Abstract
A previous RNA interference (RNAi) screen identified filamin A (FLNA) as a potential biomarker to predict chemosensitivity in triple-negative breast cancer (TNBC). However, its ability to modulate chemosensitivity and the underlying mechanism has not been investigated. Genetic manipulation of FLNA expression has been performed in an immortalized noncancerous human mammary epithelial cell line and four TNBC cell lines to investigate its effect on chemosensitivity. Western blot analysis was performed to identify the potential signaling pathway involved. Xenograft mouse model was used to examine the in vivo role of FLNA in modulating chemosensitivity. Overexpression of FLNA conferred chemoresistance to docetaxel in noncancerous human mammary epithelial cells. Knockdown of FLNA sensitized four TNBC cell lines, MDA-MB-231, HCC38, Htb126, and HCC1937 to docetaxel which was reversed by reconstituted FLNA expression. Decreased FLNA expression correlated with decreased activation of ERK. Constitutive activation of ERK2 reversed siFLNA-induced chemosensitization. Inhibition of MEK1 recapitulates the effect of FLNA knockdown. MDA-MB-231 xenograft with FLNA knockdown showed enhanced response to docetaxel compared with control xenograft with increased apoptosis. FLNA can function as a modulator of chemosensitivity to docetaxel in TNBC cells through regulation of the MAPK/ERK pathway both in vitro and in vivo. FLNA may serve as a novel therapeutic target for improvement of chemotherapy efficacy in TNBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer (TNBC) is a specific type of breast cancer that lack the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 or HER2 gene amplification. TNBC is identified in approximately 20 % of all breast cancer cases in the USA and is regarded as a very heterogeneous disease with various molecular, genetic, and clinical subgroups [1, 2]. TNBC cases lack targeted therapy and despite initial good response to chemotherapy, it often recurs with chemotherapy resistance and visceral and brain metastasis [3]. Patients with residual TNBC disease post-neoadjuvant chemotherapy have been shown to have a worse prognosis than those presenting with non-TNBC [4]. The risk of relapse for TNBC patients in the first 3–5 years is significantly higher than for women with hormone-positive breast cancer, which makes it extremely challenging from a clinical stand point of view to find the optimal chemotherapy for these women [5, 6]. Therefore, there is an urgent need for identification of biomarkers to predict chemosensitivity in TNBC as well as novel targets as chemotherapy sensitizer.
Filamins, classically known as cytoplasmic structural proteins, are large actin-binding proteins that stabilize actin networks and link them to cellular membranes during cell movements [7]. In addition to actin, recent studies have identified over 90 filamin-binding proteins involved in cell signaling, cell migration and adhesion, phosphorylation, proteolysis, ion channel regulation, transcription regulation, muscle development, and other important cellular processes [8]. Filamin A (FLNA), also known as actin-binding protein 280 (ABP 280), is one of the three members of the filamin family. Many studies have reported increased expression of FLNA in human cancer tissues such as hepatic, breast, and astrocytoma as well as in different cancer cell lines [9]. It has been identified as a biomarker for tumor progression in several cancer types, such as hepatocellular carcinoma, breast cancer, and prostate cancer, etc [10–12]. Although the primary role of FLNA in tumor development has been proposed to regulate cell migration and invasion, emerging evidence suggest that it may be involved in the tumorigenesis process through different mechanisms [8]. For example, lack of FLNA has been shown to lead to susceptibility of DNA damage and G2/M arrest and an increase in gH2AX nuclear foci with resultant increased cell death in several cancer types [13, 14]; FLNA has been implicated in angiogenesis through links with vascular endothelial growth factor A (VEGF A) [15]. Recently, FLNA has been identified as a potential biomarker to predict chemosensitivity in triple-negative breast cancer (TNBC) [16]. However, its ability to modulate chemosensitivity and the underlying mechanism has not been investigated.
In this study, we investigated the potential role of FLNA in modulating chemoresistance in TNBC. We found that knockdown of FLNA conferred chemosensitization to docetaxel in TNBC cell lines which can be reversed by reconstitution of FLNA expression. This regulation was mediated through activation of the MAPK/ERK pathway. Depletion of FLNA sensitized MDA-MB-231 xenograft to docetaxel in vivo. Therefore, FLNA may represent a novel therapeutic target for improvement of chemotherapy in TNBC.
Methods
Cell culture
MDA-MB-231, HCC38, Htb126, HCC1937, and HME1 cell lines were purchased from ATCC and were cultured in basal medium supplemented with 10 % serum at 37 °C and 5 % CO2. HME1 cells were cultured in serum-free condition as described elsewhere [17].
Chemosensitivity assay
Cells were seeded at a density of 5 × 103 cells/well in 96-well microtiter plates and allowed to attach overnight. Docetaxel or doxorubicin alone was then added and cultured for an additional 72 h. Cell viability was assessed using CellTiter-Glo® assay. Each value was normalized to cells treated with DMSO, and the IC50 values are calculated using Graphpad Prism software. Each assay was performed in biological triplicates.
Plasmids and cell transfection
Cells in logarithmic growth phase were prepared for cell transfection. pWZLblasti-HA-ERK2 GOF was a gift from Christopher Counter (Addgene plasmid # 53174). MEK1DN (dominant negative) recombinant adenovirus (ADV-118) was purchased from Cell Biolabs and infected cells as per manufacturer’s protocol. pCMV-AC-GFP FLNA complementary DNS (cDNA) (RG221764) and three individual small interfering RNAs (siRNAs) (SR301624) targeting FLNA were obtained from OriGene. siRNAs were transfected with Lipofectamine RNAi Max reagent (Invitrogen, Grand Island, NY, USA) as per manufacturer’s protocol. cDNA transfections were performed with Lipofectamine LTX reagent (Invitrogen) as per manufacturer’s protocol.
Viral transductions and stable selections
For lentivirus production, 1 μg of FLNA small hairpin RNS (shRNA) (Dharmacon) together with 1 μg of helper plasmids (0.4 μg pMD2G and 0.6 μg psPAX2) were transfected into 293FT cells with an Effectene reagent (Qiagen, Valencia, CA, USA). Viral supernatants were collected 48 h after transfections and cleared through a 0.45-μm filter. Cells were infected with viral supernatants containing 4 μg/mL polybrene (Sigma, St. Louis, MO) and selected with puromycin for 7 days.
Quantitative reverse transcription-PCR (qRT-PCR)
Total RNA was isolated from mouse tissue using RNeasy Plus Universal Mini kit (Qiagen) according to the manufacturer’s protocol. Then, 1-μg RNA was converted to cDNA using a First Strand cDNA Synthesis Kit (Roche). Real-time quantitative PCR reactions were set up in triplicate with Ssofast Master Mix (Biorad) and run on a LightCycler® 480 (Roche).
Immunoblot analysis
Total cell lysates were prepared by harvesting cells in Laemmli SDS reducing buffer (50 mM Tris-HCl (pH 6.8), 2 % SDS, and 10 % glycerol), boiled and resolved on an 8 to 10 % polyacrylamide gel, and transferred to polyvinylidinefluoride. Antibodies against FLNA (Abcam, Cambridge, USA), phospho-ERK1/2 (Abcam), total ERK1/2 (Abcam), MEK (Abcam), and β-actin (Sigma-Aldrich) were used. The blots were incubated with horseradish peroxidase–conjugated donkey anti-rabbit or anti-mouse IgG (Santa Cruz Biotechnology, Dallas, USA) at a dilution of 1:5000 and detected with SuperSignalWest Pico or Femto Chemiluminescent Substrate Kit (Thermo Scientific, Grand Island, USA).
Immunohistochemistry (IHC) staining
The paraffin-embedded sections were subjected to antigen retrieval by heating the slides in a microwave at 100 °C for 10 min in 0.1 M citric acid buffer (pH = 6.0), and then incubated with cleaved caspase 3 antibody (Cell Signaling) at 4 °C overnight. After secondary antibody incubation at room temperature for 1 h, the slides were developed in 0.05 % diaminobenzidine containing 0.01 % hydrogen peroxidase. For negative controls, specific antibodies were replaced with normal goat serum by co-incubation at 4 °C overnight preceding the immunohistochemical staining procedure.
Xenograft experiments
All animal experiments were approved by the Institutional Animal Care and Use Committee of the National Cancer Center. The protocol was approved by the Committee on the Ethics of Animal Experiments of The Second Hospital of Hebei Medical University. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. MDA-MB-231 cells (1 × 106 cells/injection) were subcutaneously injected into both flanks of the 5-week-old female nude mice group. Vehicle or docetaxel (10 mg/kg) alone or combined were injected i.p. into mice weekly for 4 weeks. Tumor volumes were measured using caliper and determined by a formula (volume = (length × width2)/2) from week1 to week 8 postimplantation. The results were expressed as mean tumor volumes with SD.
Statistical analysis
Quantitative data are expressed as mean ± SD. Statistical significance was assessed by the two-tailed Student’s t test. Differences were considered to be significant when P < 0.05. For xenograft experiment, ANOVA test followed by post hoc analysis was performed.
Results
FLNA is implicated in regulating chemosensitivity in TNBC cell lines
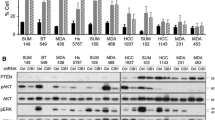
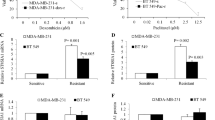
To investigate if FLNA plays a role in regulating chemoresistance, we first evaluated if overexpression of FLNA is sufficient to confer chemoresistance in HME1 cells to docetaxel and doxorubicin, two of the most commonly used chemotherapy in breast cancer patients. HME1 is a normal breast epithelial cell line immortalized with hTERT. We found that increased FLNA expression made the cells more resistant to docetaxel, but not doxorubicin, probably because of the different mechanism of action of these two drugs (Fig. 1a, b). To confirm its chemosensitivity regulatory function, we transiently knocked down FLNA expression in four TNBC cell lines using siRNA. One of the three tested siRNAs against FLNA that showed best knockdown efficiency was used in the following study (Fig. 2a). As shown in Fig. 1c–f, decreased expression of FLNA significantly sensitized these cells to docetaxel. To further confirm the causal role of FLNA in conferring chemoresistance, we restored FLNA expression in cells transfected with FLNA siRNA. As shown in Fig. 2b, reconstitution of FLNA protein expression eliminated the chemosensitizing effect of FLNA knockdown in these cells exposed to 4 or 8 nM of docetaxel.
FLNA is implicated in regulating chemosensitivity in TNBC cell lines. a and b Dose response curves of docetaxel (a) and doxorubicin (b) in HME1 cells expressing the pEGFP vector or pEGFP FLNA. Data represent mean ± s.d., n = 3. c–f Dose response curves of docetaxel in TNBC cell lines MDA-MB-231 (c), HCC38 (d), Htb126 (e), and HCC1937 (f) expressing scramble siRNA or siRNA targeting FLNA. Data represent mean ± s.d., n = 3
Restoration of FLNA protein reverses chemosensitizing effect of FLNA knockdown in TNBC cell lines. a Quantification of mRNA level of FLNA in MDA-MB-231, HCC38, Htb126, and HCC1937 cells expressing siRNA against FLNA with or without reconstituted FLNA expression. b Surviving fraction of MDA-MB-231, HCC38, Htb126, and HCC1937 cells expressing siRNA against FLNA with or without reconstituted FLNA expression in the presence of 4 or 8 nM of docetaxel. Values are normalized to control cells for each cell line. Data represent mean ± s.d., n = 3. *P < 0.05; **P < 0.01
FLNA modulates chemosensitivity by regulating ERK1/2 activation in MDA-MB-231 cells
The MAPK/ERK pathway is well known to play a role in cell survival. Importantly, elevated ERK1/2 activity (phosphorylation) has been observed in metastatic sites relative to primary breast tumors and is more common in TNBC [18]. We tested if FLNA modulates chemosensitivity through activation of ERK1/2. Western Blot analysis showed that in MDA-MB-231 cells, knockdown of FLNA led to a decrease in FLNA protein level with a concomitant decrease in phosphorylation of ERK1/2 whereas restoration of FLNA expression resulted in activation of ERK. To examine if activated ERK can reverse siFLNA-induced chemosensitization, we expressed an ERK2 mutant construct, ERK2 GOF, which contains both R67S and D321N mutations that can lead to hyperactivation of ERK1/2 [19]. As shown in Fig. 3c, constitutively active ERK2 can rescue docetaxel sensitivity as effectively as reconstituted FLNA expression. Additionally, knockdown of ERK2 by siRNA mimicked the chemosensitizing effects of siFLNA (Fig. 3d). The knockdown efficiency was confirmed by Western blot (Fig. 3b). These results suggest that FLNA modulate chemoresistance through by activation of ERK.
FLNA modulates chemosensitivity by regulating ERK1/2 activation in MDA-MB-231 cells. a Immunoblot analysis of phosphorylation of ERK1/2 in MDA-MB-231 cells with knockdown of FLNA and reconstituted FLNA expression. b Immunoblot analysis of phosphorylation of ERK1/2 and total ERK in MDA-MB-231 cells with knockdown of ERK2. c Dose response curves of docetaxel in MDA-MB-231 cells transfected with siControl, siFLNA, pEGFP FLNA, or ERK2 GOF. Data represent mean ± s.d., n = 3. d Dose response curves of docetaxel in MDA-MB-231 cells transfected with siControl, siFLNA, or siERK2. Data represent mean ± s.d., n = 3
FLNA exerts its chemosensitivity modulating effect through the MAPK/ERK pathway
To further probe the involvement of upstream kinase MEK1 in this regulation, we ectopically expressed MEK1 dominant negative form in the four TNBC cell lines. As shown in Fig. 4a–d, expression of this mutant MEK1 also sensitized these cells to docetaxel, not as effectively as siFLNA, though. The expression of this dominant negative form of MEK1 led to downregulation of ERK phosphorylation as demonstrated by Western blot in MDA-MB-231 cells (Fig. 4e). In addition, cotreatment of the MEK1 inhibitor, U0126, also sensitized these four cell lines to a sub-lethal dose of docetaxel (Fig. 4f). These results suggest that FLNA exerts its effects through the MAPK/ERK pathway.
FLNA exerts its chemosensitivity modulating effect through the MAPK/ERK pathway. a–d Dose response curves of docetaxel in MDA-MB-231 (a), HCC38 (b), Htb126 (c), and HCC1937 (d) cells transfected with siControl, siFLNA, or MEK1 DN. Data represent mean ± s.d., n = 3. e Immunoblot analysis of MEK and phosphorylation of ERK1/2 in MDA-MB-231 cells with ectopic expression of MEK DN. f Surviving fraction of MDA-MB-231, HCC38, Htb126, and HCC1937 cells expressing treated with docetaxel (1 nM), U0126 (1 μM), or combined. Values are normalized to control cells for each cell line. Data represent mean ± s.d., n = 3. *P < 0.05; **P < 0.01
Knockdown of FLNA sensitized MDA-MB-231 xenografts to docetaxel in vivo
To investigate the in vivo chemosensitizing effect of FLNA knockdown in vivo, we stably knocked down FLNA in MDA-MB-231 cells using shRNA and injected control and shFLNA cells into nude mice. In consistence with in vitro data, administration of docetaxel inhibited shFLNA xenograft growth more effectively than control xenograft (Fig. 5a). IHC staining showed that docetaxel induced significantly more cleavage of caspase 3 in shFLNA tumor sections compared with control (Fig. 5b), indicative of more apoptotic cell death.
Knockdown of FLNA sensitized MDA-MB-231 xenografts to docetaxel in vivo. a Tumor growth curves for control or stable FLNA knockdown MDA-MB-231 xenografts treated with vehicle or docetaxel (10 mg/kg). Data represent mean ± s.d., n = 5. **P < 0.01. Arrow indicates docetaxel dosing. Nsi non-silencing, Veh vehicle. b Immunohistochemical staining for cleaved caspase 3 in tumors derived from the mice above. The right bar graph shows the quantification of the cleaved caspase 3 staining intensity
Discussion
Despite their well-known function as a cytoplasmic structural protein, filamins have emerged as essential scaffolding proteins that interact with a number of proteins with roles in signaling and cytoskeletal reorganization and is regulated by phosphorylation [8]. Alterations in FLNA expression may contribute to cancer development, and overexpression of FLNA has been observed in a variety of malignancies, including hepatocellular carcinoma, breast cancer, colon cancer, melanoma, and prostate cancer, etc [10, 11, 20, 21]. A recent study using ex vivo breast cancer tissues revealed that FLNA protein was overexpressed in cancer tissues compared with distant normal mammary gland and benign breast tissues. This overexpression was associated with advanced stage, lymph node metastasis, and vascular or neural invasion of breast cancer, suggesting that FLNA may contribute to breast cancer development and progression [12]. It has been proposed that FLNA is implicated in tumorigenesis by regulation of various cell functions. Several studies showed that overexpression of FLNA promotes cancer cell growth and metastasis when it is localized to the cytoplasm and plasma membrane [8]. FLNA has also been reported to be involved in regulating double stranded breaks repair (DSBR) which may contribute to the anti-apoptotic mechanism in cancer development [8]. Moreover, FLNA has been implicated in angiogenesis, which is another crucial aspect of tumorigenesis, through its association with VEGF [15]. A recently performed RNA interference (RNAi) screen of breast cancer genome has identified FLNA as a potential biomarker to predict chemosensitivity in TNBC [16]. However, its ability to modulate chemosensitivity and the underlying mechanism has not been investigated. In the present study, we found that depletion of FLNA sensitized four TNBC cell lines to docetaxel which was reversed by reconstitution of FLNA expression (Figs. 1 and 2). Additionally, MDA-MB-231 xenograft with stable knockdown of FLNA showed better response to docetaxel with concomitant increased induction of apoptosis compared to control xenograft (Fig. 5). These results support that FLNA is directly involved in regulating chemoresistance in TNBC both in vitro and in vivo.
Mitogen-activated protein kinase (MAPK) cascades, which couple extracellular signal from cell surface receptor to transcriptional factors, are key signaling pathways involved in the regulation of cell proliferation, survival, and differentiation [22]. Specifically, the Raf-MEK-ERK pathway is a key downstream effector of the Ras small GTPase, the most frequently mutated oncogene in human cancers [22]. Aberrant activation of the Ras/MAPK pathway is known to play important roles in tumor initiation and progression [22]. Although oncogenic mutation in this pathway is infrequent in breast cancer, alternative mechanism for Ras/MAPK activation has been proposed in TNBC, including copy number alterations in canonical Ras/MAPK pathway component (i.e., amplifications or gains of KRAS and BRAF) and loss of negative regulation of this pathway (e.g., loss of NF1) [23, 24]. Interestingly, a role of Raf/MEK/ERK in regulating drug resistance has been suggested due to its interactions with the apoptosis pathway [25]. Several pieces of evidence suggest that FLNA may be implicated in MAPK signaling induced by a variety of extracellular stimuli. It has been shown that FLNA can interact with the MAPK kinases MEK1 and MKK4 and is phosphorylated by ribosomal S6 kinase, an ERK target [26, 27]. It has also been reported that filamin A can act cooperatively with β-arrestins to regulate ERK activation and actin cytoskeleton reorganization [28]. In our study, we observed a correlation between FLNA expression and ERK activation in TNBC cells. Specifically, knockdown of FLNA reduced phosphorylation of ERK whereas restoration of FLNA expression led to ERK activation (Fig. 3a). Correspondingly, constitutive activation of ERK mimicked the effect of FLNA restoration on chemosensitivity of siFLNA cells and ERK2 knockdown also sensitized these cells to docetaxel (Fig. 3b, c). Additionally, expression of dominant negative MEK1, which lacks the ability to activate ERK1/2, or cotreatment of MEK1 inhibitor, exhibited similar chemosensitizing effect in MDA-MB-231 cells (Fig. 4). Taken together, these results support that FLNA mediate chemosensitivity through the MAPK/ERK pathway in TNBC cells. Considering its role as a scaffolding protein for numerous signaling molecules, it is reasonable to postulate that FLNA may activate the MAPK/ERK pathway through its interaction with components of this signaling pathway. Further investigation is warranted as to the detailed mechanism underlying this regulation.
In conclusion, our study demonstrates that FLNA can function as a modulator of chemosensitivity to docetaxel in TNBC cells through regulation of the MAPK/ERK pathway both in vitro and in vivo. FLNA may serve as a novel therapeutic target for improvement of chemotherapy efficacy in TNBC. Unraveling the molecular pathways associated with FLNA chemoresistance is of great importance for further understanding its clinical implication in the treatment of TNBC.
References
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67.
Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–9.
Blanchard Z, Paul BT, Craft B, ElShamy WM. BRCA1-IRIS inactivation overcomes paclitaxel resistance in triple negative breast cancers. Breast Cancer Res. 2015;17:5.
Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34.
Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16 Suppl 1:1–11.
Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–76.
Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–45.
Savoy RM, Ghosh PM. The dual role of filamin a in cancer: can’t live with (too much of) it, can’t live without it. Endocr Relat Cancer. 2013;20:R341–56.
Nallapalli RK, Ibrahim MX, Zhou AX, Bandaru S, Sunkara SN, Redfors B, et al. Targeting filamin A reduces K-RAS-induced lung adenocarcinomas and endothelial response to tumor growth in mice. Mol Cancer. 2012;11:50.
Ai J, Huang H, Lv X, Tang Z, Chen M, Chen T, et al. FlNA and PGK1 are two potential markers for progression in hepatocellular carcinoma. Cell Physiol Biochem. 2011;27:207–16.
Lin JF, Xu J, Tian HY, Gao X, Chen QX, Gu Q, et al. Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int J Cancer. 2007;121:2596–605.
Tian HM, Liu XH, Han W, Zhao LL, Yuan B, Yuan CJ. Differential expression of filamin A and its clinical significance in breast cancer. Oncol Lett. 2013;6:681–6.
Meng X, Yuan Y, Maestas A, Shen Z. Recovery from DNA damage-induced G2 arrest requires actin-binding protein filamin-A/actin-binding protein 280. J Biol Chem. 2004;279:6098–105.
Yue J, Wang Q, Lu H, Brenneman M, Fan F, Shen Z. The cytoskeleton protein filamin-A is required for an efficient recombinational DNA double strand break repair. Cancer Res. 2009;69:7978–85.
Uramoto H, Akyurek LM, Hanagiri T. A positive relationship between filamin and vegf in patients with lung cancer. Anticancer Res. 2010;30:3939–44.
Singel SM, Cornelius C, Batten K, Fasciani G, Wright WE, Lum L, et al. A targeted RNAi screen of the breast cancer genome identifies KIF14 and TLN1 as genes that modulate docetaxel chemosensitivity in triple-negative breast cancer. Clin Cancer Res. 2013;19:2061–70.
Shay JW, Van Der Haegen BA, Ying Y, Wright WE. The frequency of immortalization of human fibroblasts and mammary epithelial cells transfected with SV40 large T-antigen. Exp Cell Res. 1993;209:45–52.
Adeyinka A, Nui Y, Cherlet T, Snell L, Watson PH, Murphy LC. Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin Cancer Res. 2002;8:1747–53.
Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, et al. Copper is required for oncogenic braf signalling and tumorigenesis. Nature. 2014;509:492–6.
Larriba MJ, Martin-Villar E, Garcia JM, Pereira F, Pena C, de Herreros AG, et al. Snail2 cooperates with snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis. 2009;30:1459–68.
Flanagan LA, Chou J, Falet H, Neujahr R, Hartwig JH, Stossel TP. Filamin a, the arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J Cell Biol. 2001;155:511–7.
Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310.
Craig DW, O’Shaughnessy JA, Kiefer JA, Aldrich J, Sinari S, Moses TM, et al. Genome and transcriptome sequencing in prospective metastatic triple-negative breast cancer uncovers therapeutic vulnerabilities. Mol Cancer Ther. 2013;12:104–16.
Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70.
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84.
Marti A, Luo Z, Cunningham C, Ohta Y, Hartwig J, Stossel TP, et al. Actin-binding protein-280 binds the stress-activated protein kinase (sapk) activator SEK-1 and is required for tumor necrosis factor-alpha activation of sapk in melanoma cells. J Biol Chem. 1997;272:2620–8.
Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24:3025–35.
Scott MG, Pierotti V, Storez H, Lindberg E, Thuret A, Muntaner O, et al. Cooperative regulation of extracellular signal-regulated kinase activation and cell shape change by filamin A and beta-arrestins. Mol Cell Biol. 2006;26:3432–45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by Hebei Medical Development Funding (HMDF-KJJ).
Conflicts of interest
None
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Zhao, P., Ma, W., Hu, Z. et al. Filamin A (FLNA) modulates chemosensitivity to docetaxel in triple-negative breast cancer through the MAPK/ERK pathway. Tumor Biol. 37, 5107–5115 (2016). https://doi.org/10.1007/s13277-015-4357-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4357-3