Abstract
Resveratrol, a natural polyphenolic compound found in foods and beverages, has attracted increasing attention in recent years because of its potent chemopreventive and anti-tumor effects. In this study, the effects of resveratrol on the expression of P-glycoprotein/multi-drug resistance protein 1 (P-gp/MDR1), and the underlying molecular mechanisms, were investigated in oxaliplatin (L-OHP)-resistant colorectal cancer cells (HCT116/L-OHP). Resveratrol downregulated MDR1 protein and mRNA expression levels and reduced MDR1 promoter activity. It also enhanced the intracellular accumulation of rhodamine 123, suggesting that resveratrol can reverse multi-drug resistance by downregulating MDR1 expression and reducing drug efflux. Resveratrol treatment also reduced nuclear factor-κB (NF-κB) activity, reduced phosphorylation levels of IκBα, and reduced nuclear translocation of the NF-κB subunit p65. Moreover, downregulation of MDR1 expression and promoter activity was mediated by resveratrol-induced AMP-activated protein kinase (AMPK) phosphorylation. The inhibitory effects of resveratrol on MDR1 expression and cAMP-responsive element-binding protein (CREB) phosphorylation were reversed by AMPKα siRNA transfection. We found that the transcriptional activity of cAMP-responsive element (CRE) was inhibited by resveratrol. These results demonstrated that the inhibitory effects of resveratrol on MDR1 expression in HCT116/L-OHP cells were closely associated with the inhibition of NF-κB signaling and CREB activation in an AMPK-dependent manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multi-drug resistance (MDR), in which cells are resistant to multiple structurally and functionally unrelated anti-cancer drugs, is considered a major reason for the failure of cancer chemotherapy [1]. In clinical situations, MDR is often the consequence of P-glycoprotein (P-gp) over-expression in tumor cell membranes. This protein, encoded by multi-drug resistance protein 1 (MDR1), is the most important membrane transporter for reducing intracellular accumulation of anti-cancer drugs [2]. MDR1 is expressed in tissues including kidney tubules, colon, pancreas, and adrenal gland, and tumors derived from these tissues often demonstrate a broad spectrum of substrate specificities to chemotherapeutic drugs [3]. Inhibition of P-gp expression or function has become an important method for improving the efficacy of chemotherapy.
Nuclear factor-κB (NF-κB) is the prototype member of a family of transcription factors, and is largely retained by an inhibitor (IκB) in the cytoplasm of non-stimulated cells. After stimulation, for example with cytokines or chemotherapeutic drugs, IκB undergoes phosphorylation and degradation by the proteosome. NF-κB then translocates to the nucleus, where it binds to a specific DNA consensus sequence, resulting in the induction of its target genes [4]. In cancer cells, NF-κB activity has been shown to protect against apoptosis [5] and to induce resistance to chemotherapeutic drugs. Several studies suggest that the actions of NF-κB involve control of P-gp expression [3, 6–8].
AMP-activated protein kinase (AMPK) is an important energy sensor involved in cellular metabolism. It is activated under conditions of metabolic stress that lower intracellular levels of ATP [9]. AMPK limits de novo synthesis of fatty acid and cholesterol through phosphorylation and inhibition of acetyl-CoA carboxylase (ACC) and 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase [10]. AMPK is also implicated in cancer development and is considered as a potential anti-tumor target molecule [11]. Activation of AMPK with 5′-aminoimidazole-4-carboxamide ribose (AICAR) has been shown to cause death of, or attenuate the growth of, cancer cells [12]. cAMP-dependent protein kinase A (PKA) regulates AMPK activity [13, 14], and it has been reported that cAMP and PKA are involved in regulating MDR1 expression in some cancer cells [14, 15]. Moreover, in rat liver, MDR1 expression is induced by PKA and cAMP-responsive element-binding protein (CREB) [16, 17]. Therefore, it is of interest to clarify the relationship between AMPK and MDR1.
Herbal medicine is now widely recognized worldwide for preventing and treating diseases [18, 19]. Recently, some compounds present in fruits, vegetables and herbs have been identified as potential chemopreventive agents [20]. For example, 3,5,4′-trihydroxy-trans-stilbene, also known as resveratrol (Res), has been demonstrated to play positive roles in chemotherapy, including suppression of proliferation and invasion, arrest of the cell cycle, and induction of apoptosis [21]. Previous studies have shown that Res can reverse chemoresistance of some tumors, including acute myeloid leukemia [22], oral epidermoid carcinoma [23], and breast cancer [24], and has a role in inhibiting NF-κB activation [25]. However, the effect of Res on reversing drug resistance in colon cancer cells has not yet been fully defined. In this study, we investigated the effects of resveratrol on MDR in oxaliplatin (L-OHP)-resistant HCT116 (HCT116/L-OHP) cells. Resveratrol reversed MDR via AMPK activation and inhibition of the NF-κB-dependent MDR1 signaling pathway. In addition, Res inhibited CRE transcriptional activity through AMPK upregulation, suggesting that CREB plays an important role in the regulation of MDR1 expression by Res in HCT116/L-OHP cells.
Materials and methods
Cell culture
HCT116 human colorectal carcinoma cells were obtained from the Cell Bank of Chinese Academy of Sciences. Cells were maintained in RPMI 1640 medium (Gibco, CA, USA) with 10 % fetal bovine serum (Gibco) at 37 °C in a humidified atmosphere with 5 % CO2. HCT116/L-OHP cells were established by our laboratory. The HCT116/L-OHP cells were seeded in medium containing 5 μg/ml L-OHP to maintain the drug-resistance phenotype and incubated in drug-free medium for at least 1 week before use [26].
Materials
Resveratrol, verapamil, rhodamine 123 (Rh123), H89, and forskolin were purchased from Sigma (Saint Quentin-Fallavier, France). A 100 mmol/L stock of Res was prepared with dimethyl sulfoxide (DMSO) and freshly diluted in culture medium for all in vitro experiments. DMSO alone was used as a vehicle control. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma (St Louis, MO, USA). Compound C was purchased from Calbiochem (La Jolla, CA, USA). Monoclonal antibodies reactive against human phospho-AMPK (Thr 172), AMPK, phospho-IκBα, IκBα, phospho-CREB (Ser133), and CREB were from Cell Signaling Technology (Beverly, MA, USA). Antibodies against MDR1, NF-κB p65, GAPDH, and Lamin B were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The Dual-Luciferase Assay Kit was from Vigorous Biotechnology (Beijing, China). BAY 11–7082 was from Selleck Chemicals (Houston, TX, USA).
Assessment of cell viability
The effect of L-OHP, with or without Res, on the proliferation of HCT116 and HCT116/L-OHP cells was determined by MTT assay. Cells were seeded into 96-well culture plates (1 × 105 cells/well) and allowed to attach for 12 h before treatment. The cells were exposed to L-OHP with or without Res. After incubation for 48 h, cell viability was evaluated by the MTT assay. The optical densities in control and drug-treated wells were measured at a wavelength of 570 nm in an Automated Microplate Reader (Multiskan Ex, Lab systems, FIN). The chemosensitivity of L-OHP was expressed as IC50 (concentration inducing 50 % cytotoxicity).
Quantitative real-time reverse transcription-PCR analysis
Total RNA was extracted from cells treated with different concentrations of Res for 48 h, or 50 μM Res for 0–48 h, using an RNA extraction kit (Promega Corporation) according to the instructions and reverse transcribed using the Reverse Transcription System (Takara, Dalian, China). We used SYBR Green Supermix with an iCycler thermal cycler (Bio-Rad, Hercules, CA, USA) to perform the real-time PCR assay. Primers were obtained from Shanghai Sangon Biological Engineering Technology & Services Co. Ltd (Shanghai, China) and the PCR primers were as follows: 5′-GCTCCTGACTATGCCAAAGC-3′ (sense) and 5′-TCTTCACCTCCAGGCTCAGT-3′ (antisense) for MDR1, 5′-GGTCGGAGTCAACGGATTTG-3′ (sense) and 5′-ATGAGCCCCAGCCTTCTCCAT-3′ (antisense) for GAPDH.
The data were collected and analyzed using the comparative Ct (threshold cycle) method, using GAPDH as the reference gene.
Rh123 accumulation assay
HCT116/L-OHP cells at a density of 105/well in exponential growth were used for the test. Cells were incubated in various concentrations of Res and 20 μM verapamil for 48 h. Verapamil was used as a positive control MDR inhibitory agent. After treatment, cells were exposed to 10 μg/ml Rh123 at 37 °C for 90 min. Trypsinized cells were resuspended in PBS. After washing with PBS, the intracellular mean fluorescence intensity (MFI) associated with Rh123 accumulation was measured by FACS (Becton Dickinson). Excitation was performed by an argon ion laser operating at 488 nm and the emitted fluorescence was collected through a 530-nm pass filter. Data analysis was performed using Cell Quest software.
Western blot analysis
Whole cell extractions and nuclear and membrane protein extractions were performed as described previously [27]. Protein concentrations were measured using the BCA assay (Pierce Biotechnology, Rockford, IL, USA). Western blot analysis was performed according to standard procedures as previously described [28]. GAPDH and Lamin B were used as loading controls for total/cytoplasmic fraction extracts and nuclear fraction extracts, respectively.
Transient transfection and luciferase assay
The luciferase activities were determined with the Dual-Luciferase Assay Kit using an FB12 Luminometer (Berthold Detection Systems). Briefly, cells were plated into each well of 12-well plates, and cultured for 24 h. The cells were co-transfected with 0.5 mg MDR1-Luc, NF-κB-Luc, or CRE-Luc constructs, and 0.15 mg pRL-TK Renilla luciferase construct (Promega) using Lipofectamine 2000 (Invitrogen Life Technologies). After 6 h incubation, the medium was replaced and cells were cultured overnight in DMEM supplemented with 10 % FBS. Following transfection for 24 h, the cells were washed with fresh medium, and pretreated with Res, compound C, and 20 ng/ml tumor necrosis factor-α (TNF-α). After 24 h incubation, cells were lysed and the promoter activities of NF-κB, MDR1, and CRE were calculated and represented as relative luciferase units of firefly luciferase activity per Renilla luciferase activity.
Immunocytochemical analysis
Cells were seeded on cover glasses and fixed with 4 % paraformaldehyde in PBS for 10 min at room temperature. The cells were permeabilized three times for 5 min with 0.1 % Triton X-100 in PBS. After blocking for 30 min with 10 % normal goat serum and 0.1 % Triton X-100 the cells were incubated with the rabbit antibodies overnight at 4 °C. The following day, the cells were washed three times with PBS and then incubated with horseradish peroxidase (HRP)-anti-rabbit IgG for 1 h. Nuclei were stained with DAPI for 5 min, and the cells were visualized by fluorescence microscopy (LEICA DM IRB; Leica, Wetzlar, Germany).
RNA interference assay
The sense-strand sequence used for AMPKα siRNA was 5′-GAUAUCAGGGAACAUGAAUdTdT-3′. The cells were plated at 5 × 105 cells/ml in a six-well plate. Twenty-four hours after plating, the cells were transfected with either the control siRNA or the AMPKα siRNA (50 nM) using Lipofectamine 2000.
Statistical analysis
Each experimental value was expressed as the mean ± standard deviation (SD). The significance of differences between experimental groups and controls was determined using the unpaired Student’s t test. Statistical analysis was performed using Origin 8.1 software. In all cases, p < 0.05 was considered significant.
Results
Characterization of HCT116 and HCT116/L-OHP cells
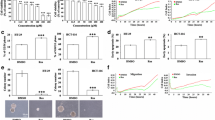
P-gp/MDR1 is a common biomarker of MDR [29]. To confirm this in our cell lines, western blotting, and real-time PCR were performed on cell extracts. As shown in Fig. 1a, b, expression levels of MDR1 protein and mRNA were higher in HCT116/L-OHP cells than in HCT116 cells. This is consistent with a previous study that demonstrated over-expression of MDR1 in HCT116/L-OHP cells [30]. Next, HCT116 and HCT116/L-OHP cells were treated with different concentrations of L-OHP (0–150 μg/ml) for 48 h. In the parental cell line HCT116, the IC50 value of L-OHP was 14.03 μg/ml. In the drug-resistant cell line, the IC50 value of L-OHP in HCT116/L-OHP was 144 μg/ml. The resistance index (RI), the ratio of the IC50 of L-OHP in HCT116/L-OHP cells to that of the parental HCT116 cells, was 10.3 (Fig. 1c). Since the RI value of the cells was greater than 3, the HCT116/L-OHP was considered to display chemoresistant characteristics.
Characterization of HCT116 and oxaliplatin (L-OHP)-resistant HCT116 (HCT116/L-OHP) cells. a Western blot analysis of MDR1 in drug-resistant HCT116 cell lines and parental HCT116 cells. GAPDH was used as a loading control. MDR1 was expressed at a higher level in HCT116/L-OHP cells than in the parental cells. b Quantitative real-time reverse transcription-PCR was performed on cDNA generated from total RNA. Data are shown as fold changes of mRNA levels in the HCT116/L-OHP cell line relative to HCT116. *p < 0.05, for MDR1 expression in HCT116/L-OHP cells versus HCT116 cells. c Effects of L-OHP (0–150 μg/ml) on HCT116/L-OHP and HCT116 cells. The two cell lines were treated with L-OHP for 48 h. Cell viability was then determined by MTT assay. Data are expressed as mean ± SD for three separate experiments
Resveratrol enhanced L-OHP toxicity in HCT116/L-OHP cells
After treatment with Res (0–50 μM) for 48 h, viabilities were all above 90 % (Fig. 2a) in HCT116/L-OHP cells. As our aim was to study Res as an adjuvant to intensify the potency of L-OHP, we chose treatment with 50 μM Res for 48 h to investigate the combination effect. The reversal activity of Res was measured using the MTT assay in HCT116/L-OHP and HCT116 cells treated with various concentrations of L-OHP. Res significantly increased L-OHP toxicity in HCT116/L-OHP cells, but did not affect the cytotoxicity of L-OHP in HCT116 cells (Fig. 2b).
Resveratrol enhanced the sensitivity of oxaliplatin (L-OHP)-resistant HCT116 (HCT116/L-OHP) cells to L-OHP. a HCT116/L-OHP cells were treated with resveratrol (0–200 μM) for 48 h. Cell viability was then determined by MTT assay and was expressed as mean ± SD for three separate experiments. *p < 0.05 for treated cells compared with the control. b Effects of co-incubation of HCT116/L-OHP and HCT116 cells with resveratrol and L-OHP. The IC50 value (concentration inducing 50 % cytotoxicity) of L-OHP in HCT116/L-OHP cells was higher than that in the parental cell line HCT116. Resveratrol significantly decreased the IC50 value of L-OHP in HCT116/L-OHP cells, but not in the parent HCT116 cell line. **p < 0.01, for the combination treated group versus the L-OHP group. All data are expressed as mean ± SD for three separate experiments
The effect of resveratrol on the expression levels of MDR1
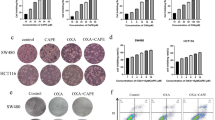
To determine whether Res inhibits MDR1, we examined the effect of Res on MDR1 protein and mRNA expression in HCT116/L-OHP cells. After treatment with various concentrations of Res for 48 h, or 50 μM Res for 6–48 h, expression of MDR1 protein was decreased in Res-treated HCT116/L-OHP cells, compared with controls, in a dose- and time-dependent manner (Fig. 3a), with similar trends observed for mRNA expression (Fig. 3b). Taken together, these results demonstrated that Res inhibited MDR1 expression in HCT116/L-OHP cells.
Effects of resveratrol on protein expression and mRNA levels of multi-drug resistance protein 1 (MDR1) in oxaliplatin (L-OHP)-resistant HCT116 (HCT116/L-OHP) cells. a HCT116/L-OHP cells were treated with resveratrol (12.5, 25, or 50 μM) for 48 h or with 50 μM resveratrol for 6–48 h. MDR1 was detected by western blotting with an MDR1 antibody. GAPDH was used as a loading control. b The mRNA levels were measured by real-time PCR. GAPDH was used as the loading control. Data are representative of three independent experiments. *p < 0.05, **p < 0.01, compared with the control. c Effect of resveratrol and verapamil on the accumulation of rhodamine 123 in HCT116/L-OHP cells. All data are expressed as mean ± SD of three separate experiments. *p < 0.05, **p < 0.01, compared with the control
The effect of resveratrol on intracellular accumulation of Rh123
Rh123 is a fluorescent P-gp substrate frequently employed to evaluate P-gp activity [31]. To further investigate the effect of Res on P-gp activity, the accumulation of Rh123 was measured in HCT116/L-OHP cells. Following pre-treatment with various concentrations of Res (12.5, 25, or 50 μM) or 20 μM verapamil, HCT116/L-OHP cells were incubated with 10 μg/ml Rh123 for 90 min. Figure 3c shows that the accumulation of Rh123 was significantly increased in Res-treated HCT116/L-OHP cells.
Resveratrol reduced the transcriptional activities of NF-κB and MDR1 in HCT116/L-OHP cells
There is growing evidence indicating that NF-κB is a major transcription factor involved in modulating the expression of MDR1 [8, 32, 33]. Therefore, we predicted that Res might inhibit the transcriptional activity of NF-κB and MDR1. A reporter gene assay was performed using pNF-κB-luc and pMDR1-luc plasmids. HCT116/L-OHP cells were transfected with pNF-κB-luc and pMDR1-luc, then stimulated with TNF-α either in the presence or in the absence of Res. As shown in Fig. 4a,b, Res inhibited the transcriptional activation of NF-κB and MDR1 in a dose-dependent manner.
Resveratrol inhibited multi-drug resistance protein 1 (MDR1)- and nuclear factor-κB (NF-κB)-luciferase activity. Cells seeded into 24-well plates were transfected with MDR1 (a) or NF-κB (b) reporter plasmids. After transfection, the cells were treated with various concentrations of resveratrol (12.5, 25, or 50 μM) and 20 ng/ml tumor necrosis factor-α (TNF-α) for 24 h. Cells were then harvested and luciferase activity was determined using a Dual-Luciferase Assay Kit. Results are representative of three independent experiments. Each value represents the mean ± SD. *p < 0.05, compared with the untreated group, and # p < 0.05, compared with the TNF-α group. HCT116/L-OHP cells were incubated with 50 μM resveratrol for 5–90 min (c) or resveratrol (12.5, 25, or 50 μM) and TNF-α (20 ng/ml) for 24 h (d). The cells were lysed and subjected to western blot analysis using anti-phospho-IκBα, anti-IκBα, and anti-GAPDH antibodies. e The translocation of p65 was detected by immunofluorescence using an anti-p65 antibody and Alexa Fluor 488-goat anti-rabbit IgG. DAPI was used as a nuclear stain. f Effect of resveratrol on nuclear translocation of p65 in HCT116/L-OHP cells. Cytoplasmic and nuclear extracts were generated and then analyzed by western blotting with an anti-p65 antibody. g Cells were co-treated with resveratrol (50 μM) and TNF-α (20 ng/ml) or BAY 11–7082 (5 μM) for 24 h. MDR1 was detected by western blotting with an MDR1 antibody. GAPDH was used as a loading control
Resveratrol suppressed MDR1 expression by inhibiting degradation and phosphorylation of IκBα and p65 translocation in HCT116/L-OHP cells
NF-κB is regulated by the TNF signaling pathway, and is largely retained by an inhibitor (IκB) in the cytoplasm of non-stimulated cells. Following stimulation, IκB is phosphorylated and degraded [34], followed by translocation of NF-κB to the nucleus where it induces its target genes [4]. To determine whether Res inhibits NF-κB and MDR1 activation by blocking phosphorylation and degradation of IκBα, we performed a time-course experiment to determine the effect of Res on the phosphorylation and degradation of IκBα. Cells treated with Res showed a marked reduction in the phosphorylation level of IκBα at 60 min (Fig. 4c). Cells exposed to TNF-α showed enhanced phosphorylation of IκBα, and Res markedly downregulated the phosphorylation of IκBα protein in a dose-dependent manner in the concentration range 12.5–50 μM (Fig. 4d). Next, to evaluate the effects of Res on nuclear translocation of the NF-κB p65 subunit, we performed immunocytochemistry for p65, stained nuclei with DAPI, and performed western blot analysis of p65 in cytosolic and nuclear extracts. As shown in Fig. 4e, nuclear co-staining for p65 and DAPI was observed in TNF-α-treated HCT116/L-OHP cells, but nuclear translocation of p65 was inhibited by the addition of Res. In parallel with this result, p65 translocation into the nucleus of cells was significantly inhibited by Res in a dose-dependent manner and increased by TNF-α (Fig. 4f). We performed western blot analysis of MDR1 to explore whether Res-inhibited MDR1 expression was altered by TNF-α or BAY 11–7082 (NF-κB inhibitor) treatment. MDR1 protein expression was significantly increased in TNF-α-treated cells, and decreased in BAY 11-7082-treated cells. However, Res or BAY 11–7082 markedly inhibited TNF-α-induced MDR1 expression (Fig. 4g). These results suggest that Res suppresses MDR1 expression by blocking phosphorylation and degradation of IκBα and p65 translocation.
Resveratrol downregulated MDR1 through phosphorylation of AMPK in HCT116/L-OHP cells
We performed western blot analysis to examine whether, as reported in murine 3T3-L1 preadipocytes [11], Res increased phospho-AMPKα (p-AMPKα) levels in HCT116/L-OHP cells. In our study, cells exposed to Res displayed dose-dependent phosphorylation of AMPKα (Fig. 5a). p-AMPKα staining was stronger in the cytoplasm and nucleus of HCT116/L-OHP cells treated with 50 μM Res than in untreated cells (Fig. 5b). In addition, compound C, an AMPK inhibitor, significantly attenuated Res-induced AMPK activation in HCT116/L-OHP cells (Fig. 5c). To explore whether the inhibitory effect of Res on MDR1 expression was mediated by the AMPK pathway, HCT116/L-OHP cells were treated with 50 μM Res and 10 μM compound C. As shown in Fig. 5c, Res inhibited MDR1 expression, whereas in cells co-incubated with compound C plus Res a marked increase in MDR1 expression was observed. No changes were observed in cells treated with compound C only. Furthermore, compound C markedly reduced Res-inhibited nuclear translocation of p65 compared with the control. Co-incubation with compound C plus Res abrogated Res-inhibited MDR1 promoter activity (Fig. 5d). These results suggested that Res reduces the expression of MDR1 in HCT116/L-OHP cells in an AMPK-dependent manner.
Resveratrol downregulated multi-drug resistance protein 1 (MDR1) via AMP-activated protein kinase (AMPK) activity. a Western blot showing phospho-AMPK protein expression level in cells in response to various concentrations of resveratrol treatment for 24 h. GAPDH was used as a loading control. b Effect of resveratrol on phospho-AMPKα activity in HCT116/L-OHP cells. Cells were treated with 50 μM resveratrol for 24 h and then resveratrol-treated and untreated cells were stained with anti-phospho-AMPKα antibody (red fluorescence), and the nuclei were stained with DAPI (blue fluorescence). Images were obtained using fluorescence microscopy. c HCT116/L-OHP cells were treated with compound C (10 μM) and resveratrol (50 μM) for 24 h. The cells were lysed, and total, membrane, and nuclear extracts were prepared for western blot analysis with anti-phospho-AMPKα, anti-AMPKα, anti- MDR1, anti-p65, and anti-lamin B antibodies. d Effect of resveratrol and compound C on MDR1 promoter activity in HCT116/L-OHP cells. Cells were transiently transfected with an MDR1 reporter plasmid and then treated with indicated concentrations of resveratrol and 10 μM compound C for 24 h. The cells were lysed, and luciferase activity was measured. Results are representative of three independent experiments. Each value represents the mean ± SD. *p < 0.05, compared with untreated cells. # p < 0.05 compared with compound C-treated cells
An AMPK-mediated decrease in CRE transcriptional activity is responsible for inhibition of MDR1 expression by resveratrol
A previous study using AICAR, an activator of AMPK, provided evidence for a potential role for AMPK in decreasing phosphorylation of CREB in the liver [35]. We aimed to determine whether AMPK-dependent CREB activity is involved in Res-mediated MDR1 inhibition in HCT116/L-OHP cells. Treatment of HCT116/L-OHP cells with 50 μM Res for 0–90 min induced a decrease of CREB phosphorylation. This was associated with a concomitant increase in AMPK phosphorylation (Fig. 6a). To elucidate the role of AMPK in CREB-dependent MDR1 down-regulation, siRNA was used to selectively knockdown AMPKα expression in HCT116/L-OHP cells before Res treatment. Western blotting confirmed that transfection with AMPKα siRNA significantly decreased Res-induced AMPK phosphorylation, and increased Res-mediated inhibition of CREB phosphorylation and MDR1 expression, compared with the controls (Fig. 6b). Intracellular Rh123 accumulation was lower in Res-treated cells transiently transfected with AMPKα siRNA than in cells treated with Res alone (Fig. 6c). In addition, CRE transcriptional activity was decreased in Res- or H89 (PKA/CRE inhibitor)-treated cells. Co-treatment with Res plus forskolin (PKA/CRE activator) attenuated forskolin-induced CRE transcriptional activity (Fig. 6d). These results indicate that resveratrol reduces the expression of MDR1 via CRE transcriptional activity dependent on upregulation of AMPK in HCT116/L-OHP cells.
Resveratrol downregulated multi-drug resistance protein 1 (MDR1) via cAMP-responsive element (CRE) transcriptional activity. a HCT116/L-OHP cells were treated with 50 μM resveratrol for 0–90 min, and cell extracts were analyzed by western blotting with anti-phospho-AMPKα, anti-AMPKα, anti-phospho- cAMP-responsive element-binding protein (CREB) (Ser 133), and anti-GAPDH antibodies. b Cells were transfected with an siRNA control or AMPKα siRNA for 24 h before treatment with 50 μM resveratrol for 24 h. AMPKα, CREB, and MDR1 expression and phosphorylation levels of AMPKα and CREB were detected by western blotting. c Cells were transfected with an siRNA control or AMPKα siRNA for 24 h before treatment with 50 μM resveratrol for 48 h, and then exposed to 10 μg/ml rhodamine 123 (Rh123) for 90 min. The intracellular accumulation of Rh123 was measured. All data are expressed as mean ± SD for three separate experiments. **p < 0.01, compared with the untreated group and # p < 0.05, compared with the resveratrol-treated group. d Cells seeded into 12-well plates were transfected with a CRE reporter gene. After transfection, the cells were treated with 50 μM resveratrol, 10 μM H89, and 10 μM forskolin for 24 h, and then cells were harvested and luciferase activity was determined. Results are representative of three independent experiments. Each value represents the mean ± SD. *p < 0.05, compared with the untreated group and # p < 0.05, compared with the forskolin-treated group
Discussion
The emergence of MDR has made many of the currently available chemotherapeutic agents ineffective. MDR in tumor cells is often associated with over-expression of MDR1. There are many reports of the use of verapamil in clinical trials in an attempt to overcome MDR. Although this drug is considered effective for reversing resistance, it is still not used in clinical practice because of its toxicities and side effects. Therefore, identifying compounds to overcome MDR to conventional and targeted therapies remains a key challenge in the fight against cancer. Some natural compounds found in the diet and beverages have been extensively studied because of their reversal effects on many cancers through inhibition of MDR1 [36–38]. Res is one such compound and has been recognized as a potent chemopreventive agent in many cancers [39]. Res has been shown to suppress cell growth and induce apoptosis in HT29 cells resistant to etoposide [40], and to modify apoptotic regulatory proteins in lymphoma and multiple myeloma cells resistant to paclitaxel [41]. However, only a few studies have used chemoresistant cell lines, in particular drug-resistant colon cancer cells, as a model. In this study, we established the HCT116/L-OHP cell line in the presence of L-OHP. This cell line exhibited functional P-gp/MDR1 over-expression and was employed as our cell model. The concentration of Res used in our study was based on the non-toxic range determined by MTT assay. Res significantly increased the chemosensitivity of HCT116/L-OHP cells to L-OHP at levels of 0–50 μM for 48 h, but had no effect on the chemosensitivity of the parental cells. In addition, Res treatment reduced the expression of MDR1 protein and mRNA in HCT116/L-OHP cells in a dose- and time-dependent manner. To further explore the suppression of MDR1 mRNA expression by Res, we examined its effects on MDR1-luciferase activity. Res markedly repressed MDR1 promoter activity. Furthermore, we found that Res treatment increased the level of intracellular Rh123 in HCT116/L-OHP cells, and this inhibition of drug efflux occurred in a dose-dependent manner. These findings suggest that Res has an effect on the restoration of sensitivity to L-OHP by reducing not only MDR1 transcriptional and translational levels but also its function.
Evidence has accumulated that transcription of MDR1 is controlled by many transcription factors, including SP1, NF-Y, YB1, MEF1, p53, and NF-R1 [42]. Ueda et al. reported that the MDR1 promoter region contains a consensus CAAT box and two GC box-like sequences, and a protein complex consisting of NF-κB p65 and c-Fos transcription factors interacts with the CAAT promoter region to negatively regulate MDR1 promoter activity [43]. Previous studies have shown that NF-κB protects kidney proximal tubule cells from cadmium and oxidative stress by increasing MDR1 expression [44], and NF-κB is also required for TNF-α-induced MDR1 expression in hepatocytes and constitutive MDR1 expression in drug-resistant cells [8, 33]. In the present study, we determined that the reversal effect of Res is associated with inhibition of NF-κB activity, which seems to play a role in Res-induced MDR1 suppression in HCT116/L-OHP cells. Reporter gene assays for NF-κB transcription activity revealed that Res inhibited NF-κB activation. Western blot analysis demonstrated that Res inhibited phosphorylation and degradation of IκBα, and nuclear translocation of the NF-κB p65 subunit, which was confirmed by immunocytochemistry analysis. Of note, Res inhibited NF-κB-dependent MDR1 promoter activity. These findings strongly suggest a potential role for NF-κB signaling in Res-regulated MDR1 expression.
HCT116/L-OHP cells were significantly enriched for p-AMPKα expression after treatment with Res. Many studies have investigated the role of AMPK in regulating the malignant phenotype of cancer cells, which is characterized by an increase in lipid production, DNA and protein synthesis, and cell proliferation and migration [45]. However, it is unclear whether MDR1 upregulation is caused by a direct effect of AMPK signaling. Previous studies have indicated that phosphorylation of AMPK on Thr172 is prevented by PKA [46], and drug sensitivity in cancer cells over-expressing P-gp is restored by inhibiting PKA activity [47]. Moreover, the activity of the kinase-inducible domain (KID) of the CREB transactivation domain was found to be regulated by PKA [48]. More recently, Yamagishi et al. demonstrated that MDR1 expression is induced by sorcin through binding of CREB to the promoter region of MDR1 [49]. In addition, it has been reported that CREB, as a downstream target of the cAMP pathway, is activated by phosphorylation at Ser133 via both AMPK and PKA [50]. Therefore, it is possible that CREB is responsible for MDR1 suppression via effects on AMPK activity. According to our data, after treatment with Res, the time-courses of decreased CREB phosphorylation and increased AMPK phosphorylation were similar, which is in agreement with previous studies [50]. Additionally, our data obtained after compound C treatment supports the notion that Res treatment triggers AMPK activation and leads to an AMPK-dependent downregulation of MDR1. Res-mediated inhibition of CREB phosphorylation and MDR1 expression were recovered in AMPKα siRNA-transfected cells. An Rh123 accumulation assay further confirmed a role for AMPK. Furthermore, Res and H89 inhibited CRE transcriptional activity, showing that AMPK is required for CREB-dependent suppression of MDR1.
In summary, our study provides further insight into the molecular mechanism underlying Res-mediated inhibition of MDR1 expression in HCT116/L-OHP cells. We propose that Res inhibits MDR1-mediated drug efflux through the upregulation of AMPK, which inhibits the nuclear translocation of NF-κB p65 by phosphorylation and degradation of IκBα, and suppresses CRE transcriptional activity, with consequent suppression of MDR1. This study is the first to demonstrate a novel mechanism of Res-induced MDR1 suppression in HCT116/L-OHP cells, which might suggest new strategies for treating and preventing drug resistance in human colon cancer cells.
References
Stein WD. Kinetics of the multidrug transporter (p-glycoprotein) and its reversal. Physiol Rev. 1997;77:545–90.
Steinbach D, Legrand O. Abc transporters and drug resistance in leukemia: Was p-gp nothing but the first head of the hydra? Leukemia. 2007;21:1172–6.
Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, et al. Nf-kappab transcription factor induces drug resistance through mdr1 expression in cancer cells. Oncogene. 2003;22:90–7.
Baldwin Jr AS. Series introduction: the transcription factor nf-kappab and human disease. J Clin Invest. 2001;107:3–6.
Wu M, Lee H, Bellas RE, Schauer SL, Arsura M, Katz D, et al. Inhibition of nf-kappab/rel induces apoptosis of murine b cells. EMBO J. 1996;15:4682–90.
Cusack Jr JC, Liu R, Baldwin Jr AS. Inducible chemoresistance to 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothe cin (cpt-11) in colorectal cancer cells and a xenograft model is overcome by inhibition of nuclear factor-kappab activation. Cancer Res. 2000;60:2323–30.
Um JH, Kang CD, Lee BG, Kim DW, Chung BS, Kim SH. Increased and correlated nuclear factor-kappa b and ku autoantigen activities are associated with development of multidrug resistance. Oncogene. 2001;20:6048–56.
Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe S, Mills GB, et al. Induction of human mdr1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate nf-kappab signaling. Oncogene. 2002;21:1945–54.
Svensson RU, Shaw RJ. Cancer metabolism: tumour friend or foe. Nature. 2012;485:590–1.
Luo Z, Zang M, Guo W. Ampk as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6:457–70.
Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L, et al. Resveratrol induces sirt1-dependent apoptosis in 3t3-l1 preadipocytes by activating ampk and suppressing akt activity and survivin expression. J Nutr Biochem. 2012;23:1100–12.
Kim YM, Hwang JT, Kwak DW, Lee YK, Park OJ. Involvement of ampk signaling cascade in capsaicin-induced apoptosis of ht-29 colon cancer cells. Ann N Y Acad Sci. 2007;1095:496–503.
Rohlff C, Glazer RI. Regulation of multidrug resistance through the camp and egf signalling pathways. Cell Signal. 1995;7:431–43.
Scala S, Budillon A, Zhan Z, Cho-Chung YS, Jefferson J, Tsokos M, et al. Downregulation of mdr-1 expression by 8-cl-camp in multidrug resistant mcf-7 human breast cancer cells. J Clin Invest. 1995;96:1026–34.
Wartenberg M, Fischer K, Hescheler J, Sauer H. Redox regulation of p-glycoprotein-mediated multidrug resistance in multicellular prostate tumor spheroids. Int J Cancer. 2000;85:267–74.
Ziemann C, Riecke A, Rudell G, Oetjen E, Steinfelder HJ, Lass C, et al. The role of prostaglandin e receptor-dependent signaling via camp in mdr1b gene activation in primary rat hepatocyte cultures. J Pharmacol Exp Ther. 2006;317:378–86.
Walton KM, Rehfuss RP, Chrivia JC, Lochner JE, Goodman RH. A dominant repressor of cyclic adenosine 3′,5′-monophosphate (camp)-regulated enhancer-binding protein activity inhibits the camp-mediated induction of the somatostatin promoter in vivo. Mol Endocrinol. 1992;6:647–55.
Takata T, Motoo Y, Tomosugi N. Effect of Saikokeishito, a Kampo medicine, on hydrogen peroxide-induced premature senescence of normal human dermal fibroblasts. J Integr Med. 2014;12:495–503.
Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med. 2014;12:331–5.
Li Y, Fang H, Xu W. Recent advance in the research of flavonoids as anticancer agents. Mini Rev Med Chem. 2007;7:663–78.
Sun C, Hu Y, Liu X, Wu T, Wang Y, He W, et al. Resveratrol downregulates the constitutional activation of nuclear factor-kappab in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet Cytogenet. 2006;165:9–19.
Kweon SH, Song JH, Kim TS. Resveratrol-mediated reversal of doxorubicin resistance in acute myeloid leukemia cells via downregulation of mrp1 expression. Biochem Biophys Res Commun. 2010;395:104–10.
Quan F, Pan C, Ma Q, Zhang S, Yan L. Reversal effect of resveratrol on multidrug resistance in kbv200 cell line. Biomed Pharmacother. 2008;62:622–9.
Huang F, Wu XN, Chen J, Wang WX, Lu ZF. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp Ther Med. 2014;7:1611–6.
Ren Z, Wang L, Cui J, Huoc Z, Xue J, Cui H, et al. Resveratrol inhibits nf-kb signaling through suppression of p65 and ikappab kinase activities. Die Pharmazie. 2013;68:689–94.
Wang Z, Liang X, Cheng Z, Xu Y, Yin P, Zhu H, et al. Induction of apoptosis and suppression of ercc1 expression by the potent amonafide analogue 8-c in human colorectal carcinoma cells. Anti-Cancer Drugs. 2013;24:355–65.
Israf DA, Khaizurin TA, Syahida A, Lajis NH, Khozirah S. Cardamonin inhibits cox and inos expression via inhibition of p65nf-kappab nuclear translocation and ikappa-b phosphorylation in raw 264.7 macrophage cells. Mol Immunol. 2007;44:673–9.
Wang J, Wu A, Xu Y, Liu J, Qian X. M(2)-a induces apoptosis and g(2)-m arrest via inhibiting pi3k/akt pathway in hl60 cells. Cancer Lett. 2009;283:193–202.
Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of atp-dependent transporters. Nat Rev Cancer. 2002;2:48–58.
Shen K, Cui D, Sun L, Lu Y, Han M, Liu J. Inhibition of igf-ir increases chemosensitivity in human colorectal cancer cells through mrp-2 promoter suppression. J Cell Biochem. 2012;113:2086–97.
Ludescher C, Thaler J, Drach D, Drach J, Spitaler M, Gattringer C, et al. Detection of activity of p-glycoprotein in human tumour samples using rhodamine 123. Br J Haematol. 1992;82:161–8.
Deng L, Lin-Lee YC, Claret FX, Kuo MT. 2-acetylaminofluorene up-regulates rat mdr1b expression through generating reactive oxygen species that activate nf-kappa b pathway. J Biol Chem. 2001;276:413–20.
Ros JE, Schuetz JD, Geuken M, Streetz K, Moshage H, Kuipers F, et al. Induction of mdr1b expression by tumor necrosis factor-alpha in rat liver cells is independent of p53 but requires nf-kappab signaling. Hepatology. 2001;33:1425–31.
Karin M. How nf-kappab is activated: the role of the ikappab kinase (ikk) complex. Oncogene. 1999;18:6867–74.
Horike N, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Kamata H, et al. Amp-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces camp-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase c gene expression in the liver. J Biol Chem. 2008;283:33902–10.
Chung SY, Sung MK, Kim NH, Jang JO, Go EJ, Lee HJ. Inhibition of p-glycoprotein by natural products in human breast cancer cells. Arch Pharm Res. 2005;28:823–8.
Ikegawa T, Ushigome F, Koyabu N, Morimoto S, Shoyama Y, Naito M, et al. Inhibition of p-glycoprotein by orange juice components, polymethoxyflavones in adriamycin-resistant human myelogenous leukemia (k562/adm) cells. Cancer Lett. 2000;160:21–8.
Patanasethanont D, Nagai J, Matsuura C, Fukui K, Sutthanut K, Sripanidkulchai BO, et al. Modulation of function of multidrug resistance associated-proteins by Kaempferia parviflora extracts and their components. Eur J Pharmacol. 2007;566:67–74.
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20.
Hwang JT, Kwak DW, Lin SK, Kim HM, Kim YM, Park OJ. Resveratrol induces apoptosis in chemoresistant cancer cells via modulation of ampk signaling pathway. Ann N Y Acad Sci. 2007;1095:441–8.
Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin’s lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2004;3:71–84.
Ogura M, Takatori T, Tsuruo T. Purification and characterization of nf-r1 that regulates the expression of the human multidrug resistance (mdr1) gene. Nucleic Acids Res. 1992;20:5811–7.
Ogretmen B, Safa AR. Negative regulation of mdr1 promoter activity in mcf-7, but not in multidrug resistant mcf-7/adr, cells by cross-coupled nf-kappa b/p65 and c-fos transcription factors and their interaction with the caat region. Biochemistry. 1999;38:2189–99.
Thevenod F, Friedmann JM, Katsen AD, Hauser IA. Up-regulation of multidrug resistance p-glycoprotein via nuclear factor-kappab activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J Biol Chem. 2000;275:1887–96.
Brown KA, Samarajeewa NU, Simpson ER. Endocrine-related cancers and the role of ampk. Mol Cell Endocrinol. 2013;366:170–9.
Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, et al. Pka phosphorylates and inactivates ampkalpha to promote efficient lipolysis. EMBO J. 2010;29:469–81.
Parissenti AM, Gannon BR, Villeneuve DJ, Kirwan-rhude AF, Chadderton A, Gluck S. Lack of modulation of mdr1 gene expression by dominant inhibition of camp-dependent protein kinase in doxorubicin-resistant mcf-7 breast cancer cells. Int J Cancer. 1999;82:893–900.
Delghandi MP, Johannessen M, Moens U. The camp signalling pathway activates creb through pka, p38 and msk1 in nih 3t3 cells. Cell Signal. 2005;17:1343–51.
Yamagishi N, Nakao R, Kondo R, Nishitsuji M, Saito Y, Kuga T, et al. Increased expression of sorcin is associated with multidrug resistance in leukemia cells via up-regulation of mdr1 expression through camp response element-binding protein. Biochem Biophys Res Commun. 2014;448:430–6.
Branvold DJ, Allred DR, Beckstead DJ, Kim HJ, Fillmore N, Condon BM, et al. Thyroid hormone effects on lkb1, mo25, phospho-ampk, phospho-creb, and pgc-1alpha in rat muscle. J Appl Physiol. 2008;105:1218–27.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81473482), the Putuo District Committee of Science and Technology, Shanghai, China (No. 201102), and Xinglin Scholars of Shanghai University of Traditional Chinese Medicine. This research was also supported by the construct program of the key discipline of State Administration of Traditional Chinese Medicine of the People’s Republic of China.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ziyuan Wang and Long Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Z., Zhang, L., Ni, Z. et al. Resveratrol induces AMPK-dependent MDR1 inhibition in colorectal cancer HCT116/L-OHP cells by preventing activation of NF-κB signaling and suppressing cAMP-responsive element transcriptional activity. Tumor Biol. 36, 9499–9510 (2015). https://doi.org/10.1007/s13277-015-3636-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3636-3