Abstract
Downregulation of microRNA-218 (miR-218) is found in various human cancers, including non-small cell lung cancer (NSCLC). However, the involvement of chemosensitivity to cisplatin (DDP) and the underlying molecular mechanism remain unclear. In this study, we investigate whether miR-218 mediates NSCLC cell functions associated with chemoresistance. Quantitative real-time polymerase chain reaction (qRT-PCR) was utilized to detect miR-218 expression in NSCLC cell lines A549/DDP and/or A549. The cell activity was measured by MTT assay. Cell cycle and cell apoptosis were detected by flow cytometry. Luciferase reporter assays and Western blots were used to validate runt-related transcription factor 2 (RUNX2) as a direct target gene of miR-218. miR-218 was significantly reduced in A549/DDP cells compared with parent A549 cells. Upregulation of miR-218 altered cell cycle-induced cell apoptosis and enhanced the sensitivity of A549/DDP cells to cisplatin. Mechanistically, RUNX2 was identified as a direct and functional target of miR-218, and RUNX2 executed the former on lung cancer chemoresistance. Our present study demonstrated for the first time that downregulation of miR-218 may contribute to the chemoresistance of NSCLC cells to cisplatin, which leads to upregulation of RUNX2. Uncovering the mechanism represents a novel approach to enhance the efficacy of chemotherapy during cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most common type of malignant cancer and leading cause of tumor-related mortality in the world with a survival rate of 15 %. There are more than 22,400 new cases of lung cancer emerging and nearly 1.4 million deaths every year [1]. Among different types of lung cancers, non-small cell lung cancer (NSCLC) weighs more than 80 % of all the cases. Despite great progress in treatment strategies in recent decades, two thirds of NSCLC patients are diagnosed in advanced stages and are normally associated with poor prognosis. The 5-year survival rate of individuals with stage IV NSCLC is approximately less than 1 % [2]. With only a few patients being treated by surgical resection, NSCLC patients are mostly treated with chemotherapy and/or radiation therapy. The platinum-based chemotherapeutic drug such as cisplatin is one of the most frequently used agents in curing or controlling NSCLC. However, the development of chemoresistance is a major inevitable incidence limiting the effect of treatment in numerous lung cancer patients succumb to the disease [3]. Although previous studies have identified a variety of molecules associated with the drug-resistance in NSCLC, the underlying molecular mechanisms of therapeutic resistance remain to be elucidated, and biologic markers with high sensitivity and specificity for the prediction of chemotherapeutic response to NSCLC are still insufficient.
MicroRNAs (miRNAs) are a class of single-stranded, endogenously expressed, non-coding RNAs of 19–25 nucleotides that can interact with 3′untranslated regions (3′UTRs) of the target genes [4]. miRNAs play a considerably important role in various biological and pathological processes including proliferation, morphogenesis, apoptosis, and carcinogenesis. The imperfect pairing with target genes and regulating transcription or post-transcription of subsequent gene expression triggers either miRNA degradation or translational inhibition [5, 6]. Accumulating evidence demonstrates that miRNAs have a vital role in regulating drug sensitivity and/or resistance to chemotherapeutic agents in various cancer types [7–9].
Increasing interests and studies indicate that the miR-218 is depleted in some human solid cancers, such as glioma, bladder cancer, oral cancer, cervical cancer, and lung cancer where tumor cell invasion and proliferation are enhanced [10–14]. Reports also showed that miR-218 might act as tumor suppressor in a variety of human cancer types [12, 15, 16]. In addition, increased expression of miR-218 can enhance the chemosensitivity to cisplatin [17, 18]. These results indicated that miR-218 may have a correlation with the resistance during lung cancer chemotherapy. The impact and the molecular mechanism of miR-218 impacts on the cisplatin chemoresistance in NSCLC remain elusive.
In this present study, we determined that miR-218 enhances apoptosis and induces cell cycle arrest in cisplatin-resistant human lung adenocarcinoma cell line A549/cisplatin (DDP). Additional experiments indicated that miR-218 contributes to chemoresistance in the cisplatin-treated NSCLC cells by negatively regulating the expression of runt-related transcription factor 2 (RUNX2) through direct interaction with its 3′UTR. Our results demonstrated that downregulation of miR-218 plays critical roles in the development of cisplatin chemoresistance in NSCLC cells, targeting the highlighted oncogenic gene, RUNX2.
Materials and methods
Cell culture
The human lung adenocarcinoma cell line A549 was purchased from Biochemistry and Cell Biology Institute of Shanghai, Chinese Academy of Science. The cells were cultured in DMEM supplemented with 10 % fetal bovine serum (Invitrogen, Carlsbad, CA, USA; 100 units/mL penicillin and 100 μg/mL streptomycin (Hyclone, Logan, UT, USA)) in a humidified incubator at 37 °C with 5 % CO2. The cisplatin-resistant A549 cells (A549/DDP) were established by our laboratory and maintained in a 41-μ/L final concentration of cisplatin.
IC50 determination
Cells at the logarithmic phase of growth were seeded onto 96-well plates at 3000 cells per well in triplicates and incubated for 24 h. Then, they were transfected with negative control (NC) or RUNX2 siRNA (si-RUNX2) and treated with a serious diluted drug, cisplatin. Cell viability was measured after 96 h using the Cell Counting Kit 8 (CCK8, Dojindo, Japan) according to the manufacturer’s instructions. IC50 were calculated as the concentration of drug required for 50 % cells killed.
RNA extraction and qRT-PCR analyses
Total RNA was extracted from cells using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. For RUNX2 expression, reverse transcription was performed with Superscript first-strand synthesis system (Invitrogen) following the manufacturer’s handbook. The messenger RNA (mRNA) level was measured on an ABI 7900HT instrument (Applied Biosystems, NY, USA). The following primers were used: RUNX2 forward, 5′-CCGCCTCAGTGATTTAGGGC-3′ and reverse, 5′-GGGTCTGTAATCTGACTCTGTCC-3′; GAPDH forward, 5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse 5′-GCCATCACGCCACAGTTTC-3′. GAPDH was used as a loading control.
To quantify miR-218, the expression level of miR-218 was measured by the TaqMan miRNA Assay (ABI) according to the manufacturer’s protocol. U6 snRNA were used as an internal loading control. All reactions were run in triplicate and all experiments were carried at least three independent times.
Construction of miR-218 expression vectors and cellular transfection
To force expression of miR-218, the sequence containing the miR-218 pre-miRNA was amplified by PCR from human genomic DNA using the forward primer 5′-AAAGGATCCGCGGGAAGAATGCATGTCA-3′ and the reverse primer 5′-AAAAGAATTCTCTCCCTCTCACATAATCTCG-3′. The resulting PCR fragment was cloned into the BamHI/EcoRI sites of the pCDH-CMV-EF1-copGFP vector (SBI, Mountain View, CA, USA) and successful cloning was confirmed by DNA sequencing.
The lentiviruses were produced by co-transfecting HEK293T with pPACKH1 Lentivector Packaging KIT (SBI) and lentiviruses expressing miR-218 or control viruses expressing GFP according to the manufacturer’s instructions. The efficacy of miR-218 overexpression was confirmed by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) 72 h after transfection.
Cell transfection
DNA fragment coding RUNX2 protein was amplified by PCR from HEK293T cell cDNA and cloned into the expression vector pcDNA3.1 (Clontech, Palo Alto, CA, USA) with primers: forward: 5′-AAAGCTAGCATGGCATCAAACAGCCTCTTC-3′; reverse: 5′-AAACTCGAGTCAATATGGTCGCCAAACAGAT-3′. The sequence of negative control small interfering RNA (siRNA) is 5′-UUCUCCGAACGUGUCACGU-3′. All the miR-218 inhibitors (anti-miR-218), inhibitor negative control (inhibitor NC), siRNA, and the scramble sequence (negative control, NC) were obtained from GenePharma (Shanghai, China).
Cells were transfected with 50 nM RUNX2 overexpression plasmid or RUNX2 siRNA and their relative control, and the cells were harvested 48 h after transfection. The sequence of siRNA targeting RUNX2 is 5′-CUGUGAUAGGUAGCUACUUdTdT-3′. The sequence of siRNA negative control is 5′-UUCUCCGAACGUGUCACGUdTdT-3′. At least three independent experiments were carried out. The expression levels of RUNX2 in A549/DDP transfected cells were detected by Western blot analysis.
Cell invasion assays
The cells (4 × 105/well) were suspended in 100 μl of serum-free DMEM medium on 8-μm pore size Transwell inserts (Corning, NY, USA) coated with 25 μg of Matrigel (BD Bioscience, Bedford, MA, USA) for invasion assays. DMEM medium with 10 % fetal bovine serum was added in the lower chamber. The cells on the upper surface of the filter were fixed with 4 % paraformaldehyde and stained with 0.5 % crystal violet following 48 h of incubation at 37 °C. The stained cells were counted in five random fields per filter under a microscope (×100 magnification), and quantification was performed by manually counting the stained cells.
Flow cytometry analysis of apoptosis and cell cycle
Apoptotic cells were examined using an Annexin V-PE Apoptosis Detection Kit (Millipore). The samples were harvested and then stained with 5 μl of annexin V-PE and 5 μl of PI for 15 min at room temperature in the dark. Flow cytometer was performed to detect cellular apoptosis, and the results were analyzed using the Cell-QuestTM Pro software (BD Biosciences). Pretreated cells were harvested, fixed in ice-cold 70 % ethanol at 4 °C overnight, and then resuspended in 1 mL of PBS containing 1 mg/mL RNase (Sigma, St. Louis, MO) and 50 μg/mL PI (Sigma) in a dark box for 30 min at room temperature. The percentage of cells in different phases of the cell cycle was calculated by the Cell-QuestTM Pro software (BD Biosciences).
Western blot assay
The cultured cells were washed three times in PBS and lysed with RIPA lysis buffer for 30 min at 4 °C. Equal amounts of protein were loaded into 10 % SDS-PAGE and were transferred electrophoretically onto a PDVF membrane (Millipore, Billerico, Massachusetts, USA). The primary antibodies against MDR 1 (Millipore), BCL-2, CyclinD 1, β-catenin (Cell Signaling Technology, Beverly, MA), RUNX2 (Abcam Inc., Cambridge, MA), and GAPDH (CST) were incubated overnight at 4 °C, and the corresponding secondary antibodies were incubated for 1 h at room temperature. The blots were then re-probed with horseradish peroxidase-conjugated secondary antibody and visualized by the SuperSignal® West Pico chemiluminescent substrate (Pierce, Rockford, IL, USA) and detected using ImageQuant LAS 4010 Imaging System (GE Healthcare Life Sciences, Piscataway, NJ).
Dual-luciferase activity assay
A full length of human RUNX2 3′UTR with a wild-type and mutant target sequence for miR-218 was cloned into the downstream of the Renilla psi-CHECK2 (Promega, Madison, WI, USA) to construct RUNX2-3′UTR-WT and RUNX2-3′UTR-MUT, respectively. The following primers were used: forward: 5′-AAACTCGAGAATTCCTCAGCAGTGGCCC-3′; reverse: 5′-AAAGCGGCCGCGCCAAATAATTCAATTTACAGTTGC-3′. The cells were cultured to approximately 60 % confluence in a 96-well plate and transfected with a mixture of 50 ng RUNX2-3′UTR-WT WT or RUNX2-3′UTR-MUT and 5 ng miR-218 or control vector using the FuGENE HD transfection reagent (Roche Applied Science, Manheim, Germany) according to the manufacturer’s instruction. After 48 h of transfection, the cells were lysed and the luciferase activities were measured by synergy™ HT microplate reader (Biotek, Beijing, China) with the Dual-Luciferase Reporter Assay reagent (Promega). Renilla luciferase activity was normalized to Firefly luciferase activity for each transfected well. All the data were obtained by averaging the results from three independent repeats.
Statistical analysis
All the result data were representative of at least three independent experiments and shown as mean ± standard deviation (SD). Student’s t tests were used to analyze the results, and P < 0.05 was considered statistically significant. All of the statistical analyses were performed using GraphPad Prism v5.0 software (GraphPad Software, Inc.).
Results
miR-218 expression is significantly reduced in A549/DPP compared with A549
To confirm the association of miR-218 with the development of chemoresistance in NSCLC cells, the expression levels of miR-218 were examined in the A549/DDP cells compared with their parent cell line, A549 by qRT-PCR. The data indicated that A549/DDP cell line had a significantly lower expression level of miR-218 than the parental cells, A549 (**P < 0.01, as represented in Fig. 1a). Moreover, the cell lines (A549 and A549/DDP) were found to exhibit changes in cell morphology (Fig. 1b).
Expression of miR-218 in A549 and A549/DDP. a A549/DDP cell line had a significantly lower expression level of miR-218 than the parental cells, A549 (**P < 0.01). The expression level of miR-218 was determined by qRT-PCR and normalized to an endogenous control (U6 snRNA). b Morphology of parental A549 and A549/DDP as observed by microscope
Effects of miR-218 on A549/DDP
To further investigate the function of miR-218 in NSCLC cell lines, cisplatin-resistant A549/DDP cells were transfected with miR-218 or vectors by lentivirus. Results from qRT-PCR confirmed that miR-218 induced significant expression compared with mock vector group (Fig. 2a). MTT assay showed that overexpression through miR-218 was dramatically more sensitive to cisplatin than vector-transfected cells (Fig. 2b). Furthermore, the apoptosis was detected by flow cytometric assay, revealing that overexpressed miR-218 attained a significantly lower survival rate than that in the vector group. This result suggested that increased expression of miR-218 altered cisplatin sensitivity in A549/DDP cells (Fig. 2c). To further validate the effect of miR-218 on cell cycle, flow cytometric analysis demonstrated that miR-218 decreased the number of cells in G0 phase and increased the number of cells in S phase in A549/DDP cells, implying an inhibition of G0/S transition (Fig. 2d). These results indicated that upregulation of miR-218 could remarkably suppress the cell cycle progression as well as induce apoptosis reinforcement in A549/DDP cells. Metastasis is an invasive nature of tumor cells that are the leading cause of cancer-related death. We tested whether overexpression of miR-218 affected cell invasion in vitro, and our results showed that miR-218 overexpression significantly suppressed cell invasion (Fig. 2e). MDR-associated genes, including the encoded MDR1, BCL-2, and CyclinD1, have been reported as a predominant cause of NSCLC patients who succumb to the disease due to drug-resistance. Accordingly, the effects of overexpressed miR-218 on these genes in A549/DDP cells were analyzed via Western blot assay (Fig. 2f). This observation suggested that miR-218 mimics led to a significantly decreased level of MDR1, BCL-2, and CyclinD1 in A549/DDP cells than that in vector cells. All the results indicated that overexpression of miR-218 facilitates the chemosensitivity to DDP in the A549 NSCLC cancer cell line.
Effects of miR-218 on A549/DPP. a The miR-218 induced significant expression compared with mock vector group. b MTT assay showed that overexpression through miR-218 was dramatically more sensitive to cisplatin than vector-transfected cells. c Flow cytometric results revealed that overexpressed miR-218 attained a significantly lower survival rate than that in vector group. d Flow cytometric analysis demonstrated that miR-218 decreased the number of cells in G0 phase and increased the number of cells in S phase in A549/DDP cells, implying an inhibition of G0/S transition. e Representative images showed A549/DDP after transfections of vector or miR-218. Quantification of the invaded cell number. f Western blot assay suggested that miR-218 led to a significantly decreased level of MDR1, BCL-2, and CyclinD1 in A549/DDP cells than that in vector cells. GAPDH was used as an internal control. The data were obtained in three independent experiments and are presented as the means ± standard deviation of three experiments. **P < 0.01
RUNX2 was directly and negatively regulated by miR-218
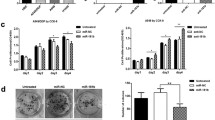
The cellular functions of miRNAs are revealed through their target genes. RUNX2 was chosen as a favored target gene of miR-218 by using open access software, Target Scan, because a conserved sequence was found in the 3′UTR of RUNX2 mRNA with a perfect match to the seed region of miR-218 (Fig. 3a). To confirm whether RUNX2 was a functional downstream target of miR-218, luciferase reporters were cloned with a fragment of RUNX2 3′UTR containing the putative wild-type or mutated miR-218 binding site. Luciferase reporter assays showed that upregulation of miR-218 decreased the relative luciferase activity of RUNX2 3′UTR noticeably but no difference to the mutated 3′UTR (Fig. 3b). When blocking the expression of miR-218 with anti-miR-218 (miR-218 inhibitor), an increased Luciferase intensity was obtained in A549 cells (Fig. 3c). Similar results were found that miR-218 overexpression significantly reduced the endogenous mRNA and protein of RUNX2 (Fig. 3c, e). In contrast, blocking the expression of miR-218 increased the expression of RUNX2 (Fig. 3e). These results indicated that RUNX2 is a direct target of miR-218 and is negatively regulated by miR-218 in A549 cells.
RUNX2 was directly and negatively regulated by miR-218. a RUNX2 was chosen as a favored target gene of miR-218 by using open access software, Target Scan, because a conserved sequence was found in the 3′UTR of RUNX2 mRNA with a perfect match to the seed region of miR-218. b Luciferase reporter assays showed that upregulation of miR-218 decreased the relative luciferase activity of RUNX2 3′UTR noticeably but no difference to the mutated 3′UTR. c When blocking the expression of miR-218 with anti-miR-218 (miR-218 inhibitor), an increased luciferase intensity was obtained in A549 cells. d In both A549 and A549/DDP cells, overexpressed miR-218 decreased the mRNA level of RUNX2. e The protein level of RUNX2 was detected with transfection of miR-218 or anti-miR-218 in A549/DDP cells or A549, respectively. GAPDH was used as an internal control. **P < 0.01
Knockdown of RUNX2 increases sensitivity of A549/DPP cells to cisplatin in vitro
Our previous serial study has proven that miR-218 negatively regulates the expression of RUNX2 mRNA and protein by targeting its 3′UTR (supplementary Figs. 1–3 and Fig. 4a). To explore whether RUNX2 is involved in the chemosensitivity of A549/DDP cells to cisplatin in vitro, we performed analyses towards the loss-of-function and gain-of-function of genes. In A549/DDP cells that were transfected with small-interfering (si) RUNX2 or negative control (NC), the protein levels of RUNX2 were examined by Western blot and showed suppressed expression of RUNX2 (Fig. 4a). With the downregulation of RUNX2 expression by siRNAs, A549/DDP cells showed significantly more sensitive to the cisplatin therapy than NC cells (Fig. 4b). Flow cytometric assay revealed that the apoptosis was observably enhanced via knocking down RUNX2 in A549/DDP cells (Fig. 4c). In addition, the cell cycle assay demonstrated decreased number of cells in the G0 phase, along with increased number of S phase, indicating blockage on G0/S transition. These data was consistent in miR-218 overexpressing A549/DDP cells, which implied that miR-218 regulates chemosensitivity of NSCLC cells in vitro by targeting RUNX2.
The knockdown of RUNX2 increases sensitivity of A549/DPP cells to cisplatin in vitro. a In A549/DDP cells that were transfected with short-interfering (si) RUNX2 or negative control (NC), the protein levels of RUNX2 were examined by Western blot and showed suppressed expression of RUNX2. GAPDH was used as an internal control. b With the downregulation of RUNX2 expression by siRNAs, A549/DDP cells showed significantly more sensitive to the cisplatin therapy than NC cells. c Flow cytometry showed that the apoptosis was observably enhanced via knocking down RUNX2 in A549/DDP cells. d The cell cycle assay demonstrated decreased number of cells in the G0 phase, along with increased number of S phase, indicating blockage on G0/S transition. Data are means of three separated experiments ± SD. **P < 0.01
Overexpression of RUNX2 rescues chemoresistance in A549/DPP cells
To further verify the function of miR-218-targeted RUNX2 in modulating chemosensitivity to A549/DDP cells, the effects of overexpressed target gene on the growth of A549/DDP cells were examined. Western blot analyses indicated that miR-218 effectively decreased the protein levels of RUNX2 in A549/DDP cells. Moreover, ectopic expression of RUNX2 rescued the miR-218-induced downregulation of RUNX2 (Fig. 5a). In addition, transfection of RUNX2 into A549/DDP cells restored the chemoresistance to cisplatin and apoptosis rate induced by overexpression of miR-218 (Fig. 5b, c). Flow cytometric assay revealed that the blockage of G0/S transition due to miR-218 could be reversed by transfecting RUNX2 ectopic expression (Fig. 5d). To sum up, these results suggested that miR-218 may play an important role in regulating cisplatin chemosensitivity directly through adjustment of anti-apoptosis activity by targeting RUNX2 in NSCLC cells.
Overexpression of RUNX2 rescues chemoresistance in A549/DPP cells. a Western blot analyses indicated that miR-218 effectively decreased the protein levels of RUNX2 in A549/DDP cells. Moreover, ectopic expression of RUNX2 rescued the miR-218-induced downregulation of RUNX2 GAPDH that was used as an internal control. b, c Transfection of RUNX2 into A549/DDP cells restored the chemoresistance to cisplatin and apoptosis rate induced by overexpression of miR-218. d Flow cytometric assay revealed that the blockage of G0/S transition due to miR-218 could be reversed by transfecting RUNX2 ectopic expression. Data are means of three separated experiments ± SD. *P < 0.05
Discussion
Lung cancer is a predominant contributor to cancer-related mortality in the world. Chemotherapeutic agents, cisplatin in particular, are widely used in the non-small cell lung cancer treatment. The main mechanism of chemo-drug action is to form intra- and inter-strand with tumor cell DNA and in turn induce tumor apoptosis [19]. However, the resistance to cisplatin represents a significant barrier to improve the long-term overcome of patients with NSCLC [20]. Factors that enhance the sensitivity of NSCLC cells to chemotherapeutic drugs may highlight predictive biomarkers or targets for therapy. Extensive studies have indicated that tumor-targeting therapies using miRNAs are becoming a novel diagnostic and therapeutic tool [21]. A number of studies have indicated that miR-218 is involved in the chemosensitivity to cisplatin process in certain tumors and that upregulation of miR-218 may partially enhance cell death [17, 18]. Consistently, in our present study, miR-218 was noteworthy downregulated in the A549/DDP cisplatin-resistant lung cancer cell lines compared with that in non-resistant A549 cells. When miR-218 expression was upregulated in A549/DDP cells, overexpressed miR-218 significantly increased cisplatin chemosensitivity, accelerated the cell death, and induced G0/S phase cell cycle arrest. These findings suggested that dysregulation of miR-218 contributes to the regulation of cisplatin chemosensitivity in non-small cell lung cancer.
The underlying action of miRNAs is to regulate their targets by direct cleavage of mRNA or by inhibition of protein synthesis [22]. To address the regulatory mechanism of miR-218 in NSCLC A549 cells to DDP, a number of additional miR-218 targets were predicted in NSCLC. Based on the bioinformatics analysis, RUNX2 was selected. RUNX2 has been known to play important roles in angiogenesis by enhancing endothelial cell proliferation, invasion, and tube formation. RUNX2 also indirectly contributes to the acquisition of apoptosis-resistant phenotype in certain cancer, such as prostate cancer and the attenuation of the sensitivity in cancer cells to anticancer agents [23–25]. In our study, siRNA-mediated silencing of RUNX2 yielded effects similar to those induced by the upregulation of miR-218 expression in NSCLC cells. Moreover, the inverse correlation between miR-218 and RUNX2 expression was also detected in the study of RUNX2 overexpression (Figs. 3, 4, and 5). To the best of our knowledge, we provided the first insight into the roles and possible mechanisms of miR-218 downregulation in chemosensitivity of A549 cells to DDP. Overall, the sequential validation studies suggested that miR-218 could target RUNX2 through direct 3′UTR interaction in NSCLC A549/DDP cells, which may illustrate the miR-218-mediated regulatory mechanism of cisplatin chemosensitivity in the A549 NSCLC cell line.
In conclusion, the present study provided confident evidence that downregulation of miR-218 strengthens human drug-resistant lung cancer cells to cisplatin, by upregulating RUNX2 and biomedical role of miR-218 in NSCLC cells. Therefore, the results demonstrated that targeting miR-218-RUNX2 interaction may be a potential therapeutic strategy for reversing the chemoresistance to cisplatin. miR-218 can be used as an assessment of a patient’s response prior to chemotherapy as well as can serve as a novel potential biomaker in human non-small lung cancer.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: Cancer J Clin. 2014;64:9–29.
Soria JC, Kim ES, Fayette J, Lantuejoul S, Deutsch E, Hong WK. Chemoprevention of lung cancer. Lancet Oncol. 2003;4:659–69.
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34.
Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5.
van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11:644–56.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
Boren T, Xiong Y, Hakam A, Wenham R, Apte S, Chan G, et al. MicroRNAs and their target messenger RNAs associated with ovarian cancer response to chemotherapy. Gynecol Oncol. 2009;113:249–55.
Blower PE, Chung JH, Verducci JS, Lin S, Park JK, Dai Z, et al. MicroRNAs modulate the chemosensitivity of tumor cells. Mol Cancer Ther. 2008;7:1–9.
Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–83.
Song L, Huang Q, Chen K, Liu L, Lin C, Dai T, et al. miR-218 inhibits the invasive ability of glioma cells by direct downregulation of IKK-beta. Biochem Biophys Res Commun. 2010;402:135–40.
Tatarano S, Chiyomaru T, Kawakami K, Enokida H, Yoshino H, Hidaka H, et al. miR-218 on the genomic loss region of chromosome 4p15.31 functions as a tumor suppressor in bladder cancer. Int J Oncol. 2011;39:13–21.
Uesugi A, Kozaki K, Tsuruta T, Furuta M, Morita K, Imoto I, et al. The tumor suppressive microRNA miR-218 targets the mTOR component Rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res. 2011;71:5765–78.
Martinez I, Gardiner AS, Board KF, Monzon FA, Edwards RP, Khan SA. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene. 2008;27:2575–82.
Davidson MR, Larsen JE, Yang IA, Hayward NK, Clarke BE, Duhig EE, et al. MicroRNA-218 is deleted and downregulated in lung squamous cell carcinoma. PLoS One. 2010;5:e12560.
Venkataraman S, Birks DK, Balakrishnan I, Alimova I, Harris PS, Patel PR, et al. MicroRNA 218 acts as a tumor suppressor by targeting multiple cancer phenotype-associated genes in medulloblastoma. J Biol Chem. 2013;288:1918–28.
Yamamoto N, Kinoshita T, Nohata N, Itesako T, Yoshino H, Enokida H, et al. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion by targeting focal adhesion pathways in cervical squamous cell carcinoma. Int J Oncol. 2013;42:1523–32.
Li J, Ping Z, Ning H. MiR-218 impairs tumor growth and increases chemo-sensitivity to cisplatin in cervical cancer. Int J Mol Sci. 2012;13:16053–64.
Tian H, Hou L, Xiong YM, Huang JX, She YJ, Bi XB, et al. miR-218 suppresses tumor growth and enhances the chemosensitivity of esophageal squamous cell carcinoma to cisplatin. Oncol Rep. 2015;33:981–9.
Panchuk R, Skorokhyd N, Chumak V, Lehka L, Omelyanchik S, Gurinovich V, et al. Specific antioxidant compounds differentially modulate cytotoxic activity of doxorubicin and cisplatin: in vitro and in vivo study. Croatian Medical J. 2014;55:206–17.
Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer. 2011;71:3–10.
Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol : Off J Am Soc Clin Oncol. 2009;27:5848–56.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Lee SH, Che X, Jeong JH, Choi JY, Lee YJ, Lee YH, et al. Runx2 protein stabilizes hypoxia-inducible factor-1alpha through competition with von Hippel-Lindau protein (pVHL) and stimulates angiogenesis in growth plate hypertrophic chondrocytes. J Biol Chem. 2012;287:14760–71.
Browne G, Nesbitt H, Ming L, Stein GS, Lian JB, McKeown SR, et al. Bicalutamide-induced hypoxia potentiates RUNX2-mediated Bcl-2 expression resulting in apoptosis resistance. Br J Cancer. 2012;107:1714–21.
Goloudina AR, Tanoue K, Hammann A, Fourmaux E, Le Guezennec X, Bulavin DV, et al. Wip1 promotes RUNX2-dependent apoptosis in p53-negative tumors and protects normal tissues during treatment with anticancer agents. Proc Natl Acad Sci U S A. 2012;109:E68–75.
Acknowledgments
This study was supported by the health system advanced appropriate technology promotion project of Shanghai in China (2013SY036).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Authors’ contributions
JX, FY, and LZW conceived and designed the experiments. JX and ZXC performed the experiments. JX, DL, and FY analyzed the data. ZW and ZXC contributed the reagents/materials/analysis tools. JX wrote the paper. LZW and ZXC reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jing Xie, Fei Yu and Dan Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xie, J., Yu, F., Li, D. et al. MicroRNA-218 regulates cisplatin (DPP) chemosensitivity in non-small cell lung cancer by targeting RUNX2. Tumor Biol. 37, 1197–1204 (2016). https://doi.org/10.1007/s13277-015-3831-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3831-2