Abstract
Non-small cell lung cancer (NSCLC) remains the most common cause of cancer-related death worldwide. Patients presenting with advanced-stage NSCLC have poor prognosis, while metastatic spread accounts for >70 % of patient’s deaths. The major advances in the treatment of lung cancer have brought only minor improvements in survival; therefore, novel strategic treatment approaches are urgently needed. Accumulating data allocate a central role for the cancer microenvironment including mesenchymal stem cells (MSCs) in acquisition of drug resistance and disease relapse. Furthermore, studies indicate that translation initiation factors are over expressed in NSCLC and negatively impact its prognosis. Importantly, translation initiation is highly modulated by microenvironmental cues. Therefore, we decided to examine the effect of bone marrow MSCs (BM-MSCs) from normal donors on NSCLC cell lines with special emphasis on translation initiation mechanism in the crosstalk. We cultured NSCLC cell lines with BM-MSC conditioned media (i.e., secretome) and showed deleterious effects on the cells’ proliferation, viability, death, and migration. We also demonstrated reduced levels of translation initiation factors implicated in cancer progression [eukaryotic translation initiation factor 4E (eIF4E) and eukaryotic translation initiation factor 4GI (eIF4GI)], their targets, and regulators. Finally, we outlined a mechanism by which BM-MSCs’ secretome affected NSCLC’s mitogen-activated protein kinase (MAPK) signaling pathway, downregulated the cell migration, and diminished translation initiation factors’ levels. Taken together, our study demonstrates that there is direct dialogue between the BM-MSCs’ secretome and NSCLC cells that manipulates translation initiation and critically affects cell fate. We suggest that therapeutic approach that will sabotage this dialogue, especially in the BM microenvironment, may diminish lung cancer metastatic spread and morbidity and improve the patient’s life quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide. Non-small cell lung cancer (NSCLC) accounts for 85 % of all lung cancer cases. Despite several novel therapeutic approaches, the majority of NSCLC patients present locally advanced or metastatic disease, which are at present incurable [1, 2]. Thus, there is great need for new therapies.
The genetics of lung cancer remains a therapeutic challenge [3]. In-depth analyses of lung cancer genomes have further defined NSCLCs as a group of distinct diseases with genetic and cellular heterogeneity [4].
On the other hand, unifying traits of NSCLC are suggested to be their tumor microenvironment characteristics [5] that have been documented to play an important role in disease initiation and progression [6]. It was reported that the stromal cells, growth factors, and cytokines surrounding the tumor can affect the proliferation, survival, migration, and drug resistance of the lung cancer cells [6].
Tumor microenvironment is very complex and includes various soluble factors, excessive extracellular matrix (ECM) deposition, and multiple cell types. This orchestrated network contributes to the disease tumorigenesis, progression, metastases, and recurrence [7]. Interestingly, distinct types of cells, such as mesenchymal stem cells (MSCs), contribute to these phenomena through a continuous crosstalk with each other directly or indirectly (secretome, ECM) [8]. MSCs are a subset of adult stem cells that possess abilities of self-renewal and multilineage differentiation (chondrocytes, osteocytes, and adipocytes).
Recent studies have demonstrated that MSCs derived from pathological settings exhibit genetic and functional abnormalities compared to their normal counterparts [9]. Specifically, MSCs in lung cancer tissue have demonstrated accelerated growth and reduced sensitivity to drugs [10].
MSCs secrete a variety of biologically active cytokines/growth factors, ECM proteins, and tissue remodeling enzymes that play important roles in various aspects of tissue function, repair, and homeostasis [11]. The factors secreted from MSCs (referred hereafter as MSC’s secretome) include cytokines such as HGF, IGF-I, and VEGF [12].
Control of protein translation is a crucial aspect of cancer development and progression [13]. Translational control includes the regulation of global protein synthesis rate as well as selective translation of specific mRNAs that promote tumor cell survival, angiogenesis, invasion, and metastasis. Indeed, deregulation of protein translation has been observed in various human malignancies with both elevated global translation and increased synthesis of proteins involved in malignant characteristics [14]. Translation initiation is the most regulated step of protein synthesis and the rate-limiting phase of the process [15]. In concordance, high expression of the translation initiation factors, eukaryotic translation initiation factor 4E (eIF4E) and eukaryotic translation initiation factor 4GI (eIF4GI), was reported in various tumors [16], including lung cancer [17, 18].
Previous publications by others and us [13, 19–21] have demonstrated the critical role of protein translation in lung cancer cells [3] with specific emphasis on translational machinery [22]. Specifically, it was shown that the small molecule 4EGI-1 inhibitor of eIF4E/eIF4G association inhibited growth and induced TRAIL-mediated apoptosis of human lung cancer cells [23].
Previously, we have investigated different aspects of protein homeostasis in NSCLC and showed that manipulation of protein regulatory networks such as proteasome and ER homeostasis impaired lung cancer cells, making them an attractive therapeutic target [20]. In the present study, we aimed to examine the role of protein synthesis in the crosstalk between the NSCLC cells and their metastatic microenvironment, the bone. Since bone marrow MSCs (BM-MSCs) are an important component of the bone niche, we focused on the BM-MSCs’ secretome that affects translation initiation and cell fate. Generally, our findings substantiate that BM-MSCs’ secretome actively modulates NSCLC, particularly translation initiation and consequently migration. Future studies should identify select influential soluble BM-MSCs’ secretome components and explore the potential they may hold as new therapeutic targets in lung cancer treatment.
Materials and methods
NSCLC cell lines
H1299 and H460 were cultured in RPMI 1640 and A549 in HAM medium, all supplemented with 10 % heat-inactivated fetal bovine serum and antibiotics (Biological Industries, Israel).
BM-MSC isolation and propagation
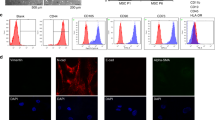
BM samples were obtained from femur head samples of normal donors undergoing elective full hip replacement due to orthopedic purposes (n = 15, 11 females and 4 males, aged 74 ± 2.5 years). Mononuclear cells were isolated from BM samples on Ficoll (Sigma, Israel). Non-adherent cells were removed from the media of culture, leaving the adhered MSCs in the culture dish. Cells were tested for the following MSC markers: positivity for vimentin (DakoCytomation, Denmark) and negativity for keratin (Zymed, CA, USA) by immunocytochemistry and presence of human stromal BM-MSC marker CD271 antibody (>80 %) (non-hematopoietic) and lack of hematopoietic markers CD34 and CD45 (MACS; Miltenyi Biotec, Germany) by flow cytometry (Navios; Beckman Coulter, USA). The cells’ multipotency was tested by assessing their capacity to differentiate into adipocytes and/or osteoblasts using StemPro adipogenesis and osteogenesis differentiation kit (Invitrogen, Carlsbad, CA, USA). Adipogenic and osteogenic differentiations were demonstrated by Sudan IV staining and Alizarin Red (Sigma) staining, respectively (Supplementary Fig. 1).
BM-MSCs’ secretome model
BM-MSCs’ conditioned medium (secretome) was collected every 72 h from MSC culture flasks with 80 % confluence. Conditioned medium was centrifuge at 172g for 5 min, and upper fluids were collected into fresh tubes. Conditioned media tubes were stored at −20 °C until use. Upon experiment, the medium was mixed with fresh media (7:1 ratio, respectively) and applied to NSCLC cell lines. The cells’ response was compared to two types of controls: 72-h secretome of NSCLC cells mixed with fresh media (7:1) and fresh media to monitor the differences between used and fresh media. No significant changes were measured between the NSCLC cells treated with fresh media and NSCLC’s secretome (data not shown). We conclude that all observed effects were attributed to components of the MSCs’ secretome. Thus, all observations were normalized to respective NSCLC’s secretome.
Trypan blue
Trypan blue was used as described before [19].
Immunoblotting
NSCLC cells were lysed, and Western blot was preformed as described elsewhere [24]. The proteins were detected with the following rabbit/mouse antihuman antibodies: peIF4E(Ser209)/total eIF4E, peIF4GI(Ser1108)/total eIF4GI, p4EBP(Ser65)/total 4EBP, pmTOR(Ser2448)/total mTOR, pSAPK/JNK (Thr183/Tyr185)/total JNK, phospho-p44/42 MAP kinase, and extracellular signal-regulated kinase (ERK) (Thr202/Tyr204)/total ERK (Cell Signaling, Danvers, MA, USA); pMNK(Thr197/Thr202)/total MNK, SMAD5, and HSC-70 (Epitomics, CA, USA); and cMyc, hypoxia-inducible factor 1 alpha (HIF1α), and nuclear factor kappa B (NFkB) (Santa-Cruz, CA, USA).
Cell viability assay
Assessment of viability was performed on NSCLC cell lines using cell proliferation reagent WST-1 (Roch) as described before [25].
Scratch assay
Scratch assay was conducted as described before [19]. Wound closure was monitored by microscopy immediately after cell scratching (0 h) and at 6/24/48 h post wounding.
Inhibitors
Mitogen-activated protein kinase (MAPK) inhibitors SP600125 (20 μM, JNK inhibitor; Biomol Int., USA), U0126 (10 μM, MEK1/2 inhibitor; CST, USA), and 4EGI-1 (35 μm, eIF4E/eIF4G interaction inhibitor; EMD Millipore) were used. All were dissolved in DMSO.
Statistical analysis
Student’s paired t tests were used in analysis of differences between cohorts. Effects were considered significant when p value is <0.05.
Institutional Helsinki approval
MSCs were isolated from healthy donor samples as approved by the Meir Medical Center’s Helsinki Committee and complying with Helsinki regulations.
Results
BM-MSCs’ secretome inhibited NSCLC cells’ viability and proliferation and induced death
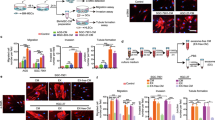
Initially, we assessed the effect of BM-MSCs’ secretome on the NSCLC phenotype. We cultured NSCLC with BM-MSC conditioned media (secretome) and determined significantly reduced viability after 72 h (30–60 %↓, p < 0.01) (Fig. 1a). All observations were normalized to respective NSCLC’s control secretome (as explained in the “Materials and methods” section).
NSCLC cells display reduced viability and proliferation and elevated death upon exposure to BM-MSCs’ secretome. NSCLC cells were cultured with BM-MSCs’ secretome. After 72 h, the cells were harvested and assessed for changes in the cells. a Viability, b proliferation, c death rate. Next, d NSCLC cell lines were lysed and immunoblotted for the PCNA protein. Results are presented as percent (mean ± SE) of control cells (dotted line), and immunoblot results are normalized to HSC-70 loading control (n ≥ 3) in bar graphs (a–c) and representative immunoblots (d). Statistically significant differences between cohorts (*p < 0.05; **p < 0.01) are indicated
Total and death cell counts were also assessed. Findings demonstrated significantly decreased total cell counts (30–35 %↓, p < 0.01) (Fig. 1b). Examination of the death rates showed elevated percentage following the exposure to BM-MSCs’ secretome (85–255 %, p < 0.01) (Fig. 1c). A more detailed representation of the phenotypic changes of the different donors is presented in Supplementary Fig. 2. In order to determine whether the decreased cell counts stemmed from changes in cell proliferation on top of elevated cell death, we assayed the expression of the proliferation marker proliferating cell nuclear antigen (PCNA). Indeed, decreased PCNA levels were determined in NSCLC cells exposed to BM-MSCs’ secretomes (40 %↓, p < 0.05) (Fig. 1d).
BM-MSCs’ secretome inhibited NSCLC cell line migration
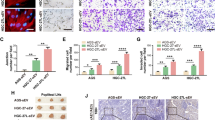
The capability of tumor cells to migrate and invade is associated with high metastatic potential and advanced stage of cancers, as frequently developed in lung cancer patients. Accumulating data underscore the importance of microenvironmental cues to cell migration [26]. Thus, we tested the effect of BM-MSCs’ secretome on NSCLCs’ migratory capability by applying the scratch assay. Our experiments indicated that BM-MSCs’ secretome caused a significant retardation in the cell migration [H1299 45 % (6 h), H460 55 % (24 h), A549 30 % (18 h) of scratch closure compared to control cells; p < 0.05] (Fig. 2a, b). The dissimilarity between the cell line migration rates may be explained by the different tissue origins and, as a consequence, different metastatic potentials (H1299: metastatic site, H460: pleural effusion, A549: lung tissue).
BM-MSCs’ secretome inhibited NSCLC cell line migration: NSCLC cell lines were cultured with BM-MSCs’ secretome, and the effect on cell migration was assessed by scratch assay. Scratch closure was photographed immediately (0 h) and after 6 h for H1299, 24 h for H460, or 18 h for A549 (magnification, ×40) of cultured cells’ area. Results are presented as a percent in bar graphs (mean ± SE, n ≥ 3) of control cells (dotted line) and b representative images. Statistically significant differences between cohorts (*p < 0.05; **p < 0.01) are indicated. c Morphology changes of NSCLC cells treated with BM-MSCs’ secretome (representative images). Control cells display spindle-shaped appearance and fibroblast-like shape, while treated cells reserved their epithelial morphology
In concurrence with the altered cell migration, we also observed changes in the cell morphology. Indeed, while control migrating NSCLC cells cultured alone exhibited a more fibroblast-like spindle appearance, the more stationary NSCLC cells treated with BM-MSCs’ secretome displayed an epithelial-like more-round morphology (Fig. 2c).
BM-MSCs’ secretome reduced translation initiation in NSCLC cell lines
The unambiguous changes in the cell proliferation and migration and the established importance of protein synthesis to both functions [15, 27–29] led us to further investigate protein synthesis-related pathways. Therefore, we examined whether major translation factors are involved. Indeed, we observed profoundly decreased levels of the factors eIF4E and eIF4GI following 72 h of culture with BM-MSCs’ secretome (30–50 %/↓, p < 0.05) (Fig. 3a).
BM-MSCs’ secretome downregulated both translation initiation and MAPK signaling. NSCLC cells were cultured with BM-MSCs’ secretome (sec). Following 48/72 h of culturing, the cells were lysed and immunoblotted for a translation initiation factors eIF4E/eIF4GI, b the factors’ regulators [eIF4E (4EBP, MNK), eIF4GI (mTOR)] and targets [eIF4E (NFkB, cyclin D1), eIF4GI (SMAD5, ERα), both (HIF1α, cMyc)]. c Next, following 1.5 h of culturing, the cells were immunoblotted for the MAPKs pJNK and pERK. d Finally, we explored short-term effects of the BM-MSCs’ secretome on NSCLC cells and immunoblotted the cells for regulators after 2.5 h of exposure and eIF4E/eIF4GI after 5 h of exposure. The results are presented as percent (mean ± SE) of control cells (dotted line) and normalized to HSC-70 loading control (n ≥ 3). Representative immunoblots are presented under each graph. Statistically significant differences between cohorts (*p < 0.05; **p < 0.01) are indicated
We also determined decreased levels of eIF4E direct regulators 4EBP (40 %/↓, p < 0.05) and MNK (35–40 %/↓, p < 0.05) (Fig. 3b) and eIF4GI regulator mTOR (50 %/↓, p < 0.05) after 48 h of exposure to BM-MSCs’ secretomes (Fig. 3b).
Finally, we assayed the expression levels of protein targets known to depend specifically on eIF4E (NFkB [30]), eIF4GI (SMAD5 and ERα [31, 32]), or both (HIF1α and cMyc [33, 34]) as indication that eIF4E/eIF4GI reduced activity de facto. Indeed, expression of all targets was decreased after 72 h (20–80 %/↓, p < 0.01) (Fig. 3b).
Altogether, these observations indicate that the BM-MSCs’ secretomes contained elements that decreased translational activity of eIF4E and eIF4GI in NSCLC cells.
BM-MSCs’ secretome affected MAPKs/translation initiation-dependent migration in NSCLC cell lines
While the majority of BM-MSCs’ secretomes affecting NSCLC phenotype and translation initiation were recorded after 72 h, we also witnessed earlier changes in cell migration as soon as 6–24 h. Thus, we speculated that there may be a common earlier upstream regulator of both phenomena (i.e., migration and translation initiation). The MAPKs JNK and ERK present as appropriate candidates for this role because they have both been reported to regulate cell motility in various cell types [35] and regulate the upstream regulators of eIF4E and eIF4GI [36]. Thus, we cultured NSCLC cell lines with BM-MSCs’ secretome for 1.5 h and then assayed the levels of active MAPKs pERK and pJNK. Indeed, reduced levels of pJNK and pERK were observed in both NSCLC cell lines (pJNK, 20/−30 %/↓; pERK, 60/−80 %/↓; p < 0.05) (Fig. 3c). Once we determined an early signaling response in MAPKs, we wondered whether there was an earlier response cycle in the translation initiation cascade as well. Therefore, we examined the effect of BM-MSCs’ secretome on eIF4E/eIF4GI regulators at a reasonable time point post MAPK activation. Indeed, after 2.5 h, we determined decreased levels of p4EBP, pMNK, and pmTOR (30–55 %↓, p < 0.05) (Fig. 3d). Finally, in concordance with all of our observations, we also determined decreased levels of peIF4E and peIF4GI as soon as 5 h after exposure to BM-MSCs’ secretomes (40–55 %↓, p < 0.01) (Fig. 3d).
In summary, our results indicate that BM-MSCs’ secretome attenuates MAPKs, eIF4E/eIF4GI, and migration in sequence. This has been suggested to us that attenuated NSCLC migration upon treatment of BM-MSCs’ secretome may also be regulated by this early response cascade.
NSCLC migration and translation initiation are regulated by MAPK
To further establish the regulatory connection between MAPK signals, eIF4E/eIF4GI phosphorylation, and NSCLC migration, we treated NSCLC cells with JNK and ERK inhibitors (SP600125 and U0126, respectively). Following 45 min of exposure, we validated their activity by assaying the levels of the respective phosphorylated MAPKs (Supplementary Fig. 3). After 1.5 h of NSCLC exposure to either MAPK inhibitors, we determined reduced peIF4E/peIF4GI levels as well as the levels of their respective regulators (regulators, 25–60 %↓; peIF4E/peIF4GI, 30–60 %↓; p < 0.05) (Fig. 4a). Finally, we performed scratch assays in NSCLC cell lines while JNK and ERK were inhibited. Indeed, we determined reduced motility rates of NSCLC cells upon either MAPK inhibition presented in retarded scratch closure (JNKi, 60–65 %; ERKi, 65–90 %; p < 0.05) (Fig. 4b, c).
MAPK inhibitors affected the translation initiation factors’ phosphorylation and cell migration in NSCLC cells. a NSCLC cell lines were treated with JNK inhibitor (SP600125, 20 μM, upper panel) and ERK inhibitor (U0126, 10 μM, lower panel). After 1.5 h, the cells were harvested and immunoblotted for peIF4E/peIF4GI (white bars) and their regulators [eIF4E (4EBP, MNK), eIF4GI (mTOR) (gray bars)]. The results are presented in graphs (left) as percent (mean ± SE) of control cells (dotted line) and normalized to HSC-70 loading control (n ≥ 3). Representative immunoblots are presented (right). b, c Next, the effect of MAPKs on NSCLC cell migration was assessed by scratch assay. The cells were treated with the inhibitors, and scratch closure was photographed immediately (0 h) and after 6 h for H1299 or 24 h for H460 (magnification, ×40) of cultured cells’ area. Results are presented as b percent in bar graphs (mean ± SE, n ≥ 3) of control cells (dotted line) and c representative images. Statistically significant differences between cohorts (*p < 0.05; **p < 0.01) are indicated
Taken together, our results demonstrate that JNK and ERK control the phosphorylation levels of eIF4E and eIF4GI in NSCLC. Moreover, inhibition of these MAPKs reduces the migration levels of NSCLC cells.
Disassociation of eIF4E/eIF4GI complex inhibited NSCLC cell migration
In order to close the loop and further establish the connection between the translation initiation factors and NSCLC cell migration, we tested the significance of eIF4E/eIF4GI inhibition to NSCLC migration. For this purpose, we used the small molecule 4EGI-1 inhibitor of translation initiation that disrupts eIF4E/eIF4G association by binding eIF4E. Thus, we performed scratch assays while the NSCLC cells were treated with 4EGI-1. Indeed, we measured reduced motility rates of NSCLC cells upon eIF4E/eIF4GI disassociation presented in retarded scratch closure (40–50 %, p < 0.05) (Fig. 5). These findings suggest that manipulation of translation initiation pathways indeed affect NSCLC migration.
Disassociation of eIF4E/eIF4GI complex inhibited NSCLC cell migration. NSCLC cell lines were treated with the small molecule 4EGI-1 (inhibit eIF4E/eIF4GI association (35 μM)). The effect of 4EGI-1 on NSCLC cell migration was assessed by scratch assay. Scratch closure was photographed immediately (0 h) and after 6 h for H1299, 24 h for H460, and 18 h for A549 (magnification, ×40) of cultured cells’ area. Results are presented as a percent in bar graphs (mean ± SE, n ≥ 3) of control cells (dotted line) and (b) representative images. Statistically significant differences between cohorts (*p < 0.05) are indicated
Discussion
In this study, we have demonstrated that the BM-MSCs’ secretome was able to suppress NSCLC cell growth and elevate their death. We showed inhibitory effect on the lung cancer cell migration that is regulated both by MAPK signaling pathways and by translation initiation mechanisms. We concentrated on fundamental factors that assemble the eIF4F translation initiation complex, eIF4E and eIF4GI. Additional evidence for the factors’ functional inhibition was afforded by the reduction in their established target levels. Finally, examination of MAPK signaling pathway in NSCLC exposed to MSCs’ secretome revealed downregulation of ERK/JNK signals that also led to reduced levels of downstream targets pMNK/pmTOR/p4EBP and peIF4E/peIF4GI (timeline is summarized in Fig. 6).
Timeline of the secretome effect on NSCLC cells. The figure presents the signaling activation sequence and phenotypic alterations following exposure of NSCLC cells to BM-MSCs’ secretome. The schematic presentation describes the timeline of events that emerged from BM-MSCs’ secretome, while the thin arrows indicate the trend of change. The gray lines represent the inhibitors’ effects at the same points. The left arrow depicts the time in which the effects were detected. Here too, the black font represents the time of the effects in the cells that were exposed to the BM-MSCs’ secretome while the gray font represents the time for cells treated with the inhibitors. On the right side of the schema, what was affected in each time point is specified
Recently, more attention has been paid to the interaction between MSCs and tumor cells. Studies that investigated MSCs in the lung cancer microenvironment revealed that they exhibit genetic and functional abnormalities compared to their normal counterparts manifested in accelerated growth kinetics, reduced sensitivity to cisplatin, and differential gene expression [10]. A number of studies have reported that MSCs inhibited lung cancer tumor progression and function by specifically limiting the tumor cell propagation [37]. On the other hand, contradictory studies have demonstrated that MSCs are an NSCLC-inductive microenvironment that has the ability to protect lung cancer cells from apoptosis [6]. A possible explanation for these conflicting in vitro outcomes lies in the ratio of MSC numbers to cancer cells that were used in different studies [6].
Despite these two contradictory outcomes, both strongly support the fundamental role played by MSCs in lung carcinogenesis and present a promising cellular substrate to dissect the function/role of the tumor microenvironment in lung carcinogenesis and to develop new targeted therapies.
A major role has been previously depicted for microenvironmental cues in cancer cell motility and metastasis [38]. In NSCLC, the median survival from the time patients develop bone metastasis is less than 6 months [39]. Early detection of NSCLC is difficult; thus, 30–40 % of patients with NSCLC develop bone metastases during the course of their disease. Prognosis of lung cancer patients with bone metastases is poor, and they experience reduced quality of life [40].
In cancer metastasis, epithelial-to-mesenchymal transition (EMT) and mesenchymal-to-epithelial transition (MET) are recognized as critical events for metastasis initiation and culmination. Published studies mainly focus on mechanisms underlying EMT, but relatively less is known about the role of MET. MET is believed to participate in the establishment and stabilization of distant metastases by allowing cancerous cells to regain epithelial properties and settle at distant organs [41]. Given the critical role of MET in metastatic tumor formation, better knowledge regarding the process will facilitate the identification of novel therapeutic targets and offer new hope for inhibiting metastatic tumor formation.
It is commonly known that the bone microenvironment is a fertile soil for tumor cells due to factors released by the bone matrix. Local bone conditions such as acidosis and hypoxia are recognized as cancer-supporting surroundings [40]. Specifically in lung cancer, a subset of disseminated tumor cells (DTCs) expressing CD133 and CXCR4 have been located in the bone tissue and expressed cancer-initiating cell (CIC) features that are essential for metastasis formation [40].
In our opinion, our findings may shed new light on bone metastasis formation in NSCLC. We propose that DTCs that spread from the primary tumor arrive at the bone marrow where they are exposed to the soluble factors secreted from the MSCs. As a result, the migration rate of the cancer cells is reduced and the cells can settle in the bone tissue and initiate a metastasis. These phenotypic characteristics coincide with the process of MET. Based on these findings, we put forward that interrupting the crosstalk between the cancer cells and the secreted factors from MSCs in the bone marrow may lead to reduced metastasis formation in lung cancer patients’ bone and improve survival and quality of life. In accordance, research by Vallone et al. [42] proposed that the primary tumor could alter the plasticity of MSCs and suggested that BM-MSCs in cancer patients may be responsible for creating a pre-metastatic niche.
Conditioned media may consist of free agents such as cytokines, growth factors, and even microRNAs and/or cargo-carrying membrane-wrapped vesicles such as exosomes (50–100 nm) and microvesicles (MVs) (100–1000 nm) [43]. Indeed, recent studies by others and us have demonstrated that factors in MSCs’ conditioned medium, including exosomes and soluble factors, can mediate biological functions and suppress tumor progression [44–46]. Specifically, MSCs’ secreted factors such as VEGF, MCP-1, MIG, MIP-1a, and MIP-1b were able to reduce MAPK signaling pathways, which affected the cancer cells’ malignant phenotype [47].
Many lines of evidence support the idea that disruption of eIF4F activity has antioncogenic consequences [48], especially in breast cancer. For example, KD of eIF4E in breast cancer metastatic cells induced MET and inhibited metastasis [49]. Additional research in breast cancer showed that inhibition of cap-dependent translation downregulated Snail expression and suppressed cell migration and invasion.
Nevertheless, no study addressed the participation of translation initiation machinery in lung cancer cell migration or the influence of neighboring cell populations on these signals. Here, we demonstrated for the first time the effect of lung cancer exposure to BM-MSCs’ secretome, specifically the inhibited translation initiation factors, the connection to MAPK regulation, and the influence on the cell fate and migration. Of note, the consequences of translation initiation modulation may have different consequences in different settings. For instance, while in the primary tumor site inhibition of translation initiation may limit the cell detachment from the tumor bulk and deplete metastatic cells, it may be the metastasis-promoting event when it promotes the migratory cells resettling at distant new sites. In summary, our current study uncovered microenvironmental cues that critically affected NSCLC cell fate via translation factor signals. We specifically shed light on the cell migration as the progression of cancers from primary tumors to invasive and metastatic stages accounts for the overwhelming majority of cancer deaths. Understanding the molecular events which promote metastasis and improve the means of foretelling their development is a major goal of the current clinical research.
References
Sun S, Schiller JH, Spinola M, Minna JD. New molecularly targeted therapies for lung cancer. J Clin Invest. 2007;117:2740–50.
Higgins MJ, Ettinger DS. Chemotherapy for lung cancer: the state of the art in 2009. Expert Rev Anticancer Ther. 2009;9:1365–78.
Legrier ME, Yang CP, Yan HG, Lopez-Barcons L, Keller SM, Perez-Soler R, et al. Targeting protein translation in human non small cell lung cancer via combined MEK and mammalian target of rapamycin suppression. Cancer Res. 2007;67:11300–8.
Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–46.
Graves EE, Maity A, Le QT. The tumor microenvironment in non-small-cell lung cancer. Semin Radiat Oncol. 2010;20:156–63.
Liu R, Wei S, Chen J, Xu S. Mesenchymal stem cells in lung cancer tumor microenvironment: their biological properties, influence on tumor growth and therapeutic implications. Cancer Lett. 2014;353:145–52.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Korkaya H, Wicha MS. Breast cancer stem cells: we’ve got them surrounded. Clin Cancer Res. 2013;19:511–3.
Klaus M, Stavroulaki E, Kastrinaki MC, Fragioudaki P, Giannikou K, Psyllaki M, et al. Reserves, functional, immunoregulatory, and cytogenetic properties of bone marrow mesenchymal stem cells in patients with myelodysplastic syndromes. Stem Cells Dev. 2010;19:1043–54.
Gottschling S, Granzow M, Kuner R, Jauch A, Herpel E, Xu EC, et al. Mesenchymal stem cells in non-small cell lung cancer—different from others? Insights from comparative molecular and functional analyses. Lung Cancer. 2013;80:19–29.
Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64.
Kim S, Kim HS, Lee E, Kim HO. In vivo hepatic differentiation potential of human cord blood-derived mesenchymal stem cells. Int J Mol Med. 2011;27:701–6.
Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nat Rev Cancer. 2010;10:254–66.
Barnhart B, Simon M. Taking aim at translation for tumor therapy. J Clin Invest. 2007;117:2385–8.
Thornton S, Anand N, Purcell D, Lee J. Not just for housekeeping: protein initiation and elongation factors in cell growth and tumorigenesis. J Mol Med. 2003;81:536–48.
Agnelli L, Fabris S, Bicciato S, Basso D, Baldini L, Morabito F, et al. Upregulation of translational machinery and distinct genetic subgroups characterise hyperdiploidy in multiple myeloma. Br J Haematol. 2007;136:565–73.
Li Y, Fan S, Koo J, Yue P, Chen ZG, Owonikoko TK, et al. Elevated expression of eukaryotic translation initiation factor 4E is associated with proliferation, invasion and acquired resistance to erlotinib in lung cancer. Cancer Biol Ther. 2012;13:272–80.
Bauer C, Brass N, Diesinger I, Kayser K, Grasser FA, Meese E. Overexpression of the eukaryotic translation initiation factor 4G (EIF4G-1) in squamous cell lung carcinoma. Int J Cancer. 2002;98:181–5.
Zismanov V, Drucker L, Gottfried M. ER homeostasis and motility of NSCLC cell lines can be therapeutically targeted with combined Hsp90 and HDAC inhibitors. Pulm Pharmacol Ther. 2013;26:388–94.
Zismanov V, Drucker L, Gottfried M. Combined inhibition of Hsp90 and the proteasome affects NSCLC proteostasis and attenuates cell migration. Anti Cancer Drugs. 2014;25:998–1006.
Meric F, Hunt KK. Translation initiation in cancer: a novel target for therapy. Mol Cancer Ther. 2002;1:971–9.
Jacobson BA, Alter MD, Kratzke MG, Frizelle SP, Zhang Y, Peterson MS, et al. Repression of cap-dependent translation attenuates the transformed phenotype in non-small cell lung cancer both in vitro and in vivo. Cancer Res. 2006;66:4256–62.
Fan S, Li Y, Yue P, Khuri FR, Sun SY. The eIF4E/eIF4G interaction inhibitor 4EGI-1 augments trail-mediated apoptosis through c-FLIP down-regulation and DR5 induction independent of inhibition of cap-dependent protein translation. Neoplasia. 2010;12:346–56.
Zismanov V, Lishner M, Tartakover-Matalon S, Radnay J, Shapiro H, Drucker L. Tetraspanin-induced death of myeloma cell lines is autophagic and involves increased UPR signalling. Br J Cancer. 2009;101:1402–9.
Attar-Schneider O, Drucker L, Zismanov V, Tartakover-Matalon S, Rashid G, Lishner M. Bevacizumab attenuates major signaling cascades and eIF4E translation initiation factor in multiple myeloma cells. Lab Investig. 2012;92:178–90.
O’Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409:635–49.
Dolfi SC, Chan LL, Qiu J, Tedeschi PM, Bertino JR, Hirshfield KM, et al. The metabolic demands of cancer cells are coupled to their size and protein synthesis rates. Cancer Metab. 2013;1:20.
Zismanov V, Drucker L, Attar-Schneider O, Matalon ST, Pasmanik-Chor M, Lishner M. Tetraspanins stimulate protein synthesis in myeloma cell lines. J Cell Biochem. 2012;113:2500–10.
Robert F, Pelletier J. Translation initiation: a critical signalling node in cancer. Expert Opin Ther Targets. 2009;13:1279–93.
Yang YJ, Zhang YL, Wang JD, Lai ZS, Wang QY, Cui HH. [Role of eukaryotic initiation factor-4E (eIF-4E) in regulation of expression of NF-kappaB and its subsequent influence on transcription and activity of heparanase in human colon adenocarcinoma cell line]. Ai Zheng. 2003;22:1023–9.
Shiroki K, Ohsawa C, Sugi N, Wakiyama M, Miura K, Watanabe M, et al. Internal ribosome entry site-mediated translation of Smad5 in vivo: requirement for a nuclear event. Nucleic Acids Res. 2002;30:2851–61.
Baird SD, Turcotte M, Korneluk RG, Holcik M. Searching for IRES. RNA. 2006;12:1755–85.
Braunstein S, Karpisheva K, Pola C, Goldberg J, Hochman T, Yee H, et al. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol Cell. 2007;28:501–12.
Kaiser C, Dobrikova EY, Bradrick SS, Shveygert M, Herbert JT, Gromeier M. Activation of cap-independent translation by variant eukaryotic initiation factor 4G in vivo. RNA. 2008;14:2170–82.
Lau MT, So WK, Leung PC. Fibroblast growth factor 2 induces E-cadherin down-regulation via PI3K/Akt/mTOR and MAPK/ERK signaling in ovarian cancer cells. PLoS One. 2013;8:e59083.
Shveygert M, Kaiser C, Bradrick SS, Gromeier M. Regulation of eukaryotic initiation factor 4E (eIF4E) phosphorylation by mitogen-activated protein kinase occurs through modulation of Mnk1-eIF4G interaction. Mol Cell Biol. 2010;30:5160–7.
Ampollini L, Madeddu D, Falco A, Frati C, Lorusso B, Graiani G, et al. Lung mesenchymal cells function as an inductive microenvironment for human lung cancer propagating cellsdagger. Eur J Cardiothorac Surg. 2014;46:e103–12.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52.
Decroisette C, Monnet I, Berard H, Quere G, Le Caer H, Bota S, et al. Epidemiology and treatment costs of bone metastases from lung cancer: a French prospective, observational, multicenter study (GFPC 0601). J Thorac Oncol. 2011;6:576–82.
Roato I. Bone metastases: when and how lung cancer interacts with bone. World J Clin Oncol. 2014;5:149–55.
Campolo F, Gori M, Favaro R, Nicolis S, Pellegrini M, Botti F, et al. Essential role of Sox2 for the establishment and maintenance of the germ cell line. Stem Cells. 2013;31:1408–21.
Fernandez Vallone VB, Hofer EL, Choi H, Bordenave RH, Batagelj E, Feldman L, et al. Behaviour of mesenchymal stem cells from bone marrow of untreated advanced breast and lung cancer patients without bone osteolytic metastasis. Clin Exp Metastasis. 2013;30:317–32.
Redzic JS, Balaj L, van der Vos KE, Breakefield XO. Extracellular RNA mediates and marks cancer progression. Semin Cancer Biol. 2014;28:14–23.
Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–57.
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8:e84256.
Attar-Schneider O, Zismanov V, Dabbah M, Tartakover-Matalon S, Drucker L, Lishner M. Multiple myeloma and bone marrow mesenchymal stem cells’ crosstalk: effect on translation initiation. Mol Carcinog. 2015. doi:10.1002/mc.22378.
Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685.
Nasr Z, Pelletier J. Tumor progression and metastasis: role of translational deregulation. Anticancer Res. 2012;32:3077–84.
Shankar J, Messenberg A, Chan J, Underhill TM, Foster LJ, Nabi IR. Pseudopodial actin dynamics control epithelial-mesenchymal transition in metastatic cancer cells. Cancer Res. 2010;70:3780–90.
Authors’ contribution
The authors’ contributions were as follows: Attar-Schneider and Zismanov were responsible for the conception and design of the study, acquisition, analysis and interpretation of data, and drafting the article. Drucker and Gottfried were responsible for the conception and design of the study and for final approval of the version to be submitted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Oshrat Attar-Schneider and Victoria Zismanov contributed equally to this work.
Rights and permissions
About this article
Cite this article
Attar-Schneider, O., Zismanov, V., Drucker, L. et al. Secretome of human bone marrow mesenchymal stem cells: an emerging player in lung cancer progression and mechanisms of translation initiation. Tumor Biol. 37, 4755–4765 (2016). https://doi.org/10.1007/s13277-015-4304-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4304-3