT was performed according to the method reported previously [20]. Briefly, sections of 4-μm thick were placed on slides coated with 3-aminopropyltriethoxysilane. Paraffin sections were deparaffinized in xylene and rehydrated through decreasing concentrations of ethanol (100, 95, and 85 % for 5 min each). Antigens were retrieved by microwave irradiation for 3 min in pH 6.0 citric buffer and cooled at room temperature for 60 min. Endogenous peroxidase activity was blocked by incubation of the slides in 3 % H2O2/phosphate-buffered saline, and non-specific binding sites were blocked with goat serum. Primary antibody was as follows: rabbit polyclonal to BHMT (AV41474; Sigma-Aldrich, USA; 1:400 dilution). An EnVision Detection kit (GK500705: Gene Tech, Shanghai, China) was used to visualize tissue antigens. Tissue sections were counterstained with hematoxylin for 5 min. Negative control slides omitting primary antibody were created for all assays. Photographs of two representative fields were captured under high-power magnification (×200), and identical settings were used for each photograph. The representative fields were defined as follows: first, we glance at ×40 magnification. If there has high-expression and low-expression area, we captured at ×200 magnification, which contains both high-expression and low-expression area. Image-Pro Plus v6.0 software was used to count and measure integrated optical density (IOD), and mean IOD was calculated from two photographs per patient. X-tile plots were created for assessment of BHMT expression, and optimization of cut-points was based on the outcome of IOD value [21]. Statistical significance was assessed using the cutoff score derived from 180 cases by a standard log-rank method, with p values obtained from a lookup table.
Statistic analysis
Differences among variables were assessed by χ 2 analysis or two-tailed Student’s t test. Kaplan–Meier analysis was used to assess survival. Log-rank tests were used to compare survival of patients between subgroups. Multivariate analyses were performed by multivariate Cox proportional hazard regression model. Data were presented as mean ± SEM. Differences were considered to be statistically significant for p < 0.05.
Results
Downregulation of BHMT in HCC tissues
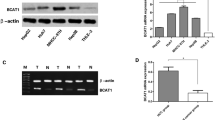
To investigate whether BHMT might play a role in the development and progression of HCC, we performed IHC analysis for BHMT using a tissue microarray, which contained 68 paired HCC tissue samples. As shown in Fig. 1a, the majorities of tumoral and peritumoral tissues showed diffuse cytoplasmic expression pattern of BHMT. Compared with paired peritumoral tissues, HCC tissues had significantly downregulated expression of BHMT (Fig. 1b). Statistical analysis of the BHMT staining density in 68 available paired tissues also revealed that the BHMT was obviously weaker in HCC group than that in peritumoral tissues group (Fig. 1c, p < 0.0001).
Downregulation of BHMT in HCC tissues. BHMT expression analyses in 68 paired samples of HCC tissues and matched peritumoral liver tissues using IHC staining. a Representative images of the primary HCC tissue and matched peritumoral tissue sample taken from the same patient (magnification ×200). b Representative image of HCC portion and peritumoral portion in the same patient (magnification ×200). c Integrated optical density (IOD) for BHMT was obtained from 68 paired samples of HCC tissues and matched peritumoral liver tissues
Correlation of BHMT expression with prognosis of HCC patients
IHC analysis was performed to assess the BHMT expression in 180 paraffin-embedded HCC tissues, in which 115 were in the primary cohort and 65 were in the validation cohort. The survival and recurrence of selected patients was analyzed with Kaplan–Meier analysis. As shown in Supplemetary Figure 1A-B, HCC patients of primary cohort with low BHMT expression had much shorter OS times (mean OS 32.0 vs 54.3 months, p < 0.0001) and a higher tendency of disease recurrence (mean TTR 26.1 vs 47.3 months, p < 0.0001). Likewise, HCC patients of validation cohort with low BHMT expression had much shorter OS times (mean OS 29.8 vs 43.9 months, p = 0.020, Supplemetary Figure 1C) and a higher tendency of disease recurrence (mean TTR 21.0 vs 36.3 months, p = 0.037, Supplemetary Figure 1D).
The association between BHMT expression and OS/TTR was also analyzed in all 180 HCC patients, including both primary cohort and validation cohort. The results strongly showed that HCC patients with low BHMT expression had much shorter OS times (mean OS 32.1 vs 52.2 months, Fig. 2a, p < 0.0001) and a higher tendency of disease recurrence (mean TTR 25.0 vs 44.5 months, Fig. 2b p < 0.0001).
Association between BHMT expression and clinicopathological features
Next, the relationship between BHMT expression and clinicopathological variables of all HCC patients in our study (n = 248) was investigated. Significant correlations were found between BHMT expression and three parameters, including serum AFP (p = 0.011), tumor size (p = 0.039), and vascular invasion (p = 0.017), respectively. HCC patients with low BHMT expression had a higher tendency to be with high level of serum AFP, large-size tumor, and frequent vascular invasion. However, there were no statistical connections between BHMT expression and the rest clinicopathological parameters, such as age, sex, HBsAg, liver cirrhosis, tumor differentiation, tumor number, Child-Pugh class, and TNM stage (all p > 0.05, Table 1).
Univariate and multivariate analyses of prognostic significance of BHMT in HCC patients
Furthermore, univariate analysis was applied to evaluate prognostic significance between BHMT and clinicopathologic parameters in primary cohort and validation cohort, respectively. BHMT expression and the clinicopathologic variables that were significant in univariate analysis of the primary cohort, such as liver cirrhosis, TNM stage, tumor size, and tumor number, were further evaluated in multivariate analysis. As shown in Supplementary Table 1, BHMT was found to be an independent predictor for OS (hazard ratio (HR) 0.457, 95 % confidence interval (CI) 0.239–0.874, p = 0.018). In the validation cohort, TNM stage, tumor size, and vascular invasion were found significant in univariate analysis. Those clinicopathologic variables and BHMT expression were also further evaluated in multivariate analysis. The result (Supplementary Table 2) showed that BHMT was also an independent predictor for OS (HR 0.438, 95 % CI 0.209–0.916, p = 0.024) and TTR (HR 0.452, 95 % CI 0.226–0.906, p = 0.025).
Multiple Cox regression analysis was further utilized to evaluate independent prognostic value of BHMT in all 180 HCC patients, including both primary cohort and validation cohort. The results also indicated that BHMT was an independent prognostic marker for OS (HR 0.433, 95 % CI 0.266–0.704, p = 0.001) and TTR (HR 0.543, 95 % CI 0.347–0.848, p = 0.007) (Table 2).
Discussion
HCC is a prevalent cancer featured with high rate of metastasis and mortality. Surgery and chemotherapy for HCC have achieved great improvements in recent years; however, the dismal prognosis for HCC patients after surgical resection is still common [22]. Although several molecular biomarkers, such as Smad4 [6], AP-4 [7], CD133 [8], and KMO [9], have been reported to have clinical significance for predicting HCC prognosis, the searching for valuable biomarkers for HCC diagnosis and prognostic prediction is still substantially needed in clinic. In this study, we aimed to investigate the expression and the prognostic value of BHMT in a large cohort of HCC patients.
Our results showed that the BHMT protein level was significantly downregulated in HCC patients. In agreement with our results, it has been reported that BHMT transcripts were significantly decreased in HepG2 cells and HCC samples [18]. Further investigation conformed that the decreased BHMT transcripts resulted from the transcription of a splicing variant that produced a frameshift in exon 4, with a premature termination codon in exon 5 [18]. In addition, BHMT messenger RNA (mRNA) was also significantly reduced in liver cirrhosis, which might result from the hypermethylation of methionine adenosyltransferase (MAT1A) promoter [23]. A recent study has shown that BHMT expression was decreased in dysplastic liver nodules (DNs) and HCCs in F344 and BN rats, compared with normal liver [24]. BHMT was also closely rated with other types of cancer, such as head and neck squamous cell carcinoma (HNSCC) [25], renal cell carcinoma (RCC) [26], and uterine cervical carcinoma [27]. For example, BHMT 742GA polymorphism associated with tobacco modulate cancer risk in HNSCC [25]. In RCC, BHMT mRNA was significantly decreased and might be involved in the development of RCC [26]. In addition, BHMT 239Gln variant played a protective role in cervical cancer incidence [27].
In results of Kaplan–Meier survival analysis, we found that HCC patients with high BHMT expression had longer survival. This might be explained by the findings that BHMT participated in HCC development in the Bhmt deletion mouse model [28]. Our current study employed large numbers of patients in two independent cohorts (n = 180). Cox regression analysis suggested independent risk factors for predicting short overall survival including liver cirrhosis, tumor size, tumor number, and BHMT expression. It is necessary to point out that BHMT acted as an independent prognostic biomarker in both primary cohort and validation cohort and had significant negative correlation with both OS and TTR of HCC patients. All these results indicated that BHMT may be a pivotal modulator involved in cancer development. Therefore, IHC detection of BHMT expression in HCC may aid in the development of new therapeutic strategies.
BHMT expression was not associated with any other clinicopathologic factors, including age, sex, HBsAg, liver cirrhosis, tumor differentiation, tumor number, Child-Pugh class, and TNM stage. Nevertheless, low BHMT expression was significantly associated with malignant tumor characteristics, such as high level of serum AFP, large-size tumor, and frequent vascular invasion. Levels of AFP increased with tumor progression in HCC patients, and high levels of AFP had a poor prognosis and exhibited multicentric growth more frequently than AFP negative patients [29, 30]. The size of tumor nodule, which represents tumor burden, was also frequently associated with the aggressiveness of HCC and determined the clinical outcome of the HCC patients [31, 32]. Moreover, vascular invasion was also recognized as a risk factor for outcome following curative resection in HCC [33, 34]. Above all, decreased BHMT expression might be served as a hallmark of advanced stage tumors. However, the detailed tumor suppressive effects of BHMT require further investigation.
In summary, to the best of our knowledge, this is the first study to report that low BHMT expression is correlated with aggressive malignant phenotype of HCC. Our data indicate that BHMT may serve as a novel prognostic marker for HCC.
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Sasaki Y, Yamada T, Tanaka H, et al. Risk of recurrence in a long-term follow-up after surgery in 417 patients with hepatitis B-or hepatitis C-related hepatocellular carcinoma. Ann Surg. 2006;244:771–80.
Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–55.
Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892–9.
Ueno M, Uchiyama K, Ozawa S, et al. Adjuvant chemolipiodolization reduces early recurrence derived from intrahepatic metastasis of hepatocellular carcinoma after hepatectomy. Ann Surg Oncol. 2011;18:3624–31.
Hiwatashi K, Ueno S, Sakoda M, et al. Strong Smad4 expression correlates with poor prognosis after surgery in patients with hepatocellular carcinoma. Ann Surg Oncol. 2009;16:3176–82.
Hu BS, Zhao G, Yu HF, et al. High expression of AP-4 predicts poor prognosis for hepatocellular carcinoma after curative hepatectomy. Tumour Biol. 2013;34:271–6.
Lu HF, Yuan WP, Li M, et al. Properly assessing CD133 as a risk factor for poor prognosis in patients with hepatocellular carcinoma after resection. Tumour Biol. 2015;36:4937–8.
Jin H, Zhang Y, You H, et al. Prognostic significance of kynurenine 3-monooxygenase and effects on proliferation, migration, and invasion of human hepatocellular carcinoma. Sci Rep. 2015;5:10466.
Millian NS, Garrow TA. Human betaine-homocysteine methyltransferase is a zinc metalloenzyme. Arch Biochem Biophys. 1998;356:93–8.
Craig SAS. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539–49.
Garcia-Perez A, Burg MB. Renal medullary organic osmolytes. Physiol Rev. 1991;71:1081–115.
Pajares MA, Perez-Sala D. Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell Mol Life Sci. 2006;63:2792–803.
Delgado-Reyes CV, Wallig MA, Garrow TA. Immunohistochemical detection of betaine-homocysteine S-methyltransferase in human, pig, and rat liver and kidney. Arch Biochem Biophys. 2001;393:184–6.
Sun J, Shannon M, Ando Y, et al. Serum metabolomic profiles from patients with acute kidney injury: a pilot study. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;893:107–13.
Shinohara M, Ji C, Kaplowitz N. Differences in betaine-homocysteine methyltransferase expression, endoplasmic reticulum stress response, and liver injury between alcohol-fed mice and rats. Hepatology. 2010;51:796–805.
Zhang B, Denomme MM, White CR, et al. Both the folate cycle and betaine-homocysteine methyltransferase contribute methyl groups for DNA methylation in mouse blastocysts. FASEB J. 2015;29:1069–79.
Pellanda H, Namour F, Fofou-Caillierez M, et al. A splicing variant leads to complete loss of function of betaine-homocysteine methyltransferase (BHMT) gene in hepatocellular carcinoma. Int J Biochem Cell Biol. 2012;44:385–92.
Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7.
Jin GZ, Li Y, Cong WM, Yu H, Dong H, Shu H, et al. iTRAQ-2DLC-ESI-MS/MS based identification of a new set of immunohistochemical biomarkers for classification of dysplastic nodules and small hepatocellular carcinoma. J Proteome Res. 2011;10(8):3418–28.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9.
Zhu AX. Systemic treatment of hepatocellular carcinoma: dawn of a new era? Ann Surg Oncol. 2010;17:1247–56.
Avila MA, Berasain C, Torres L, et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J Hepatol. 2000;33:907–14.
Frau M, Simile MM, Tomasi ML, et al. An expression signature of phenotypic resistance to hepatocellular carcinoma identified by cross-species gene expression analysis. Cell Oncol (Dordr). 2012;35:163–73.
da Silva LM, Galbiatti AL, Ruiz MT, et al. MTHFD1 G1958A, BHMT G742A, TC2 C776G and TC2 A67G polymorphisms and head and neck squamous cell carcinoma risk. Mol Biol Rep. 2012;39:887–93.
Peng JP, Zhang J, Huang HW, et al. L-arginine-glycine amidinotransferase, betaine-homocysteine S-methyltransferase, and neuropolypeptide H3 are diminished in renal clear cell carcinoma of humans. Saudi Med J. 2011;32:467–73.
Mostowska A, Myka M, Lianeri M, et al. Folate and choline metabolism gene variants and development of uterine cervical carcinoma. Clin Biochem. 2011;44:596–600.
Teng YW, Mehedint MG, Garrow TA, et al. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J Biol Chem. 2011;286:36258–67.
Oka H, Tamori A, Kuroki T, et al. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61–6.
Iida H, Honda M, Kawai HF, et al. Ephrin-A1 expression contributes to the malignant characteristics of {alpha}-fetoprotein producing hepatocellular carcinoma. Gut. 2005;54(6):843–51.
Trevisani F, Magini G, Santi V, et al. Impact of etiology of cirrhosis on the survival of patients diagnosed with hepatocellular carcinoma during surveillance. Am J Gastroenterol. 2007;102:1022–31.
Huo TI, Hsu CY, Huang YH, et al. Prognostic prediction across a gradient of total tumor volume in patients with hepatocellular carcinoma undergoing locoregional therapy. BMC Gastroenterol. 2010;10:146.
Sumie S, Nakashima O, Okuda K, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21(3):1002–9.
Cucchetti A, Piscaglia F, Grigioni AD, et al. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010;52(6):880–8.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 81100323, no. 81160256), the Fundamental Research Funds of Shan Dong University (Qilu Hospital Research Project) (2014QLKY18), and the Project Fund of Techpool (UF201330).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
None
Supplementary information
Supplementary Figure 1, Supplementary Table 1–2.
Additional information
Bin Jin and Zhiwei Gong contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Correlation of low BHMT expression with unfavorable OS and TTR in primary cohort and validation cohort, respectively. Probabilities of OS (A) and TTR (B) of 115 HCC patients (primary cohort) were analyzed using Kaplan-Meier survival analysis (log-rank test). Probabilities of OS (C) and TTR (D) of 65 HCC patients (validation cohort) were analyzed using Kaplan-Meier survival analysis (log-rank test). (DOC 274 kb)
Supplementary Table 1
Univariate and multivariate analysis of different prognostic parameters in primary cohort by Cox regression analysis (n=115). (DOC 92 kb)
Supplementary Table 2
Univariate and multivariate analysis of different prognostic parameters in validation cohort by Cox regression analysis (n=65). (DOC 88 kb)
Rights and permissions
About this article
Cite this article
Jin, B., Gong, Z., Yang, N. et al. Downregulation of betaine homocysteine methyltransferase (BHMT) in hepatocellular carcinoma associates with poor prognosis. Tumor Biol. 37, 5911–5917 (2016). https://doi.org/10.1007/s13277-015-4443-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4443-6