Abstract
Treatment failure in cancer chemotherapy is largely due to the toxic effects of chemotherapeutic agents on normal cells/tissues. The proteasome inhibitor bortezomib has been successfully applied to treat multiple myeloma (MM), but there are some common adverse reactions in the clinic including peripheral neuropathy (PN). The TAK1 selective inhibitor 5Z-7-oxozeaenol has been widely studied in cancer therapy. Here, we investigated the potential synergy of bortezomib and 5Z-7-oxozeaenol in Burkitt’s lymphoma (BL) cell lines. Cell viability assay showed that co-treatment of bortezomib at 8 nM, representing a one-eighth concentration for growth arrest, and 5Z-7-oxozeaenol at 2 μM, a dose that exhibited insignificant cytotoxic effects, synergistically induced apoptosis in the cell line Daudi. In parallel with the increasing dose of the bortezomib, and 5Z-7-oxozeaenol at 0.5 μM, lower colony formation efficiencies were seen in the cell line Daudi. Western blotting analysis verified that TAK1 inhibition by 5Z-7-oxozeaenol completely blocked JNK, p38, Erk, IKK, and IκB phosphorylation, which was almost instantly activated by TAK1 both directly or indirectly. Both agents synergistically prevented nuclear translocation of NF-κB, a characteristic of NF-κB inactivation. Moreover, a synergistic effect of bortezomib and 5Z-7-oxozeaenol on Western blotting analysis and flow cytometry was disclosed. Collectively, our results indicated that the proteasome inhibitor bortezomib and the TAK1 inhibitor 5Z-7-oxozeaenol displayed synergy on inhibiting BL cell apoptosis by inhibiting NF-κB activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Burkitt’s lymphoma (BL) is a highly aggressive non-Hodgkin’s B-cell lymphoma, and is the fastest growing human tumor with a doubling time of 24–48 h. BL represents an initially discovered virus-related cancer, which is related to chromosomal translocation. Therefore, the classification and clinical diagnosis of BL has received much attention. According to the geographical distribution and clinical manifestation, the World Health Organization (WHO) classifies BL into different types, including localized, dispersible, and immunodeficient-associated types [1]. Genetic studies on BL showed that BL cells exhibited chromosomal translocation that was induced by c-myc gene activation. The related chromosomal translocation included c-myc/IgH (present in patients with [t (8; 14)], about 80 %), c-myc/IgL (κ or λ light chains, present in patients with [t (2; 8) or t (8; 22)], about 20 %) [2]. Additional study showed that NF-κB is related to the overexpression of translocated c-myc. In addition, NF-κB can combine with the identification fragment of the Ig heavy chain regions and increase the expression of translocated c-myc, an observation which suggests that NF-κB is a potential therapeutic target for BL [3].
It is well known that the nuclear transcription factor NF-κB and the activation of its signal transduction pathway represent a key regulatory role in innate and acquired immune responses. Moreover, the NF-κB signaling pathway not only exhibits an essential role in tumorigenesis and tumor progression, but also functions as a key factor linking inflammation and cancer cells [4, 5]. Furthermore, NF-κB is the main factor underlying the mechanism of tumor cells escaping immune surveillance during tumorigenesis and progression, and it regulates tumor angiogenesis and cancer cell invasion of other tissues. Therefore, the investigation of this pathway and its associated factors may provide new cancer therapeutic target [6–8].
TAK1 (TGF-β-activated kinase 1 or MAP3K7) is a member of the MAP3K family, and is a central factor of intracellular NF-κB and MAPK signaling pathways. It plays a key role in the processes of development, cell survival, inflammation, metabolism, and tumorigenesis [9]. TAK1 is upstream of NF-κB, where it is a key intermediary that activates IKK during the stress response, and plays an important role in innate and acquired immune responses [10, 11].
Bortezomib is an inhibitor of the 26S proteasome, and IκB can be degraded by the 26S proteasome. Bortezomib is the first proteasome drug that was approved the U.S. FDA and the European Medicines Agency (EMA), which is mainly used in the treatment of newly diagnosed multiple myeloma, relapsed or refractory multiple myeloma, and mantle cell lymphoma [12]. Pre-clinical studies showed that the underlying mechanism of the anti-tumor effect of bortezomib included enhanced expression of pro-apoptotic proteins (e.g., Noxa and IκBα), inhibition of NF-κB, and suppresses the inhibition of a series of anti-apoptotic proteins (e.g., Bcl-XL and Bcl-2). Among them, degradation of IκB is the most important mechanism for the anti-tumor effects of bortezomib [12, 13].
Based on these finding, we hypothesized that inhibition of TAK1 activity can inhibit IKK and IκB, and together with bortezomib therapy, can then suppress NF-κB activity, thus enhancing the killing effect of bortezomib on cancer cells. In the present study, we used BL cell lines and found that the TAK1 inhibitor 5Z-7-oxozeaenol and the proteasome inhibitor bortezomib have synergistic effects on inhibiting NF-κB activity and apoptosis in Daudi cells in vitro. This study not only provides the basis for an in vivo study, but also broadens the potential application and clinical scope of bortezomib.
Materials and methods
Cell lines and cell culture
The primary B-cell lines of rat BaF-3 and the human BL cell lines Daudi, NAMALWA, and Raji were obtained from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Daudi, Raji, and NAMALWA cells were cultured in RPMI-1640, which was supplemented with 10 % FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C in a fully humidified atmosphere of 5 % CO2 in air. BaF-3 cells were cultured in RPMI-1640, which was supplemented with 10 % FBS, penicillin (100 U/mL), IL-3 (1 μg/mL), and streptomycin (100 μg/mL) at 37 °C in a fully humidified atmosphere of 5 % CO2 in air.
Antibodies and reagents
Anti-phospho-IKKβ (2078S), anti-IKKβ (2684S), anti-phospho-IкBα (9246S), anti-IкBα (9242S), anti-phospho-JNK (9251L), anti-JNK (9252L), anti-phospho-p38 (9211L), anti-p38 (9212L), anti-NF-κB (4764S), anti-caspase3 (9662S), anti-PARP (9532S), anti-β-actin (4967S), anti-mouse (7076S), and anti-rabbit (7074S) secondary antibodies were purchased from Cell Signaling Technology. Bortezomib was obtained from Xian-Janssen Pharmaceuticals, Ltd, and 5Z-7-oxozeaenol (499610) was purchased from Calbiochem.
Cell viability assay
Briefly, cell lines were seeded into 96-well plates at a density of 1 × 104 cells per well. After incubating the plate for 24 h at 37 °C, the cells were treated with various concentrations of bortezomib, 5Z-7-oxozeaenol or their combination, for a duration as indicated in the text. Relative cell viability was quantified by adding 10 μL of Cell Counting Kit-8 (Dojindo Laboratories) solution, and then incubating the plate for 2 h at 37 °C, and measuring the absorbance at a wavelength of 450 nm.
Soft agar assay
Soft agar colony formation assay was done in 6-well plates. Each well contained 1.2 mL of 0.6 % agar (Sigma, St. Louis, MO, USA) in complete medium in the bottom layer, and 1 mL of 0.35 % agar in complete medium with cells at a density of 1 × 103 cells per well in the middle layer. The culture system was then overlaid with 0.5 mL of complete culture medium. The cells were cultured at 37 °C with 5 % atmospheric CO2 for 2 to 3 weeks. After overnight staining with tetrazolium dye 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT reagent; Sigma) at 200 μL per well at 5 mg/mL. The colonies were then photographed and colonies counted.
Western blotting assay
Cell lysates were obtained by washing the cells twice with ice-cold PBS and then lysing the cells with RIPA lysis buffer. After centrifuging at 13,000×g for 15 min at 4 °C, supernatants were collected, resolved by SDS polyacrylamide gel electrophoresis (PAGE) and transferred to PVDF membranes. The membranes were blocked with 5 % non-fat dry milk in TBS containing 0.1 % Tween 20 for 1 h at room temperature and then incubated with corresponding primary antibodies against corresponding proteins overnight at 4 °C. Blots were washed three times in TBS-Tween 20 buffer, followed by incubation with the appropriate horseradish peroxidase-linked secondary antibodies against mouse or rabbit for 1 h at room temperature. The membranes were then visualized by the ECL-Plus Western detection system (GE Health Care, Buckinghamshire, UK).
Flow cytometry
Apoptosis was determined by the Annexin-V FITC Apoptosis Detection Kit, which was performed according to the manufacturer’s instructions (BD Pharmingen). Data were acquired on a FACSCalibur flow cytometer (BD Biosciences). Results were obtained by analyzing data with FlowJo Version 7.6.1 software (TreeStar). Results represent the mean value of three independent experiments.
Statistical analysis
All the experiments were performed at least three times. Statistical analyses were performed using the Student’s t test (two-tailed). All values are expressed as the mean ± SD, and SPSS software (version 20.0) was used for data analysis. A P value of less than 0.05 was considered statistically significant.
Results
TAK1 inhibition significantly enhances the cytotoxic effect of bortezomib on BL cells
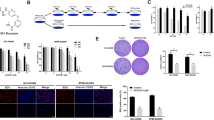
We hypothesized that the combination of 5Z-7-oxozeaenol with bortezomib should induce greater apoptosis. To test this hypothesis, we examined the cytotoxic effects of bortezomib and 5Z-7-oxozeaenol on BL cell lines (Fig. 1a–c, Supplementary Figs. S1 and S2). Multiple BL cell lines, including Daudi, NAMALWA, and Raji, were treated with bortezomib, 5Z-7-oxozeaenol, or their combination for up to 96 h, and analyzed by CCK-8 assay. All the BL cell lines were sensitive to bortezomib (Fig. 1b, Figs. S1 and S2). However, only Daudi cells exhibited a dose-dependent increase in proliferation of 5Z-7-oxozeaenol (Fig. 1a). TAK1 inhibition alone had little cytotoxic effect at a relatively high dose (10 μM) and did not enhance the cytotoxic effect of bortezomib on two other BL cell lines, i.e., NAMALWA and Raji (see Fig. S2). Compared with individual treatment with bortezomib, the BTZ/5Z-7-oxozeaenol combination induced an synergistic action of the cytotoxic effect (***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05, Fig. 1b). According to TC Chou’s study, we calculate the CI equals 0.88 and we conclude that BTZ and 5Z-7-oxozeaenol have a mild synergistic effect [14]. The cell morphology captured by light microscopy indicated that the maximal effect was achieved with the combination of drugs, as compared with that of individual (Fig. 1c).
5Z-7-oxozeaenol (5Z-7) enhances the cytotoxic effect of bortezomib (BTZ) on Burkitt lymphoma cell Daudi. a, b Daudi cells were seeded into 96-well plates at a concentration of 1.5 × 104 per well. After 16 h, cells were incubated with drugs for 24 h at indicated concentrations and cell viability was assessed by CCK-8 assay. Results presented as % vehicle ± SD (n = 5). ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05 (Student’s t test, two-tailed). c Daudi cells were seeded into 12-well plates at a concentration of 2 × 105 per well. After 16 h, cells were incubated with drugs for 24 h at indicated concentrations and cell morphology was captured by light microscopy. Arrows indicate the dead cells
TAK1 inhibition enhances the inhibitory effect of bortezomib on BL colony formation
A similar effect on cell proliferation was observed during a soft agar assay. The significant decrease in colony formation viability achieved with the combination of 5Z-7-oxozeaenol with bortezomib, as compared with that of bortezomib, despite the use of a low dose of 5Z-7-oxozeaenol (0.5 μM) in the experiment (***P ≤ 0.001, **P ≤ 0.01, Fig. 2). However, 5Z-7-oxozeaenol alone had no significant effect on cell proliferation in culture, as compared with the blank control (Fig. 2). The results of colony formation suggest a synergistic effect of these two drugs on the clonogenicity of cells.
5Z-7-oxozeaenol enhances the inhibitory effect of bortezomib on colony formation in the soft agar assay. Daudi were plated in 0.3 % agarose/RPMI-1640 media containing the indicated drugs with indicated concentration on top of a 0.5 % agarose/RPMI-1640 layer. After 3 weeks, colonies were stained with MTT and counted. The data shown are representative of values from at least three independent experiments with similar results and reported as mean with SDs. ***P < 0.001 (Student’s t test, two-tailed)
TAK1 inhibition enhances the inhibitory effect of bortezomib on NF-κB translocation to the nucleus
TAK1 is the main upstream repressor of NF-κB, JNK, p38, and Erk. Since NF-κB activation is critical for cell survival, we postulated that the combinatorial effect of 5Z-7-oxozeaenol with bortezomib might be caused by inhibition of NF-κB activation. To test this idea, we validated selected targets, including P38, P-P38, ERK, P-ERK, JNK, P-JNK, IKK, P-IKK, IκB, and P-IκB by Western blotting after cultivation of Daudi cell lines in the presence or absence of bortezomib, 5Z-7-oxozeaenol, or both in combination. Immunoblotting analyses show that P-P38, P-ERK, and P-JNK expressions are decreased in the Daudi cells treated with 5Z-7-oxozeaenol or the BTZ/5Z-7-oxozeaenol combination, and the phenomenon had not been observed in the individual treatment with bortezomib group. Compared with individual treatment with 5Z-7-oxozeaenol, the BTZ/5Z-7-oxozeaenol combination results in a higher expression. The results show that P-IKK, P-IκB expressions are increased in the Daudi cells treated with bortezomib, as compared with blank. However, the P-IKK and P-IκB expressions are decreased treated with 5Z-7-oxozeaenol or the BTZ/5Z-7-oxozeaenol combination (Fig. 3a). Compared to the blank controls, the expression of NF-κB in nucleus were downregulated in Daudi cells stimulated in the Daudi cells by bortezomib, 5Z-7-oxozeaenol, or the BTZ/5Z-7-oxozeaenol combination, and the maximal effect achieved with the combination of two drugs. Furthermore, the expressions of histone H3 in nucleus are on the contrary (Fig. 3b). These data suggest that there is a synergistic action in the process of the TAK1 inhibitor 5Z-7-oxozeaenol and bortezomib in the prevention of the nuclear translocation of NF-κB in Daudi cells.
5Z-7-oxozeaenol and bortezomib synergistically inhibits NF-κB from entering the nucleus. a 5Z-7-oxozeaenol inhibits JNK, Erk, P38, IKK, and IκB activation. Daudi cells were treated with bortezomib at the indicated time points (15, 30, 60, 120 min) with or without 5Z-7-oxozeaenol, total protein extracts were subjected to SDS-PAGE and immunoblotted with antibodies indicated. β-actin was detected as a loading control for whole cell extracts. b NF-κB p65 gene expression in the cytoplasm and nucleus of Daudi. Cells were treated with bortezomib at the concentration of 16 nM for 1 h with or without 5Z-7-oxozeaenol. Proteins were extracted from the cytoplasm and nucleus and subjected to SDS-PAGE and immunoblotted with antibodies indicated, with β-tubulin and histone 3, respectively, as the internal standards
TAK1 inhibition enhances bortezomib-induced apoptosis
As NF-κB activation upregulates anti-apoptotic molecules that inhibit cell death, we considered that 5Z-7-oxozeaenol and bortezomib might promote apoptosis by blocking NF-κB activation. To explore this, we examined cleavage of PARP, which is the key splicing substrate of core members during apoptosis by caspases. Combinatorial treatment with 5Z-7-oxozeaenol and bortezomib significantly increased the cleavage of PARP in cells compared to bortezomib treatment alone. We also found that the Daudi cell lines exhibited a time-dependent decrease in expressions of cleaved PARP treated by the BTZ/5Z-7-oxozeaenol combination (Fig. 4a). Concordant with these results, dual staining with Annexin-V and propidium iodide (PI) coupled with FACS analysis also showed that late stage apoptotic cells were increased after treatment with 5Z-7-oxozeaenol and bortezomib as compared to bortezomib treatment alone. After 48 h of incubation, bortezomib-induced apoptosis in 8.66 % of cells, and induction of apoptosis increased to 44.2 % when cells were simultaneously incubated with 5Z-7-oxozeaenol and bortezomib (Fig. 4b).
5Z-7-oxozeaenol enhances bortezomib-induced Daudi apoptosis. a Daudi cells were treated with bortezomib at the indicated time points (0, 8, 16, 24 h) with or without 5Z-7-oxozeaenol, total protein extracts were subjected to SDS-PAGE and immunoblotted with antibodies against cleaved PARP to detect the apoptotic cells. β-actin was detected as a loading control for whole cell extracts. b Daudi cells were treated with bortezomib (8 nM) and 5Z-7-oxozeaenol 2 μM) or their combination for 24 h and examined by flow cytometry using Annexin-V/PI staining to label apoptotic cells. c Working model of pharmacological synergistic action of 5Z-7-oxozeaenol and bortezomib in Burkitt lymphoma cell Daudi. TAK1 inbibitor and proteasome inhibitor synergistically inhibit NF-κB from entering the nucleus and inducing cell death
Discussion
BL is a biologically and clinically heterogeneous disease. TAK1 was activated in lymphoma cells, following which it promoted cell proliferation through the NF-κB pathway [15]. Bortezomib inhibited lymphoma proliferation by preventing NF-κB from entering the nucleus, but there are many side effects of bortezomib during its clinical application. We reasoned that pharmacological inhibition of TAK1 activity by blocking the NF-κB pathway and reducing the side effects of bortezomib. In the present study, we used three BL cell lines, including Raji, NAMALWA, and Daudi, which were derived from a local type of BL. The results showed that all cell lines were sensitive to the proteasome inhibitor bortezomib, which is an NF-κB targeting drug (Fig. 1a–c; Figs. S1 and S2). However, only Daudi cells were sensitive to the TAK1 inhibitor 5Z-7-oxozeaenol. Moreover, during combination therapy, 5Z-7-oxozeaenol enhanced the killing capacity of bortezomib against Daudi cells only. Since the Daudi cell lines multiply rapidly, we found that the biggest difference in the phenotype occurred in the Daudi cells treated by drugs after 48 h (Figs. 1b and 2; Fig. S1a). We found that it induces apoptosis in BaF-3 cell lines with the combined two drugs, however, compared with the normal cells, the BTZ/5z-7-oxozeaenol combination induce greater apoptosis in Daudi cells (Fig. 4a, b; Fig. S4). These findings imply that Daudi cells are very unique and the local type BL displays the potential for further classification.
In addition, with the development of recent combinatorial chemotherapy (such as cyclophosphamide, doxorubicin, vincristine, and cytarabine and rituximab targeted therapy), the treatment options available for pediatric patients has received great progress. However, the efficacy of these treatments has still not been optimized adults [16–18]. Further study that focuses on the biological characteristics and more precise classification of BL will not only provide greater value in the clinical diagnosis of this condition, but will also improve therapeutic efficacy via more pertinent targeted therapies.
Other investigators have shown that bortezomib inhibits NF-κB function inducing apoptosis in BL cell lines [19]. It is well known that the degradation of IκB is the most important mechanism for the anti-tumor effects of bortezomib. The classical activation mechanism of NF-κB is well known. In resting cells, NF-κB binds with matrix proteins like IκB, which is located in the cytoplasm. When cells are stimulated, IKK phosphorylates IκB, which is then ubiquitinated, and finally degraded by the proteasome [20]. The released NF-κB then translocates to the nucleus, where it exhibits biological effects after initiating transcription via binding with the promoter sequence of κB [8, 21]. These findings are consistent with the results obtained in this study, wherein we found that bortezomib and 5Z-7-oxozeaenol synergistically act on the NF-κB inhibitory protein IκB and inhibit its degradation, and thus exhibit the biological effects of NF-κB by promoting its location in the cytoplasm of Daudi cells (Fig. 3a, b). And the Daudi cell lines exhibited a time-dependent decrease in expressions of cleaved PARP treated by the BTZ/5Z-7-oxozeaenol combination (Fig. 4a). Both in vivo and in vitro studies showed that inhibition of TAK1 by RNAi silencing or oral administration of the TAK1 inhibitor (LYTAK1) can inhibit NF-κB activity and enhance the sensitivity of gemcitabine-induced pancreatic cancer cell death [22]. As the schematic representation exhibit, inhibition of TAK1 activity can inhibit IKK and IκB, can then suppress NF-κB activity. Bortezomib (BTZ), a proteasome inhibitor, performs a function for preventing tumors via degradation of IκB [12, 13]. Therefore, we conclude that bortezomib and 5Z-7-oxozeaenol synergistically inhibit Daudi cell proliferation and induce Daudi cell apoptosis via inhibition of NF-κB activity (Fig. 4c). However, we also found that 5Z-7-oxozeaenol can inhibit the activity of ERK, JNK, and p38, which suggests that the other reason for the inhibitory effect of 5Z-7-oxozeaenol on Daudi cell growth is inhibition of MAPK, which is the downstream signaling pathway of TAK1 (Fig. 3a). RNAi screening showed that TAK1 is a potential target enhancing the efficiency of topoisomerase inhibitors in breast cancer cells [23]. In addition, TAK1 exhibits an important role in cell growth of non-small cell lung cancer (NSCLC) and KRAS-dependent colorectal cancer. The persistent activation of TAK1 in tumor-associated macrophages promotes the growth of small cell lung cancer cells. Inhibition of TAK1 activity can enhance apoptosis in KRAS-dependent colorectal cancer [24, 25]. In neuroblastoma cells, TAK1 inhibition significantly enhanced the sensitivity to DNA damaging drugs [26]. Therefore, further comprehensive studies exploring the TAK1-dependent signaling pathway during 5Z-7-oxozeaenol-promoted effect of bortezomib are still needed. These studies will provide more valuable information to guide the clinical use of 5Z-7-oxozeaenol in the future. In addition, the combination strategy of using the TAK1 inhibitor and the proteasome inhibitor together will need to be verified in vivo.
The other interesting finding of this study is that the combination of the proteasome inhibitor bortezomib and the TAK1 inhibitor 5Z-7-oxozeaenol can reduce the dose of bortezomib, which may provide novel therapeutic options to reduce the toxicity of bortezomib in the clinic. It has previously been shown that clinical use of bortezomib has considerable side effects, including peripheral neuropathy, thrombocytopenia, neutropenia bite, anemia, fatigue, and diarrhea [27–29]. A phase II clinical trial of bortezomib showed that more than 80 % of patients appeared with polyneuropathy symptoms. [30] Thrombocytopenia is observed in the majority of patients during therapy, which can be sustained for 14 days after therapy, and which can be rescued until 21 days post-treatment [31]. Therefore, it is critical to reduce the side effects of bortezomib in the clinic. It is an option to reduce the dose of one drug by combinatorial therapy with other drugs. In vitro results of this study showed that the combination of bortezomib and 5Z-7-oxozeaenol (2 μM) against Daudi cells greatly reduced the dose by 40 % in the case of not affecting therapeutic efficacy. These findings not only broaden the application scope of the proteasome inhibitor bortezomib, but these studies also provide the basis for further in vivo studies.
In summary, we have found that the proteasome inhibitor bortezomib and TAK1 inhibitor 5Z-7-oxozeaenol can synergistically inhibit NF-κB activity in Daudi cells. Our study not only provides the basis for further in vivo studies, but also provides novel ideas for reducing the side effects of bortezomib in the clinic, observation that collectively support potential approaches in the clinic that might advance classification of the local type of BL.
References
Molyneux EM, Rochford R, Griffin B, et al. Burkitt's lymphoma. Lancet. 2012;379:1234–44.
Busch K, Keller T, Fuchs U, et al. Identification of two distinct MYC breakpoint clusters and their association with various IGH breakpoint regions in the t (8; 14) translocations in sporadic Burkitt-lymphoma. Leukemia. 2007;21:1739–51.
Kanda K, Hu H-M, Zhang L, Grandchamps J, Boxer LM. NF-κB activity is required for the deregulation of c-myc expression by the immunoglobulin heavy chain enhancer. J Biol Chem. 2000;275:32338–46.
Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441(7092):431–6.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Garkavtsev I, Kozin SV, Chernova O, et al. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature. 2004;428:328–32.
Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci. 1995;92:10599–603.
Yamaguchi K, Shirakabe K, Shibuya H, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science. 1995;270:2008–11.
Chen Z, Bhoj V, Seth R. Ubiquitin, TAK1 and IKK: is there a connection? Cell Death Differ. 2006;13:687–92.
Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–62.
San Miguel J, Blade J, Boccadoro M, et al. A practical update on the use of bortezomib in the management of multiple myeloma. Oncologist. 2006;11:51–61.
Cavo M. Proteasome inhibitor bortezomib for the treatment of multiple myeloma. Leukemia. 2006;20:1341–52.
Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81.
Buglio D, Palakurthi S, Byth K, et al. Essential role of TAK1 in regulating mantle cell lymphoma survival. Blood. 2012;120:347–55.
Perkins AS, Friedberg JW. Burkitt lymphoma in adults. ASH Educ Program Book. 2008;2008:341–8.
Yustein JT, Dang CV. Biology and treatment of Burkitt's lymphoma. Curr Opin Hematol. 2007;14:375–81.
Gerecitano J, Straus DJ. Treatment of Burkitt lymphoma in adults. Expert Rev Anticancer Ther. 2006;6:373–81.
Yang X, Li X, Chen Y, et al. Apoptosis of Burkitt’s lymphoma Raji cell line induced by bortezomib. Zhongguo shi yan xue ye xue za zhi/Zhongguo bing li sheng li xue hui = J Exp Hematol/Chinese Ass Pathophysiol. 2009;17:592–6.
Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Investig. 2001;107:241.
Spencer E, Jiang J, Chen ZJ. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes Dev. 1999;13:284–94.
Melisi D, Xia Q, Paradiso G, et al. Modulation of pancreatic cancer chemoresistance by inhibition of TAK1. J Natl Cancer Inst. 2011;103:1190–204.
Martin S, Wu Z-H, Gehlhaus K, et al. RNAi screening identifies TAK1 as a potential target for the enhanced efficacy of topoisomerase inhibitors. Curr Cancer Drug Targets. 2011;11:976.
Singh A, Sweeney MF, Yu M, et al. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell. 2012;148:639–50.
Ahmed N, Zeng M, Sinha I, et al. The E3 ligase Itch and deubiquitinase Cyld act together to regulate Tak1 and inflammation. Nat Immunol. 2011;12:1176–83.
Fan Y, Cheng J, Vasudevan SA, et al. TAK1 inhibitor 5Z-7-oxozeaenol sensitizes neuroblastoma to chemotherapy. Apoptosis. 2013;18:1224–34.
Ale A, Bruna J, Navarro X, Udina E. Neurotoxicity induced by antineoplastic proteasome inhibitors. Neurotoxicology. 2014;43:28–35.
Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008;112:1593–9.
Grosicki S, Barchnicka A, Jurczyszyn A, Grosicka A. Bortezomib for the treatment of multiple myeloma. Expert Rev Hematol. 2014;7(2):173–85.
Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–20.
Palumbo A, Bringhen S, Larocca A, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol. 2014;32:634–40.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81270615), and the Outstanding Discipline Leaders of Shanghai Health System Support Program (XBR2013077).
Conflicts of interest
No conflict of interest exits in the submission of this manuscript, and the manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part.
Author contributions
J. Z. and B. L.: manuscript writing and editing; H. W., J. O., R. W., J. L., W. C., X. L., S. Z., J. Y., and L. Z.: critical discussion for conception and design; S. L. and A. L.: conception and design, manuscript writing and editing, and final approval of manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jie Zhang and Bing Li contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
5Z-7-oxozeaenol enhances the cytotoxic effect of bortezomib on Burkitt lymphoma cell Daudi. Daudi cells were seeded into 96-well plates at a concentration of 1.5 × 104/well. After 16 h, cells were incubated with drugs combinational or respective for 24/48/72/96 h at indicated concentrations and cell viability was assessed by CCK-8 assay. Results presented as % vehicle ± SD (n = 5). ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05 (Student’s t test, two-tailed) (PPTX 100 kb)
Fig. S2
Cytotoxic effect of 5Z-7-oxozeaenol and bortezomib on Burkitt lymphoma cells Raji or NAMALWA. Raji or NAMALWA cells were seeded in 96-well plates at a concentration of 1.5 × 104 cells per well. After 16 h, cells were incubated with drugs for 24 h at indicated concentrations, and growth inhibition was assessed by CCK-8 assay. Results presented as % vehicle ± SD (n = 5). P > 0.05 (Student’s t test, two-tailed) (PPTX 78 kb)
Fig. S3
5Z-7-oxozeaenol inhibits Erk, p38 activation in Daudi. Daudi cells were treated with bortezomib at the indicated time points (0, 2, 5, 10, 15 min) with or without 5Z-7-oxozeaenol, total protein extracts were subjected to SDS-PAGE and immunoblotted with antibodies indicated. β-tubulin was detected as a loading control for whole cell extracts (PPTX 87 kb)
Fig. S4
BaF-3 cells were treated with bortezomib (8 nM) and 5Z-7-oxozeaenol (2 μM) for 24/48 h and examined by flow cytometry using Annexin-V/PI staining to label apoptotic cells (PPTX 233 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Li, B., Wu, H. et al. Synergistic action of 5Z-7-oxozeaenol and bortezomib in inducing apoptosis of Burkitt lymphoma cell line Daudi. Tumor Biol. 37, 531–539 (2016). https://doi.org/10.1007/s13277-015-3832-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3832-1