Abstract
Ovarian carcinoma is one of the most severe cancers in women, with a high relapse rate and limited secondary treatment options. To assist research in novel treatment technologies, including CD8+ T cell-base immunotherapy, we examined the effect of tumor-infiltrating regulatory T cells (Tregs) in inhibiting CD8+ T cell inflammation. We found that compared to their peripheral blood counterparts, tumor-infiltrating Tregs exhibited more potent inhibitory function, which was associated with higher interleukin 10 (IL-10) production in tumor-infiltrating Tregs. Blockade of T cell immunoglobulin mucin 3 (TIM3), a regulatory molecule overrepresented on tumor-infiltrating Tregs, had significantly reverted Treg-mediated suppression. Moreover, expression of TIM3 on tumor-infiltrating Tregs was directly correlated with tumor size. Together, our results demonstrated that ovarian tumor-infiltrating Treg cells were more immunosuppressive than their peripheral blood counterparts in a TIM3-dependent fashion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian carcinoma is one of the most common cancers in women, with a 2015 estimation of 21,000 new diagnoses and 14,000 ovarian cancer-caused deaths in the USA [1]. Due to the fact that the disease often progresses before symptoms appear, most ovarian cancer cases are diagnosed at an advanced stage, making the overall 5-year survival rate below 40 % [2, 3]. Although the initial response rate to conventional treatments, including surgical operations and platinum-based chemotherapy, is high, 30–40 % of patients will relapse within 12 months and become refractory to further chemotherapies [4, 5]. To solve this issue, new treatment options, including CD8+ cytotoxic T cell-based immunotherapies, have been explored in ovarian cancer [6]. Despite clear signs of promise, intratumoral suppression mechanisms, including those mediated by regulatory T cells, are thought to hamper anti-tumor CD8+ T cell activity and reduce the clinical benefit [7, 8].
Conventional regulatory T cells (Tregs), identified by CD4+CD25+Foxp3+ expression, are potent regulators of CD8+ T cell activation and inflammation through both direct suppression by inhibitory cytokines, such as interleukin 10 (IL-10), IL-35, and transforming growth factor beta (TGF-b), and indirect suppression mechanisms such as subversion of dendritic cells toward a more immunoregulatory phenotype, depletion of local IL-2, a crucial T cell growth factor, and establishing an inhibitory tumor microenvironment [8, 9]. In ovarian cancer, the number of Treg cells was associated with more advanced cancer stage, higher death hazard, and suppressed Th1 immunity [10–12]. These previous studies suggested a role of Treg cells in promoting ovarian cancer growth, but whether ovarian tumor-infiltrating Treg cells possess similar characteristics and functions has not been extensively studied.
In this study, we examined the ovarian carcinoma-infiltrating regulatory T cells, and found that they exhibit more potent regulatory effects than their peripheral blood counterparts. Interestingly, T cell immunoglobulin mucin 3 (TIM3), a transmembrane molecule associated with CD8+ T cell dysfunction and exhaustion [13], was overrepresented on tumor-infiltrating Tregs but not peripheral blood Tregs. Blockade of TIM3 reverted the inhibitory functions of tumor-infiltrating Treg cells. Furthermore, TIM3 expression on tumor-infiltrating Tregs was directly correlated with ovarian tumor size. Together, these observations further elucidated the mechanism of immune suppression in the tumor microenvironment, and revealed an additional challenge in cancer immunotherapy.
Materials and methods
Samples
Patient characteristics are described in Table 1. All patients in this study were diagnosed with serous epithelial ovarian carcinoma at The Maternal and Child Health Hospital of Jinan City, and all of their diseases were classified as high grade (G2 or G3). Age-matched healthy controls were also recruited. Written informed consent was obtained from all subjects. All study procedures were approved by the Ethics Committee of The Maternal and Child Health Hospital of Jinan City. Tumor specimens were collected by surgical resection, and peripheral blood samples were collected by venipuncture. Tumor-infiltrating lymphocytes (TILs) were prepared using Tumor Dissociation Kit, human (Miltenyi). Briefly, resected tumors were cut into small pieces of 2–4 mm in diameter. The cut pieces were then placed in a gentleMACS C tube (Miltenyi) containing 5 mL of the enzyme mix, and was placed in the gentleMACS Dissociator (Miltenyi) for dissociation, following procedures provided by the manufacturer. At the end of the dissociation, the samples were resuspended and allowed to flow through a cell strainer with 70-μm mesh size. The resulting cell mixture was subsequently processed by Percoll (Sigma-Aldrich) gradient centrifugation to extract TILs. Peripheral blood mononuclear cells (PBMCs) were prepared from peripheral blood samples by Ficoll (Life Technologies) gradient centrifugation. Samples were cryopreserved at −150 °C for less than 1 year before being thawed and used in experiments. After thawing, cells were cultured in RPMI 1640 medium (Life Technologies) supplemented with L-glutamine, penicillin/streptomycin, and 10 % fetal bovine serum (FBS) during experiments, unless otherwise noted.
Flow cytometry
The following monoclonal antibodies from BD were used: anti-human CD3 (HIT3a), CD4 (OKT4), CD8 (RPA-T8), CD25 (BC96), FoxP3 (206D), IFNg (4S.B3), IL-10 (JES3-9D7), and TIM3 (F38-2E2). For surface staining, cells were first washed with PBS + 2 % FBS, incubated with LIVE/DEAD Fixable Aqua Dead Cell Stain (Life Technologies) for 30 min, and then stained with surface antibodies. For intracellular interferon gamma (IFNg) and IL-10 staining, GolgiStop/GolgiPlug (BD) was added 6 h prior to the start of surface staining. Cells were then stained with the surface staining protocol, after which the cells were fixed and permeabilized using Cytofix/Cytoperm (BD), and the anti-IFNg and IL-10 antibodies were added in diluted PermWash (BD). For FoxP3 staining, the FoxP3/Transcription Factor Staining Buffer Set (eBioscience) was used according to the manufacturer’s protocol. All samples were fixed with 2.5 % formaldehyde in PBS at the end of the staining and immediately analyzed on a FACSCanto II cytometer (BD). For live cell sorting, CD3, CD4, CD8, and CD25 antibodies were added to the cells incubated in culture media, at 4 °C for 30 min, to identify CD4+CD25+ Tregs and CD8+ T cells. Cells were then washed twice and sorted in a FACSAria cytometer (BD).
Cell culture
For CD8+ T cell suppression assay, CD3+CD4+CD25+ Treg cells and CD3+CD8+ T cells from PBMCs and TILs were sorted and counted in FACSAria, after which they were cocultured at 1:1 (5 × 103 CD8+ T + 5 × 103 Tregs) or 5:1 (2.5 × 104 CD8+ T + 5 × 103 Tregs) ratio, in 100 μL medium. 105 irradiated autologous feeder cells (T cell-depleted PBMCs) were also placed in 100-μL medium. The Tregs, CD8+ T cells, and feeder cells were then combined in the same well of a 96-well flat-bottom plate. One microgram per milliliter staphylococcal enterotoxin B (SEB) was added in culture. Anti-TIM3 antibody (F38-2E2), anti-IL-10 antibody (JES3-19F1), and anti-IL-10R (3F9) were added in some cocultures according to previously published methods [14, 15]. Cells were incubated at 37 °C 5 % CO2 level for 72 h before proceeding to staining and flow cytometry.
Statistical analyses
All statistical analyses were performed by GraphPad Prism 6 software (GraphPad). Shapiro-Wilk test was used to analyze data distribution and to decide whether parametric or nonparametric tests should be used. Two-tailed value of P < 0.05 was considered significant.
Results
CD4+CD25+Foxp3+ Treg cells were significantly upregulated in ovarian carcinoma compared to peripheral blood
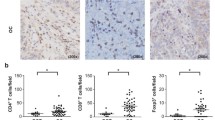
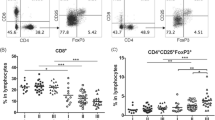
We first examined frequency of CD4+CD25+Foxp3+ Treg cells in ovarian patients and healthy controls. Peripheral blood mononuclear cells (PBMCs) were obtained from ovarian cancer patients and healthy controls. Ovarian tumor-infiltrating lymphocytes (TILs) were obtained from ovarian cancer patients. Treg markers were examined by flow cytometry (Fig. 1a). We found that the frequencies of CD4+CD25+ T cells were significantly upregulated in ovarian TILs than in PBMCs, while that in control PBMCs were significantly lower than both (Fig. 1b). The CD4+CD25+ T cells in TILs also expressed significantly higher Foxp3, both by the percentage of Foxp3+ cells and by Foxp3 mean fluorescence intensity (MFI) (Fig. 1c, e). Overall, CD4+CD25+Foxp3+ Tregs were overrepresented in ovarian cancer patients than healthy controls, with significantly further increases in TILs compared to in PBMCs (Fig. 1d). The expression of Treg transcription factor Foxp3 is considered a stronger indicator of regulatory T cell activity than CD4+CD25+ expression, since CD25 is the IL-2 receptor alpha chain and not all CD4+CD25+ T cells can be characterized as Treg cells [16]. To examine the exclusivity of Foxp3 expression in CD4+CD25+ T cells, we found that the vast majority of Foxp3+ CD4+ T cells were CD25+, in both ovarian cancer PBMCs and TILs (Fig. 1f). Collectively, these results demonstrated that CD4+CD25+Foxp3+ Treg cells were overrepresented in ovarian carcinoma than in peripheral blood.
Frequencies of Treg cell subsets were elevated in TILs compared to PBMCs in ovarian cancer patients. a Gating strategy of CD4+CD25+ Treg cells and CD4+CD25+Foxp3+ Treg cells in one representative ovarian cancer (OC) patient. Greater than 100,000 live cell events in the lymphocyte gate were shown. The differences between OC TILs, OC PBMCs, and healthy control PBMCs, in terms of b the frequencies of CD4+CD25+ cells in CD4+ T cells, c the frequencies of Foxp3+ cells in CD4+CD25+ Tregs, d the frequencies of CD4+CD25+Foxp3+ Treg cells in CD4+ T cells, e the mean fluorescence intensity (MFI) of Foxp3 in CD4+CD25+ Tregs, and f the percentage of CD25+ cells in all Foxp3+ CD4+ T cells, were compared with regular or Kruskal-Wallis one-way ANOVA followed by Tukey’s or Dunn’s test for normally or non-normally distributed data. ***P < 0.001; **P < 0.01; *P < 0.05
Ovarian TIL Tregs expressed higher levels of TIM3 than their peripheral blood counterparts
TIM3 has recently emerged as a key regulator of CD8+ T cell dysfunction and exhaustion [13, 14]. When expressed on Treg cells, TIM3 seems to promote Treg-mediated immunosuppression in anti-tumor immunity, chronic inflammation, and acute injury repair [17–20]. Here, we examined TIM3 expression in ovarian TIL Treg cells. Compared to their counterparts in PBMCs, the TIL CD4+CD25+ T cells contained significantly higher levels of TIM3+ cells and presented significantly higher TIM3 MFI (Fig. 2a). Similarly, the TIL CD4+CD25+Foxp3+ T cells also contained significantly higher levels of TIM3+ cells and presented significantly higher TIM3 MFI (Fig. 2b). Together, these data demonstrated that TIM3 upregulation on Treg cells was concentrated in the ovarian intratumoral environment.
Expression of TIM3 on Treg cells was elevated in TILs compared to PBMCs in ovarian cancer patients. a The gating strategy of TIM3 expression, the percentage of TIM3+ cells, and the TIM3 MFI on CD4+CD25+ Tregs were shown and compared with Student’s t test. b The gating strategy of TIM3 expression, the percentage of TIM3+ cells, and the TIM3 MFI on CD4+CD25+Foxp3+ Tregs were shown and compared with Student’s t test or Mann–Whitney test for normally or non-normally distributed data. ***P < 0.001
CD4+CD25+ TIL Tregs were more potent at mediating CD8+ T cell exhaustion than their peripheral blood counterparts
The higher expression of TIM3 and Foxp3 in TIL Treg cells led to our speculation that at equal numbers, TIL Treg cells were more immunosuppressive than their peripheral blood counterparts. To demonstrate this, we cocultured peripheral blood CD8+ T cells with autologous CD4+CD25+ Tregs from PBMCs or from TILs at equal numbers. Interferon gamma (IFNg) secretion from CD8+ T cells was used as the readout. We found that when CD8+ T cells and Treg cells were cocultured at 1:1 ratio, both PBMC Tregs and TIL Tregs had significantly suppressed CD8+ T cell-mediated IFNg expression (Fig. 3a, b). The suppressive effect of TIL Tregs was stronger than that of PBMC Tregs (Fig. 3b). When CD8+ T cells and Treg cells were cocultured at 5:1 ratio, no significant suppression mediated by PBMC Tregs were observed, while TIL Tregs had significantly suppressed CD8+ T cell inflammation (Fig. 3c). We also examined IL-10 production in PBMC and TIL Tregs after coculture (Fig. 3d), and found that TIL Tregs contained significantly higher frequencies of IL-10+ cells (Fig. 3e). To examine whether the higher IL-10 secretion by TIL Tregs could result in more potent CD8+ T cell suppression by TIL Tregs, in a separate experiment, IL-10 and IL-10 receptor (IL-10R) blocking antibodies were added in the CD8+ T cell-Treg cell coculture. We found that IL-10 blockade partially reverted the increased inhibition by TIL Treg cells (Fig. 3f).
TIL Treg cells were more potent at suppressing autologous CD8+ T cell IFNg production, and secreted higher levels of IL-10, than their PBMC counterparts. CD4+CD25+ Treg cells from PBMCs or TILs were live sorted and cocultured with autologous live sorted CD8+ T cells, in the presence of 5 × 104 irradiated APCs (T cell-depleted PBMCs) and 1 μg/mL SEB per 200 μL medium for 72 h. Six hours prior to harvest, GolgiStop and GolgiPlug were added into the coculture. IFNg and IL-10 expression was analyzed by flow cytometry. a Representative IFNg expression in CD8+ T cells, when CD8+ cells were cocultured alone (no Treg), with PBMC Treg (+PBMC Treg), or with TIL Treg (+TIL Treg). b Percentage of IFNg+ CD8+ T cells after coculture, at 1:1 ratio (5 × 103 CD8+ T cells plus 5 × 103 CD4+CD25+ Tregs). c Percentage of IFNg+ CD8+ T cells after coculture, at 5:1 ratio (2.5 × 104 CD8+ T cells plus 5 × 103 CD4+CD25+ Tregs). d Representative IL-10 expression in CD4+CD25+ Treg cells after coculture. e Percentage of IL-10+ cells in PBMC Tregs or autologous TIL Tregs. f Percentage of IFNg+ CD8+ T cells after coculturing with Tregs and 5 μg/mL anti-IL-10 and 2.5 μg/mL anti-IL-10R (IL-10 blocking), at 5:1 ratio (1.25 × 104 CD8+ T cells plus 2.5 × 103 CD4+CD25+ Tregs). Differences were computed by RM one-way ANOVA and Tukey’s multiple comparisons test for comparisons between three groups, and by paired t test for comparisons between two groups. ***P < 0.001; **P < 0.01; *P < 0.05. ns not significant
TIL Treg-mediated CD8+ T cell suppression and IL-10 production were TIM3-dependent
Since IL-10 by Treg cells mediates several suppression mechanisms [21], including the suppression of CD8+ T cell proinflammatory cytokine production, we examined IL-10 production by TIM3+ and TIM3− Tregs. We found that compared to PBMC Tregs under unstimulated conditions, TIL Tregs contained significantly higher frequencies of IL-10+ cells, most of which belonged to the TIM3+ compartment (Fig. 4). Staphylococcal enterotoxin B (SEB), a superantigen, was used to mimic the antigen-specific stimulation of T cells. After SEB stimulation, TIL Tregs still contained significantly higher frequencies of IL-10+ cells than PBMC Tregs. The TIM3+ compartment contained most of the IL-10+ cells in both Treg populations.
IL-10 expression by TIM3+ or TIM3− Treg cells from PBMCs or TILs. PBMCs or TILs were cultured in unstimulated culture medium or with 1 μg/mL SEB per 200 μL medium for 72 h. Six hours prior to harvest, GolgiStop and GolgiPlug were added into the coculture. IL-10 expression in TIM3+ CD4+CD25+ Tregs or TIM3− CD4+CD25+ Tregs, in PBMC Tregs or TIL Tregs, was analyzed by flow cytometry. N = 10. Two-way ANOVA and Sidak’s multiple comparisons test. ***P < 0.001. ns not significant
Since we observed significantly higher TIM3 expression and IL-10 production by TIM3+ cells in TIL Tregs compared to PBMC Tregs, we next examined whether higher TIM3 expression was responsible for the enhanced inhibitory function by TIL Tregs. Only the CD8+ T-to-Treg = 5:1 scenario was examined due to the small amount of TIL Treg cells. We found that TIM3 blockade had significantly abrogated TIL Treg-mediated suppression of CD8+ T cell IFNg production (Fig. 5a), and downregulated IL-10+ cells by TIL Treg cells (Fig. 5b).
TIM3 expression was involved in TIL Treg-mediated suppression of IFNg production in CD8+ T cells, and IL-10 expression in Treg cells. a Suppression of CD8+ T cell IFNg production by TIL Tregs were abrogated by TIM3 blockade. CD8+ T cells and autologous CD4+CD25+ Tregs were cocultured at 5:1 ratio as previously described, in the presence of 10 μg/mL TIM3 antibody (F38-2E2) or isotype control [14]. b Expression of IL-10 in TIL CD4+CD25+ Tregs was suppressed by TIM3 blockade. Differences were computed with RM one-way ANOVA and Tukey’s multiple comparisons test. **P < 0.01; *P < 0.05. ns not significant
Collectively, these data demonstrated the roles of TIM3 in TIL Treg-mediated CD8+ T cell suppression and IL-10 production.
TIM3 expression on TIL Treg cells was correlated with tumor size
To demonstrate in vivo relevance of our findings, the correlation between TIM3 expression on TILs with ovarian tumor size was examined. We found that the frequency of TIM3+ cells in CD4+CD25+ TIL Tregs and the frequency of TIM3+ cells in CD4+CD25+Foxp3+ TIL Tregs were both positively correlated with tumor size (Fig. 6a, b), suggesting an involvement of TIM3 expression on TIL Tregs in tumor growth and progression.
TIM3 expression on TIL Tregs was directly correlated with tumor size. a The percentage of TIM3+ cells in CD4+CD25+ TIL Tregs was positively correlated with tumor size. R = 0.4987. P Pearson correlation coefficient. b The percentage of TIM3+ cells in CD4+CD25+Foxp3+ TIL Tregs was positively correlated with tumor size. R = 0.4987. P Pearson correlation coefficient
Discussion
In conclusion, our results from this study supported the hypothesis that ovarian tumor-infiltrating Treg cells were more immunosuppressive than peripheral blood Treg cells. Staining of PBMCs and TILs revealed that higher frequencies of CD4+CD25+ T cells were found in TILs than in PBMCs. The expression of Foxp3, a canonical transcription factor in Treg development, was elevated in CD4+CD25+ T cells in TILs. The expression of TIM3, a negative regulator of T cell inflammation when expressed on CD8+ T cells, and a promoter of regulatory function when expressed on Treg cells, was overrepresented in TIL Tregs compared to PBMC Tregs. Coculture experiments using autologous CD8+ T cells and Treg cells of PBMC or TIL origin demonstrated a stronger suppression of CD8+ T cell-mediated IFNg expression by TIL Treg cells. Furthermore, TIL Treg cells were able to exert inhibitory function toward more CD8+ T cells than the same number of PBMC Treg cells. Interestingly, we also found that TIL Tregs expressed more IL-10 than PBMC Tregs in the coculture, which possibly explained why TIL Tregs were more inhibitory. Indeed, blocking IL-10 and IL-10R partially reverted TIL Treg-mediated suppression of CD8+ T cell IFNg production. Together, these data demonstrated that tumor-infiltrating Tregs were functionally different from peripheral blood Tregs. This difference may act as an additional obstacle in developing tumor-specific CD8+ T cell-based immunotherapies.
Our results also suggest that TIM3 expression on TIL Treg cells play an important role in suppressing CD8+ T cell responses and tumor development. Blocking of TIM3 significantly reduced the inhibitory effect of TIL Tregs and the level of IL-10 production. Moreover, the level of TIM3 expression on TIL Tregs was directly correlated with tumor size. These results revealed an additional mechanism by which TIM3 inhibits tumor immunity [17, 22]. Currently, we do not know if TIM3 expressions on CD8+ T cells and Treg cells were regulated by a shared mechanism, such that patients with high TIM3 expression on CD8+ T cells also had high TIM3+ on tumor-infiltrating Tregs. Studies so far seem to suggest that TIM3 expression suppresses proinflammatory CD8+ T cell inflammation while it promotes Treg inhibitory function, which highlighted the potential benefit of depleting TIM3-expressing cells during cancer immunotherapy.
Here, we focused on the effect of tumor-infiltrating Treg cells on CD8+ T cells. It is also known that Treg cells could potently subvert the maturation and function of antigen-presenting dendritic cells (DCs) required for effector T cell function. Treg expression of cytotoxic T lymphocyte antigen 4 (CTLA4) and CD80/CD86 signaling could condition DCs toward a more inhibitory phenotype and result in the expression of indoleamine 2,3-dioxygenase (IDO), a potent promoter of effector T cell apoptosis [23–25]. How tumor-infiltrating Tregs might differ in these suppression mechanisms is still unknown, and would require further study.
References
Lowe KA, Chia VM, Taylor A, O’Malley C, Kelsh M, Mohamed M, et al. An international assessment of ovarian cancer incidence and mortality. Gynecol Oncol. 2013;130:107–14.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics: 2011. CA Cancer J Clin. 2011;61:69–90.
Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9:730–56.
Winter WE, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7.
Du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzin. Cancer. 2009;115:1234–44.
McGray AJR, Hallett R, Bernard D, Swift SL, Zhu Z, Teoderascu F, et al. Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor. Mol Ther. 2014;22:206–18.
Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307.
Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Investig. 2007;117:1167–74.
Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9.
Govindaraj C, Scalzo-Inguanti K, Madondo M, Hallo J, Flanagan K, Quinn M, et al. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin Immunol. 2013;149:97–110.
Barnett JC, Bean SM, Whitaker RS, Kondoh E, Baba T, Fujii S, et al. Ovarian cancer tumor infiltrating T-regulatory (T(reg)) cells are associated with a metastatic phenotype. Gynecol Oncol. 2010;116:556–62.
Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;213–16.
Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–79.
Das A, Ellis G, Pallant C, Lopes ARR, Khanna P, Peppa D, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–35.
Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. 2010;10:490–500.
Sakuishi K, Ngiow SF, Sullivan JM, Teng MWL, Kuchroo VK, Smyth MJ, et al. TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2, e23849.
Song H, Zhou Y, Li G, Bai J. Regulatory T cells contribute to the recovery of acute lung injury by upregulating Tim-3. Inflammation. 2015;38:1267–72.
Moorman JP, Wang JM, Zhang Y, Ji XJ, Ma CJ, Wu XY, et al. Tim-3 pathway controls regulatory and effector T cell balance during hepatitis C virus infection. J Immunol. 2012;189:755–66.
Gautron A-S, Dominguez-Villar M, de Marcken M, Hafler DA. Enhanced suppressor function of TIM-3(+) FoxP3(+) regulatory T cells. Eur J Immunol. 2014;1–9.
Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;636–45.
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94.
Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302.
Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4 + CD25+ T cells. Immunity. 2003;19:877–89.
Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Meimei Bu and Yizhen Shen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bu, M., Shen, Y., Seeger, W.L. et al. Ovarian carcinoma-infiltrating regulatory T cells were more potent suppressors of CD8+ T cell inflammation than their peripheral counterparts, a function dependent on TIM3 expression. Tumor Biol. 37, 3949–3956 (2016). https://doi.org/10.1007/s13277-015-4237-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4237-x