Abstract
MicroRNAs (miRNAs) are small noncoding RNA molecules which regulate the target gene expression posttranscriptionally. Increasing studies have shown that microRNAs play important roles in multiple biological pathways. For instance, aberrant expression of microRNA-224 (miR-224) plays a vital role in tumor biology in various types of human cancer. Here, we aim to summarize the molecular mechanisms that lead to the overexpression of miR-224 in cancers, analyze the effect of miR-224 on tumor biology, and reveal the clinical significance of miR-224. MiR-224 regulates its targets by modulating messenger RNA (mRNA) stability and/or protein translation, and it would provide new insight into molecular targeting cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MicroRNAs (miRNAs) are highly conserved, approximately 22 nucleotide-long, and noncoding RNA molecules that play a vital role in regulating messenger RNA (mRNA) expression. MiRNAs repress translation or induce degradation of their target mRNAs through binding to the 3′untranslated region (UTR) [1–3]. Dysregulation of miRNAs participate in a wide range of biological processes such as oncogenesis, apoptosis, proliferation, metastasis, invasion, and even drug resistance [4–6]. MiR-224 is a commonly dysregulated miRNA in human cancers such as glioma, cervical cancer, lung adenocarcinoma, prostate cancer (PCa), breast cancer (BRC), hepatocellular carcer (HCC), and colorectal cancer (CRC) [7]. In this review, we will focus on the functions of miR-224 in tumor development, progression, treatment as well as prognosis. With a clear understanding of miR-224, we will partly reveal the cross talk between miR-224 and cancers.
The regulation of miR-224 expression
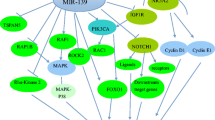
MiR-224 is a commonly dysregulated microRNA in most cancers, which affects crucial cellular processes. MiR-224-residing locus in chromosome Xq28 and the transcript expression of miR-224 and gene on chromosome Xq28 are upregulated in HCC. Histone acetylation was reported to play critical roles in the activation of the expression of miR-224 and its associated Xq28 gene. Beside, E1A binding protein p300 (EP300) may account for the upregulation of miR-224 expression in HCC. EP300 is upregulated and displays increased binding to the Xq28 locus which activates expression of miR-224 by increasing histone acetylation [8]. NF-κB inflammatory pathways may be another regulator mechanism behind miR-224 upregulated in HCC. ChIP analysis showed that p65/NF-κB is important for the miR-224 promoter and exogenously expressed p65/RelA activates the miR-224 promoter. Moreover, LPS, LTα, and TNFα increase miR-224 expression through activating transcription of the miR-224 promoter. Altogether, above findings link the inflammatory signals to NF-κB-mediated upregulated expression of miR-224 [9]. Knoll et al. reported that miR-224 was significantly increased in advanced melanoma cell and the transcription factor E2F1 may account for this phenomenon. MiR-224 is located in the gamma-aminobutyric acid (GABA), a receptor epsilon gene (GABRE), and its expression is directly activated by E2F1 through transactivation of the GABRE gene [10]. Ning et al. study showed that the expression of miR-224 could be regulated by angiotensin II (Ang II). Ang II significantly accelerated the expression of miR-224 in adult rat cardiac fibroblasts [11]. MiR-224 expression is regulated by transforming growth factor-beta (TGF-β)1/Smads pathway. The activation of TGF-β1 can induce upregulation of miR-224 through accelerating phosphorylation of the downstream effectors Smad2/3 in granulosa cell (GC) [12]. Hypoxia-inducible factor 1 alpha (HIF1A) is increased in TGF-β1 pathway-expressing melanoma cells and that HIF1A upregulates the expression of miR-224 [13]. On the contrary, the expression of miR-224 was reported to be suppressed by the tumor suppressor gene p53 and NF-κB p65 subunit in follicular granulosa cell. TGF-β1 enhanced the binding of p53 and p65 to miR-224 host gene, the proximal promoter region of GABAA receptor ε subunit (miR-224 host gene). P53 and p65 bind with miR-224 host gene and inactivate the GABAA receptor ε subunit promoter in GC [14]. Ubc9 is an E2 conjugating enzyme that transfers the activated small ubiquitin-related modifier and may be a regulator of miRNA expression. The ectopic expression of Ubc9 induces downregulated expression of miR-224. It is not clear how Ubc9 regulates miR-224 expression. Available evidence suggests that Ubc9-mediated inhibition of miR-224 is more likely to occur at the transcriptional level [15]. Elucidating the mechanism of miR-224 regulation will contribute to offering valuable insights in evaluating the clinical significance of miR-224 as a potential biomarker for cancer (Fig. 1).
The expression of miR-224 is regulated by various important signaling pathways such as NF-κB signaling pathway and TGF-β signaling pathways. A lot of regulated factors participate in inducing miR-224 expression, including EP300, LPS, LTα, TNFα, E2F1, HIF1A, P53, p65, and Ubc9. It is not clear how Ubc9 and Ang II regulate miR-224 expression. Some regulated factors, like LPS, LTα, TNFα, P53, and p65 are involved in NF-κB inflammatory pathways. TGF-β1-induced miR-224 expression through regulating Smad2/3 and HIF1A
MiR-224 affects tumor biology
MiR-224 in tumorigenesis
Tumorigenesis is a multi-step process during which the normal cells attain genetic and epigenetic alterations, which can enhance the growth and survival of normal cells, and the transformed cells may eventually obtain some characteristics, for instance apoptosis-resistant, growth-independent, tissue-invasive, and/or metastatic [16]. Many recent studies demonstrate that aberrant plasma miRNAs are found in various types of cancers, and their expression are associated with tumorigenesis by affecting posttranscriptional gene expression [17, 18]. The dysregulated miR-224 is associated with tumorigenesis of various tumors. MiR-224 plays an important role in inflammatory bowel disease (IBD) cancer carcinogenesis through participating in cell cycle regulation. MiR-224 regulates the G1–S checkpoint through targeting and inhibiting a key cell cycle regulator P21 [19]. P21 as a regulator of cyclin-dependent kinases (CDKs) was reported to be a key protein in cell proliferation suppression and play a vital role in maintaining the integrity of the genome [20]. MiR-224 promotes HCC tumorigenesis through targeting and silencing Smad4 gene. Smad4 contains two miR-224-binding sites in the 3′untranslated region and acts as a tumor suppressor role [21]. It was also reported that miR-224 acts as an oncogenic role in the cellular processes by targeting Smad4 in CRC [22]. The ectopic expressions of miRNAs may act as oncogenes which are closely related to the cancer development, and different signatures of miRNA expression distinguish the cancer cells from the normal ones [23].
MiR-224 in cell proliferation and apoptosis
Cell proliferation and apoptosis are indispensable yet opposite cellular processes. The cross talk between these processes promotes a balance between the proliferation and apoptosis. Increasing studies have shown that miRNAs have important roles in cancer processes, including cell proliferation and apoptosis [24]. MiR-224 enhances the growth ability of CRC cells through accelerating the G1–S phase transition. MiR-224 decreases levels of PH domain leucine-rich-repeat protein phosphatase 1 (PHLPP1), and PHLPP2 may be the potential mechanism. PHLPP1 and PHLPP2 inhibit PI3K/AKT/FOXO3a signaling through dephosphorylating AKT. The activation of PI3K/AKT/FOXO3a signaling restrains expression of p21Cip1 and p27Kip1 and increases expression level of cyclin D1 which may promote proliferation of CRC cells [25]. MiR-224 inhibits the expression of PPP2R1B, which most likely results in the change of the AKT signaling pathway and increasing the cell abilities of proliferation and even migration and invasion in HCC [26]. Furthermore, miR-224 promotes TGF-β-induced cell proliferation by targeting Smad4. Smad4 as a mediator of TGF-β pathway participates in the inhibition of cell proliferation [12]. The upregulated miR-224 reduces the expression of TRIB1 via targeting the mRNA of TRIB1. Either upregulated miR-224 or downregulated TRIB1 gene expression could reduce the migration activities and increase the apoptotic rates of prostate cancer cells [27]. TRIB1 belongs to the tribbles family [28] and promotes aggressive growth of cancer cell probably by interacting with the receptor tyrosine kinase/mitogen-activated protein kinase (MAPK) pathway [29]. In gastric cancer, miR-224 regulates cell apoptosis through targeting Raf kinase inhibitor protein (RKIP). The downregulated RKIP increases cell viability and the S-phase fraction, concomitant with a reduction in cell apoptosis [30]. RKIP participates in several critical signal transduction pathways, such as the MAPK signaling pathway, and regulates some important biological characteristics, including cell division, growth, proliferation, cycle, and apoptosis [31]. It is well known that miRNAs can restrain the expression of targeted genes, but recent researches confirm that they can also increase the transcription and translation of various genes [32]. It was reported that the overexpression of miR-224-mediated upregulation of MAPK1 and B cell lymphoma 2 (BCL-2) which are crucial factors for the regulation of cell survival and cell proliferation. Sustained activation of MAPK1 and BCL-2 plays an important role in cell viability at the early stage of rVacA-induced apoptosis; thus, the overexpression of miR-224 can promote survival and proliferation of cells by increasing the expression of MAPK1 and BCL-2 [33]. The upregulated miR-224 promotes apoptotic cell death via targeting apoptosis inhibitor-5 (API-5) and suppressing the expression of API-5 gene [34]. API-5 is a protein with potent antiapoptotic signaling in tumor cells and acts as a suppressor of apoptosis mediated by E2 promoter-binding factors [35]. The downregulated miR-224 suppresses malignant meningioma cell growth and results in the enhancement of cell apoptosis through activation of the ERG2-BAK-induced apoptosis pathway [36]. These findings indicate that miR-224 plays an indispensable role in not only cell proliferation but also apoptosis of tumor cells. The dysregulated miR-224 in multiple cancers affects cell proliferation and apoptosis by targeting various genes and regulates the balance of interactions in cell apoptosis. The dynamic balance determines the final observed phenotype of the miR-224 function in tumor cells (Fig. 2).
MiR-224 regulates cell proliferation and apoptosis via regulating expressions of various target genes, such as PHLPP, PPP2R1B, Smad4, TRIB1, RKIP, BCL-2, ERG2, and API-5. By targeting various genes, miR-224 participates in multiple signaling pathway, including AKT singling pathway, TGF-β singling pathway as well as MAPK singling pathway, which can influence proliferation and apoptosis abilities of cancer cell
MiR-224 regulates cell invasion and migration
The invasion and migration of tumor cells are the major risk factors to cancer patients. The phenotype of cell invasion and migration can be modulated through regulating the associated signaling pathways and functional proteins. There is increasing evidence that miRNAs could play a vital role in regulating tumor progression and invasion [37]. CDC42, PAK2, and CDH1 are targets of miR-224, and their expression levels have an inverse correlation with miR-224 in HCC. CDC42 and CDH1 can suppress cell–cell adhesion, and the downregulation of either PAK2 or CDC42 causes spindle misorientation and results in daughter cells failing to retain connection to the substratum after cell division [34]. Upregulation of miR-224 inhibits cytoskeletal composition changes from global to filamentous-actin via targeting Cdc42 and restraining the cell motility and the formation of lamellipodia, thereby suppressing CRC cell migration [38]. Cdc42 belongs to Rho family GTPases and participates in Rho family GTPase signaling pathways which play an important role in regulating cellular proliferation, morphology, and transformation. PAKs are specific downstream effectors for Rho family GTPases and that they mediate the Rho family GTPase effects on the cell metastasis [39]. The upregulated miR-224 in HCC cells alters cell migration and invasion abilities by influencing PAK4 and MMP-9 expression [40]. PAK4 can regulate cell morphology and motility while MMP-9, an inhibitor of collagen IV and laminin-5, can assist metastatic cancerous cells to pass through the basement membrane [41, 42]. Further study confirmed that miR-224-induced upregulation of the tumor invasion-associated proteins p-PAK4 and MMP-9 by targeting HOXD10. MiR-224/HOXD10/p-PAK4/MMP-9 signaling may be a regulatory pathway in the regulation of cell migration and invasion [43]. TGF-β/Smad4 signaling was reported to be regualted by miR-224, and the inhibition of TGF-β/Smad4 signaling could enhance migration and invasive ability of CRC cell [44]. MiR-224 reduces expression level of methyl-CpG-binding domain protein 2 (MBD2) and negatively correlates with metastatic abilities of cancer. MBD2 restrains the expression of metastasis suppressor maspin through silencing the gene encoding maspin in metastatic cells [45]. Maspin participates in regulating PI3K and ERK1/2 activities concomitant with a decrease in cell motility and an increase in cell adhesion [46]. MiR-224 reduces migration and invasion abilities of PCa cell through targeting oncogenic TPD52 and silence TPD52 gene expression [47]. Previous study has indicated that TPD52 activates the protein kinase B (PKB)/Akt pathway via integrin and promotes prostate cancer cell migration [48]. MiR-224-induced cancer cell metastasis and invasion are directly mediated by RKIP suppression [30, 49]. In addition, RKIP is supposed to inhibit MAP kinase signaling in response to growth factors through binding to Raf-1 and preventing Raf-1-mediated phosphorylation of MEK/MAP kinase [50, 51] (Fig. 3).
MiR-224 significantly affects cancer cell invasion and metastasis by activating/inhibiting some important signaling pathway such as Rho family GTPase signaling pathways, HOXD10/p-PAK4/MMP-9 signaling pathway, TGF-β/Smad4 signaling pathway, Akt/PKB pathway, and MEK/MAPK signaling pathway. A series of miR-224 targets play an important role in the process of invasion and metastasis such as CDC42, PAKs, CDH1, MMP-9, HOXD10, Smad4, MBD2, TPD52, and RKIP
MiR-224 regulates tumor cell invasion and metastasis through suppressing its targets and, in turn, activating/inhibiting a pro-metastatic gene. Furthermore, ectopic expression of miR-224 influences expressions of its various target genes in cancer cell lines, highlighting a miR-224-mediated regulatory network potentially important for cell invasion and migration.
MiR-224 associates with hormone release
MiR-224 that targets type 1 iodothyronine deiodinase (DIO1) may account for tissue hypothyroidism in renal cancer. DIO1 as a target of miR-224 catalyzes the conversion of prohormone thyroxine to the active thyroid hormone 3,3′,5-triiodothyronine (T3). The overexpression of miR-224 negatively correlated with expressions of DIO1 and intracellular T3 in renal cancer. T3 is a regulator of key cellular processes, including differentiation, apoptosis proliferation, and metabolism [52]. Further studies suggest the regulatory feedback loop between T3 and miR-224. T3 actions are mediated by thyroid hormone receptors (TRs), which includes thyroid hormone receptor alpha (THRA) and THRB. The upregulated T3 increases expression of TRs and DIO1 and decreases expression of GABRE gene. It was also demonstrated that downregulated expression of THRA led to overexpression of miR-224 but did not change the level of GABRE gene. While, silencing of THRB increased the expression of miR-224 and GABRE gene [53]. It was reported that miR-224 expression is regulated by TGF-β1/Smads pathway. The overexpression of miR-224 not only enhances TGF-β1-induced GC proliferation but also promotes estradiol release through targeting Smad4. In addition, both TGF-1 and miR-224 can increase estradiol release from GC partly through increasing CYP19A1 (a key enzyme in E2 biosynthesis) mRNA levels. E2 increases cell proliferation and inhibits miR-21 expression in an estrogen receptor-dependent manner in MCF-7 cells [12] (Fig. 4).
The expression of DIO1 and TRs is stimulated by T3. Beside, miR-224 downregulated expression of DIO1 results in inhibition of T3 that is associated with increased miR-224 levels in a feedback loop. What’s more, miR-224 expression is regulated by TGF-β1/Smads pathway, and the upregulated miR-224 promotes estradiol release through targeting Smad4. CYP19A1 as a shared target of TGF-β1 and miR-224 can increase E2 release. T3 and E2 are important for cellar processes
Clinical significance of miR-224
MiR-224 affects cancer therapy
As a vital emerging modulator in cellular pathways, miR-224 plays an important role in cancer therapy. Targeting miR-224 may have a dramatic effect on therapeutic strategy for human cancers in the future. Chemoresistance is one of the main drawbacks and obstacles toward cancer therapy. The chemoresistance is largely attributed to genetic and epigenetic modifications as well as the ectopic expression of miRNAs. MiR-224 promotes cisplatin resistance of lung adenocarcinoma cells via regulating G1/S cell cycle transition and apoptosis. MiR-224 influences the G1/S transition of cell cycle and apoptosis in lung adenocarcinoma cells through the intrinsic mitochondrial death pathway and the p21WAF1/CIP1-pRb pathway [54]. MiR-224 relates to methotrexate (MTX) resistance via targeting CDS2, HSPC159, and SLC4A4 in colon cancer. MiR-224-induced downregulation of CDS2, HSPC159, and SLC4A4 leads to increasing MTX sensitivity of HT29 cells [55]. MiR-224 relates to the effect of celastrol on the inhibition of migration and invasion of HCC Cells. NF-κB signaling pathway is a direct transcriptional regulator of miR-224 expression and plays an important role in tumor cell metastasis. Both miR-224 and NF-κB signaling pathway can be regulated by celastrol. Celastrol can suppress the activity of P65/NF-κB through inhibiting phosphorylation of IkB and blocking P65 entry into the nucleus. Meanwhile, celastril inhibits the activity of PI3K/Akt signaling pathway by inhibiting phosphorylation of Akt which can decrease NF-κB activity. All these results suggest that celastrol suppresses the expression of miR-224 and inhibits the abilities of migration and invasion in HCC cells directly and indirectly. Furthermore, the study also shows that the downregulation of miR-224 expression by treatment with celastrol results in the reduction of MMP-2 and MMP-9 expressions, which are important components of cancer invasion, as well as the inhibition of migration and invasion [56]. Further studies on miR-224 show that miR-224 amplifies radiation sensitivity of glioblastoma (GBM) cells. The upregulated miR-224 is discovered to cause the reduction in clonogenic potential by itself as well as further increase the efficacy of radiotherapy on clonogenic potential. API-5 gene as a target of miR-224 involves in the reduction of clonogenic potential of GBM cells and mediating radiation sensitizing effect of miR-224 [57]. Furthermore, upregulation of miR-224 expression is found to be associated with a better responsiveness to sorafenib (a effective systemic molecular-targeted treatment drug) in HCC patients. Platelet-derived growth factor receptor (PDGFR) is a target of miR-224, and its expression correlates with therapeutic response to sorafenib [58]. In ovarian cancer, ectopic expression of miR-224-5p is related to platinum-based chemoresistance. Overexpression of miR-224-5p increases chemoresistance to cisplatin via apoptosis reversion at least in part by downregulating PRKCD in ovarian cancer cells [59]. In diffuse large B cell lymphoma (DLBCL) patients, the expression levels of miR-224 and its target CD59 can predict the response and outcome of patients treated with rituximab, cyclophosphamide, adriamycin, vincristine, and prednisone (R-CHOP) [60] (Table 1).
MiR-224 reflects tumor staging
Accumulating evidence suggest that dysregulated miR-224 is related to tumor staging in a broad range of cancers. Compared with normal tissues, the expression levels of miR-224 in cervical cancer tissues are much higher. Cervical cancer patients with lymph node metastasis-positive or vascular invasion or advanced International Federation of Gynecology and Obstetrics (FIGO) stage always have higher levels of miR-224 than those with lymph node metastasis-negative (P = 0.008) or without vascular invasion patients (P = 0.01) or early FIGO stage (P = 0.02) [61]. According to a study, the expression levels of miR-224 are significantly higher in the Barcelona Clinic Liver Cancer (BCLC) stage C patients than those with stage B patients (P = 0.005) in HCC. In addition, patients grouped by the status of portal vein tumor thrombus (PVTT) are associated with significant different levels of miR-224 expression [62]. In PCa patients, downregulated miR-224 is related to PCa metastasis, high PSA level as well as high Gleason scores and thus associated with advanced clinical stage [20, 63]. Compared to the corresponding noncancerous lung tissues, the expression levels of miR-224 are much lower in nonsmall-cell lung cancer (NSCLC). In NSCLC patients, downregulated miR-224 is associated with advanced TNM stage (P < 0.001) and lymph node metastasis (P = 0.002) [64]. MiR-224 has some links with tumor staging of CRC, and its expression level is relatively low in patients with an early T classification (T1 and T2) and dramaticly increased in T3 tumors and further upregulated in T4 tumors [25]. These findings indicate that miR-224 may act as a special marker of tumor stage.
MicroRNA-224 as a biomarker for cancer prognosis
MiR-224 as a regulator of mRNAs influences recurrence, and outcome of cancer patients suggests that miR-224 may be a biomarker for cancer prognosis. Upregulated miR-224 represses cellar proliferation, invasion, migration as well as increases cell apoptosis through inhibiting TRIB1 expression in PCa patients. Moreover, either downregulated miR-224 or upregulated TRIB1 gene is related to poor prognosis [27]. HCC patients with low levels of miR-224 were reported to be insensitive to sorafenib and lead to poor outcomes; thus, miR-224 may be an independent prognostic factor for HCC patients treated with sorafenib [58]. Downregulated miR-224 was reported to be associated with insensitivity of radiotherapy in GBM patients. Male GBM patients with lower levels of miR-224 have worse overall survival and shorter median survival duration when compared to those with higher levels of miR-224. API5 as a target of miR-224 is found to have a negative relation with median survival duration of GBM patients. MiR-224 may be a biomarker for GBM prognosis because of its regulated effect on radiation sensitivity through targeting API5 [57]. Lu et al. research showed that in human GBM tissue, the level of miR-224 is higher when compared with nontumor brain tissue. Upregulated miR-224 is related to aggressive clinicopathological features as well as advanced GBM progression concomitant with poorer DFS and OS [65]. Upregulated miR-224 was reported to be associated with poorer overall survival through repressing expression of Smad4 gene in HCC patients [7]. Cervical cancer patients with high levels of miR-224 tend to have more aggressive progression and poorer prognosis than those with lower levels of miR-224 [61]. In CRC patients, miR-224 may be an independent prognostic factor for outcome because of CRC patients with downregulated miR-224 exhibit better prognosis than those with high levels of miR-224 [25].
Prospects
Increasing studies suggest functional links between miR-224 and the expression of its targets in various tumors cells. Ectopic expression of miR-224 may be related with process and prognosis of tumor. The relation between abnormal levels of miR-224 and cancer development indicates that miR-224 is a potential biomarker for molecular targeted therapy. Acting as a promising biomarker for tumors, miR-224 may therefore be a potential anticancer and a therapeutic target. In the future research, clinic studies are needed to evaluate the potential of miR-224 as a prognostic marker and a therapeutic target in tumors. Ideally, miR-224 could be a therapeutic tool, due to its ability to target various genes. However, when anti-miR-224 “drugs” translated into clinical applications would encounter several problems. It is well-known that it is difficult to identify the downstream targets of miR-224. The complication is that miR-224 could target multiple genes while various miRNAs could target the same gene; hence, detailed studies on the multiple binding sites of miR-224 and the clear mechanisms of which miR-224 participating in cell biology in tumors are indispensable. Moreover, it is essential to realize that the importance of miRNA co-regulation network act together on various signal pathways which might help in designing a more specific therapeutic target.
Conclusion
In this review, we focus on the role of miR-224 as a regulator during cancer progresses. MiR-224 is dysregulated expression in various cancers, including HCC, PCa, CRC, GBM, BCa, cervical cancer, ovarian cancer, and lung adenocarcinoma. MiR-224 participates in tumor process through regulating multiple target genes and influencing the complicated signaling pathways. In this review, these regulatory pathways are only the part of this complex regulated network, and more future studies are needed to focus on exploring mechanism of regulated effect. With a better understanding of miR-224 and its target genes, we may explore a better target for cancer therapy.
References
Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3(3):83–92.
Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013;14(8):475–88.
Berindan-Neagoe I, Monroig PC, Pasculli B, Calin GA. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64(5):311–36.
Chen WX, Hu Q, Qiu MT, Zhong SL, Xu JJ, Tang JH, et al. miR-221/222: promising biomarkers for breast cancer. Tumour Biol. 2013;34(3):1361–703.
Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. microRNAs in cancer management. Lancet Oncol. 2012;13(6):249–58.
Wang Z, Li Y, Kong D, Ahmad A, Banerjee S, Sarkar FH. Cross-talk between mirna and notch signaling pathways in tumor development and progression. Cancer Lett. 2010;292(2):141–8.
Wang Y, Ren JW, Gao Y, Ma JZ, Toh HC, Chow P, et al. MicroRNA-224 targets SMAD family member 4 to promote cell proliferation and negatively influence patient survival. PLoS One. 2013;8(7):e68744.
Wang Y, Toh HC, Chow P, Chung AY, Meyers DJ, Cole PA, et al. MicroRNA-224 is up-regulated in hepatocellular carcinoma through epigenetic mechanisms. FASEB J. 2012;26(7):3032–41.
Scisciani C, Vossio S, Guerrieri F, Schinzari V, De Iaco R, D’Onorio de Meo P, et al. Transcriptional regulation of miR-224 upregulated in human HCCs by NFkB inflammatory pathways. J Hepatol. 2012;56(4):855–61.
Knoll S, Fürst K, Kowtharapu B, Schmitz U, Marquardt S, Wolkenhauer O, et al. E2F1 induces miR-224/452 expression to drive EMT through TXNIP downregulation. EMBO Rep. 2014;15(12):1315–29.
Ning QL, Jiang XY. Angiotensin II upregulated the expression of microRNA-224 but not microRNA-21 in adult rat cardiac fibroblasts. Biomed Rep. 2013;1(5):776–80.
Yao GD, Yin MM, Jie L, Tian H, Liu L, Li X, Sun F. MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol. 2010;24(3):540–51.
Hwang HW, Baxter LL, Loftus SK, Cronin JC, Trivedi NS, Borate B, Pavan WJ. Distinct microRNA expression signatures are associated with melanoma subtypes and are regulated by HIF1A. Pigment Cell Melanoma Res. 2014;27(5):777–87.
Liang M, Yao GD, Yin MM, Lu M, Tian H, Liu L, et al. Transcriptional cooperation between p53 and NF-κB p65 regulates microRNA-224 transcription in mouse ovarian granulosa cells. Mol Cell Endocrinol. 2013;370(1–2):119–29.
Zhu S, Sachdeva M, Wu F, Lu Z, Mo YY. Ubc9 promotes breast cell invasion and metastasis in a sumoylation-independent manner. Oncogene. 2010;29(12):1763–72.
Yang W, Lee DY, Ben-David Y. The roles of microRNAs in tumorigenesis and angiogenesis. Int J Physiol Pathophysiol Pharmacol. 2011;3(2):140–55.
Zhang WY, Dahlberg JE, Tam W. MicroRNAs in tumorigenesis: a primer. Am J Pathol. 2007;171(3):728–38.
Sumbul AT, Gogebakan B, Ergun S, Yengil E, Batmaci CY, Tonyali O, Yaldiz M. miR-204-5p expression in colorectal cancer: an autophagy-associated gene. Tumour Biol. 2014;35(12):12713–9.
Olaru AV, Yamanaka S, Vazquez C, Mori Y, Cheng Y, Abraham JM, et al. MicroRNA-224 negatively regulates p21 expression during late neoplastic progression in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(3):471–80.
Waga S, Li R, Stillman B. p53-induced p21 controls DNA replication. Leukemia. 1997;11(3):321–3.
Lan SH, Wu SY, Zuchini R, Lin XZ, Su IJ, Tsai TF, et al. Autophagy suppresses tumorigenesis of hepatitis B virus-associated hepatocellular carcinoma through degradation of microRNA-224. Hepatology. 2014;59(2):505–17.
Zhang GJ, Zhou H, Xiao HX, Li Y, Zhou T. Up-regulation of miR-224 promotes cancer cell proliferation and invasion and predicts relapse of colorectal cancer. Cancer Cell Int. 2013;13(1):104.
Skaftnesmo KO, Prestegarden L, Micklem DR, Lorens JB. MicroRNAs in tumorigenesis. Curr Pharm Biotechnol. 2007;8(6):320–5.
Wu XJ, Pu XM, Zhao ZF, Zhao YN, Kang XJ, Wu WD, et al. The expression profiles of microRNAs in Kaposi’s sarcoma. Tumour Boil. 2015;36(1):437–46.
Liao WT, Li TT, Wang ZG, Wang SY, He MR, Ye YP, et al. MicroRNA-224 promotes cell proliferation and tumor growth in human colorectal cancer by repressing PHLPP1 and PHLPP2. Clin Cancer Res. 2013;19(17):4662–72.
Ma DL, Tao XC, Gao F, Fan C, Wu D. miR-224 functions as an onco-miRNA in hepatocellular carcinoma cells by activating AKT signaling. Oncol Lett. 2012;4(3):483–8.
Lin ZY, Huang YQ, Zhang YQ, Han ZD, He HC, Ling XH, et al. MicroRNA-224 inhibits progression of human prostate cancer by downregulating TRIB1. Int J Cancer. 2014;135(3):541–50.
Seher TC, Leptin M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr Biol. 2000;10(11):623–9.
Hegedus Z, Czibula A, Kiss-Toth E. Tribbles: a family of kinase-like proteins with potent signaling regulatory function. Cell Signal. 2007;19(2):238–50.
Liu H, Li P, Li B, Sun P, Zhang J, Wang B, Jia B. RKIP suppresses gastric cancer cell proliferation and invasion and enhances apoptosis regulated by microRNA-224. Tumour Biol. 2014;35(10):10095–103.
Al-Mulla F, Bitar MS, Taqi Z, Yeung KC. RKIP: much more than Raf kinase inhibitory protein. J Cell Physiol. 2013;228(8):1688–702.
Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–98.
Zhang YZ, Takahashi S, Tasaka A, Yoshima T, Ochi H, Chayama K. Involvement of microRNA-224 in cell proliferation, migration, invasion, and anti-apoptosis in hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28(3):565–75.
Wang Y, Lee AT, Ma JZ, Wang J, Ren J, Yang Y, et al. Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J Biol Chem. 2008;283(19):13205–15.
Koci L, Chlebova K, Hyzdalova M, Hofmanova J, Jira M, Kysela P, et al. Apoptosis inhibitor 5 (API-5; AAC-11; FIF) is upregulated in human carcinomas in vivo. Oncol Lett. 2012;3(4):913–6.
Wang M, Deng X, Ying Q, Jin T, Li M, Liang C. MicroRNA-224 targets ERG2 and contributes to malignant progressions of meningioma. Biochem Biophys Res Commun. 2015;460(2):354–61.
Chen W, Cai F, Zhang B, Barekati Z, Zhong XY. The level of circulating mirna-10b and mirna-373 in detecting lymph node metastasis of breast cancer: potential biomarkers. Tumour Biol. 2013;34(1):455–62.
Ke TW, Hsu HL, Wu YH, Chen WT, Cheng YW, Cheng CW. MicroRNA-224 suppresses colorectal cancer cell migration by targeting Cdc42. Dis Markers. 2014;2014:617150.
Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, et al. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem. 2002;277(1):550–8.
Li Q, Wang G, Shan JL, Yang ZX, Wang HZ, Feng J, et al. MicroRNA-224 is upregulated in HepG2 cells and involved in cellular migration and invasion. J Gastroenterol Hepatol. 2010;25(1):164–71.
Zhang HJ, Siu MK, Yeung MC, Jiang LL, Mak VC, Ngan HY, et al. Overexpressed PAK4 promotes proliferation, migration and invasion of choriocarcinoma. Carcinogenesis. 2011;32(5):765–71.
Kaneyoshi T, Nakatsukasa H, Higashi T, Fujiwara K, Naito I, Nouso K, et al. Actual invasive potential of human hepatocellular carcinoma revealed by in situ gelatin zymography. Clin Cancer Res. 2001;7(12):4027–32.
Li Q, Ding CC, Chen C, Zhang Z, Xiao H, Xie F, et al. miR-224 promotion of cell migration and invasion by targeting homeobox D 10 gene in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(4):835–42.
Xu XT, Xu Q, Tong JL, Zhu MM, Nie F, Chen X, et al. MicroRNA expression profiling identifies miR-328 regulates cancer stem cell-like SP cells in colorectal cancer. Br J Cancer. 2012;106(7):1320–30.
Yuan KF, Xie K, Fox J, Zeng H, Gao H, Huang C, Wu M. Decreased levels of miR-224 and the passenger strand of miR-221 increase MBD2, suppressing maspin and promoting colorectal tumor growth and metastasis in mice. Gastroenterology. 2013;145(4):853–64.
Bailey CM, Khalkhali-Ellis Z, Seftor EA, Hendrix MJ. Biological functions of maspin. J Cell Physiol. 2006;209(3):617–24.
Goto Y, Nishikawa R, Kojima S, Chiyomaru T, Enokida H, Inoguchi S, et al. Tumour-suppressive microRNA-224 inhibits cancer cell migration and invasion via targeting oncogenic TPD52 in prostate cancer. FEBS Lett. 2014;588(10):1973–82.
Ummanni R, Teller S, Junker H, Zimmermann U, Venz S, Scharf C, Giebel J, et al. Altered expression of tumor protein D52 regulates apoptosis and migration of prostate cancer cells. FEBS J. 2008;275:5703–13.
Huang L, Ting D, Lin X, Zimmermann U, Venz S, Scharf C, et al. MicroRNA-224 targets RKIP to control cell invasion and expression of metastasis genes in human breast cancer cells. Biochem Biophys Res Commun. 2012;425(2):127–33.
Keller ET, Fu Z, Brennan M. The role of Raf kinase inhibitor protein (RKIP)In health and disease. Biochem Pharmacol. 2004;68:1049–53.
Trakul N, Rosner MR. Modulation of the MAP kinase signaling cascade by Raf kinase inhibitory protein. Cell Res. 2005;15:19–23.
Boguslawska J, Wojcicka A, Piekielko-Witkowska A, Master A, Nauman A. MiR-224 targets the 3′UTR of type 1 5′-iodothyronine deiodinase possibly contributing to tissue hypothyroidism in renal cancer. PLoS One. 2011;6(9):e24541.
Boguslawska J, Piekielko-Witkowska A, Wojcicka A, Kedzierska H, Poplawski P, Nauman A. Regulatory feedback loop between T3 and microRNAs in renal cancer. Mol Cell Endocrinol. 2014;384(1–2):61–70.
Wang H, Zhu LJ, Yang YC, Wang ZX, Wang R. MiR-224 promotes the chemoresistance of human lung adenocarcinoma cells to cisplatin via regulating G1/S transition and apoptosis by targeting p21 (WAF1/CIP1). Br J Cancer. 2014;111(2):339–54.
Mencia N, Selga E, Noe V, Ciudad CJ. Underexpression of miR-224 in methotrexate resistant human colon cancer cells. Biochem Pharmacol. 2011;82(11):1572–82.
Li HY, Li Y, Liu D, Sun H, Liu J. miR-224 is critical for celastrol-induced inhibition of migration and invasion of hepatocellular carcinoma cells. Cell Physiol Biochem. 2013;32(2):448–58.
Upraity S, Shaikh S, Padul V, Shirsat NV. MiR-224 expression increases radiation sensitivity of glioblastoma cells. Biochem Biophys Res Commun. 2014;448(2):225–30.
Gyöngyösi B, Végh E, Járay B, Szekely E, Fassan M, Bodoky G, et al. Pretreatment microRNA level and outcome in sorafenib-treated hepatocellular carcinoma. J Histochem Cytochem. 2014;62(8):547–55.
Zhao H, Bi T, Qu Z, Jiang J, Cui S, Wang Y. Expression of miR-224-5p is associated with the original cisplatin resistance of ovarian papillary serous carcinoma. Oncol Rep. 2014;32(3):1003–12.
Song GQ, Song GR, Ni HY, Gu L, Liu H, Chen B, et al. Deregulated expression of miR-224 and its target gene: CD59 predicts outcome of diffuse large B-cell lymphoma patients treated with R-CHOP. Curr Cancer Drug Targets. 2014;14(7):659–70.
Shen SN, Wang LF, Jia YF, Hao YQ, Zhang L, Wang H. Upregulation of microrna-224 is associated with aggressive progression and poor prognosis in human cervical cancer. Diagn Pathol. 2013;8:69.
Zhuang LP, Meng ZQ. Serum miR-224 reflects stage of hepatocellular carcinoma and predicts survival. Biomed Res Int. 2015;2015:731781.
Fu H, He HC, Han ZD, Wan YP, Luo HW, Huang YQ, et al. MicroRNA-224 and its target CAMKK2 synergistically influence tumor progression and patient prognosis in prostate cancer. Tumour Biol. 2014;36(3):1983–91.
Zhu D, Chen H, Yang XG, Chen W, Wang L, Xu J, Yu L. Decreased microRNA-224 and its clinical significance in non-small cell lung cancer patients. Diagn Pathol. 2014;9(1):198.
Lu S, Wang S, Geng S, Ma S, Liang Z, Jiao B. Upregulation of microRNA-224 confers a poor prognosis in glioma patients. Clin Transl Oncol. 2013;15(7):569–74.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81272470).
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Fan, Xm., Mao, L. et al. MicroRNA-224: as a potential target for miR-based therapy of cancer. Tumor Biol. 36, 6645–6652 (2015). https://doi.org/10.1007/s13277-015-3883-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3883-3