Abstract
Breast cancer survival was associated with higher frequencies of CD8+ T cytotoxic T cells in infiltrating lymphocytes. On the other hand, the frequency of CD4+CD25+FoxP3+ regulatory T cells was inversely correlated with clinical outcomes of breast cancer. The regulation and interaction of different types of tumor-infiltrating T cells in different stages of breast cancer patients are still unclear. In this study, we examined the functions and regulations of CD8+ T cells and CD4+CD25+FoxP3+ T cells from resected tumors from 12 stage I, 24 stage II, and 20 stage III untreated breast cancer patients. We found that tumor-infiltrating CD8+ T cells from stage III patients were more refractory to T cell receptor (TCR) stimulation than those from stage I and stage II patients in terms of interferon gamma (IFN-γ) production and proliferation. On the other hand, tumor-infiltrating CD4+CD25+FoxP3+ T cells had higher proliferation in stage III tumors than in stage I and stage II tumors. In addition, we found that tumor-infiltrating CD4+CD25+ T cells can suppress CD8+ T cell inflammation ex vivo. Altogether, our data demonstrated that stage III tumors in breast cancer patients had a more immunosuppressive microenvironment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a complex and heterogeneous disease and is currently the most frequently diagnosed type of cancer in Chinese female population [1]. The 5-year survival rate of patients with breast cancer is strongly associated with the stage of discovery, from as high as 100 % in stages 0 and I to merely 22 % in stage IV [2, 3]. A growing body of literature has demonstrated that actions of tumor-infiltrating immune cells can act to either suppress or promote the development of breast cancers, depending on the specific stages and types of cancer [4, 5]. Improved patient survival and long-term positive clinical outcome have been linked with stronger antitumor immunity in tumor-infiltrating lymphocytes [6]. Studies have shown that higher frequencies of CD8+ T cytotoxic T cells in infiltrating lymphocytes are associated with better breast cancer patient survival [7, 8]. Moreover, in colorectal cancer, the presence of activated CD8+ T cells within the tumor and the peritumoral stroma may have better prognostic power than traditional staging methods in that most stage I and II patients without T cell infiltration had cancer recurrence within 5 years while stage III patients with T cell infiltration were associated with unusually long disease-free intervals [9, 10].
Effective antitumor activity mediated by CD8+ cytotoxic T cells requires adequate CD4+ T cell help [11, 12]. CD4+ T cells represent a group of heterogeneous, multifunctional lymphocytes with central roles in recruiting effector cells to the site of inflammation, providing proliferation signals to the activated T cells and B cells, modulating and structuring the surrounding microenvironment, and regulating and suppressing prolonged inflammation that may cause excessive tissue damage. Among various CD4+ T cell fractions, a particular subset with CD4+CD25+FoxP3+ expression was previously described as regulatory T cells and was shown to mediate suppression of autoimmunity in type I diabetes, rheumatoid arthritis, multiple schlerosis, and systemic lupus erythematosus [13–16]. Various mechanisms of suppression, including blocking priming of effector T cells, preventing dendritic cell maturation, and direct inhibition of tissue inflammation by production of anti-inflammatory cytokine IL-10 were observed in regulatory T cells [17]. On the other hand, regulatory T cells are thought to blunt tumor-specific immune responses [18] and were shown to induce perforin-dependent dendritic cell death in tumor-draining lymph nodes [19]. In breast cancer, studies have found that the frequency of CD4+CD25+FoxP3+ regulatory T cells is inversely correlated with clinical outcomes of breast cancer [20], possibly through inhibition of antitumor activity by CD8+ cytotoxic T cells [20]. The depletion of CD4+CD25+ T cells in a mouse model of human breast cancer was shown to reduce CD4+CD25+ T cell-mediated suppression, improve immunity, and enhance tumor regression [21].
Although the participation of tumor-infiltrating T cells in antitumor immunity has been well studied, it is still unclear why some patients had more tumor-infiltrating CD8+ T cells than others. Moreover, the interaction between tumor-infiltrating CD8+ T cells and regulatory T cells and their regulation in different breast cancer stages is unknown. To solve this problem, we designed the following study. We obtained resected tumor tissues from 12 stage I, 24 stage II, and 20 stage III breast cancer patients and examined the frequencies and functions of tumor-infiltrating T cells in different stages. We also examined the correlations and interactions between different types of tumor-infiltrating T cells in different stages. Our study provided insight into the differential regulation of intratumoral T cells in different stages of breast cancer patients.
Method
Study subjects
Peripheral blood samples and resected tumors were obtained from 12 stage I, 24 stage II, and 20 stage III untreated breast cancer patients recruited at Yuhuangding Hospital. All the patients were invasive ductal carcinoma. The demographic and clinical characteristics of the study cohort were summarized in Table 1. All patients gave written informed consent. This study was approved by Yuhuangding Hospital Research Ethics Committee.
Sample preparation
For the preparation of peripheral blood cells, 20 mL of blood was obtained by venipuncture at the upper arm at the time of diagnosis. Peripheral blood mononuclear cells were then obtained by extracting the leukocyte layer after standard Ficoll centrifugation. For isolation of tumor-infiltrating lymphocytes, fresh resected tumor samples were first examined to remove the surrounding nontumor tissues. The remaining tumor samples were then cut into fragments and placed in X-VIVO 20 medium (Lonza, Switzerland) for mechanical dissociation with the GentleMACS Dissociator (Miltenyi, Germany). Remaining connective tissues were removed by a 40 μm cell strainer. The resulting single cell suspension was then subjected to Ficoll gradient centrifugation to obtain the tumor-infiltrating lymphocytes. All cells were cultured in RPMI 1640 medium (Invitrogen, MA, USA) supplemented with L-glutamine, penicillin/streptomycin, and 10 % fetal bovine serum (FBS) during experiments.
Flow cytometry
The following monoclonal antibodies were used: anti-human CD3, CD4, CD8, CD19, CD25 (BioLegend, CA, USA), FoxP3 (eBioscience, CA, USA), and interferon gamma (IFN-γ) (BioLegend, CA, USA). An additional anti-human CD19 (mouse IgG1, kappa) were used in place of IFN-γ of the same isotype and same fluorescence to provide gating control for IFN-γ-expressing T cells. For surface staining, cells were first washed with PBS + 2 % FBS, incubated with LIVE/DEAD Fixable Violet Dead Cell Stain (Invitrogen, MA, USA) for 30 min, and then stained with surface antibodies. For intracellular IFN-γ staining, cells were first incubated in culture medium or with plate-bound anti-human CD3/CD28 monoclonal antibodies for 6 h, in the presence of GolgiStop/GolgiPlug (BD, NJ, USA), and then stained with the surface staining protocol, after which the cells were fixed and permeabilized using Cytofix/Cytoperm (BD, NJ, USA), and the anti-IFN-γ monoclonal antibody was added in diluted PermWash (BD, NJ, USA). For FoxP3 staining, the FoxP3/Transcription Factor Staining Buffer Set (eBioscience, CA, USA) was used and the staining proceeded as per manufacturer’s protocol. All samples were fixed with 2 % formalin at the end of the staining procedure and immediately analyzed on a FACSCanto II (BD, NJ, USA).
Proliferation
Cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) using the CellTrace CFSE Cell Proliferation Kit (Invitrogen, MA, USA) according to manufacturer’s protocol and stimulated with 1 μg/mL each of anti-CD3 and anti-CD28 antibodies. After 72 h, the cells were harvested and the proliferation was measured by flow cytometry.
Cell isolation and coculture
For CD4+CD25+ depletion experiments, fresh tumor-infiltrating lymphocytes were separated into three portions. Purified CD8+ T cells were obtained by magnetic negative selection using EasySep Human CD8+ T cell Enrichment Kit (Stemcell, Canada) on one portion, and whole CD4+ T cells were obtained by magnetic negative selection using EasySep Human CD4+ T cell Enrichment Kit (Stemcell, Canada). To obtain CD4+CD25+-depleted T cells, anti-human CD25 monoclonal antibody (BioLegend, CA, USA) was added in the antibody cocktail mix in the CD4 enrichment kit prior to magnetic negative selection. Purified CD8+ T cells were then cocultured with whole CD4+ T cells or CD4+CD25+-depleted T cells for 72 h, after which 1 μg/mL anti-CD3/anti-CD28 antibodies were added to the coculture to stimulate the cells and the intraceullar IFN-γ production was measured using the aforementioned method.
Statistical analysis
The significance of the differences between multiple groups was evaluated by Kruskal-Wallis one-way ANOVA followed by Dunn’s multiple comparison test. The significance of the differences between two conditions on samples from the same individual was evaluated by Wilcoxon matched-pairs signed rank test. Correlations were computed by Pearson correlation test. All statistical analyses were done using Prism software (GraphPad, CA, USA). P < 0.05 (two-tailed) is considered significant.
Result
No significant differences in the overall frequencies of tumor-infiltrating CD8+ T cells were found between different stages of breast cancer tumors
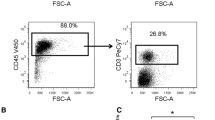
Given the positive role of CD8+ cytotoxic T cells in clinical outcomes, we first examined the frequencies of CD8+ T cells in peripheral blood and resected tumors of patients in all three stages (Fig. 1a). We found that the frequencies of intratumoral CD8+ T cells were significantly reduced compared to those in peripheral blood (Fig. 1b), consistent with previous reports demonstrating a reduction of CD8+ T cells in the tumor site of breast cancer patients [7]. When we compared the frequencies of CD8+ T cells between the three stages, no significant differences were observed in either peripheral blood or tumor.
T cell composition in peripheral blood and resected tumor of different stages of breast cancer patients. a Representative gating strategy for CD8+ T cells and CD4+CD25+FoxP3+ T cells in the peripheral blood and tumor of one stage III patient. b Frequencies of peripheral and intratumoral CD8+ T cells in stages I, II, and III patients. c Frequencies of peripheral and intratumoral CD4+CD25+FoxP3+ T cells in stages I, II, and III patients, calculated by multiplying the percentage of CD25+FoxP3+ cells and the percentage of CD4+ cells. Bar represents mean. Kruskal-Wallis one-way ANOVA. *P < 0.05. **P < 0.01. ***P < 0.001
Stage III subjects had significantly higher frequencies of intratumoral CD4+CD25+FoxP3+ T cells than stage I and II subjects
Since the numbers of CD4+CD25+FoxP3+ T cells were shown to negatively impact breast cancer prognosis, we also examined the expression of CD4+CD25+FoxP3+ T cells in study subjects (Fig. 1c). We found that the intratumoral frequencies of CD4+CD25+FoxP3+ T cells in stages II and III patients were significantly increased than the peripheral blood frequencies. Moreover, within the resected tumor tissue, stage III patients had significantly higher CD4+CD25+FoxP3+ T cells than stage I and stage II patients.
Stage III intratumoral CD8+ T cells have reduced proliferation capacity and less IFN-γ production in response to T cell receptor stimulation
IFN-γ production by tumor-infiltrating CD8+ T cells is thought to improve antitumor immunity [22]. To examine the intratumoral CD8+ T cell response in different stages of resected tumors, we examined the secretion of IFN-γ and proliferation of CD8+ T cells ex vivo. Anti-CD3/anti-CD28 monoclonal antibodies were added in some experiments to provide T cell receptor (TCR) stimulation and costimulation signals. We found that under unstimulated conditions, no significant differences were found in IFN-γ production by tumor-infiltrating CD8+ T cells in the three stages (Fig. 2b); however, after TCR stimulation by anti-CD3/CD28 monoclonal antibodies, intratumoral CD8+ T cells from stage III patients had significantly reduced frequencies of IFN-γ-producing cells than stage I and stage II patients (Fig. 2c).
Percentages of IFN-γ-producing cells in tumor-infiltrating CD8+ T cells. a Representative gating strategy for IFN-γ+ cells from one stage III patient. Control antibody was anti-human CD19 of the same fluorescence as the anti-human IFN-γ antibody. b Percentages of IFN-γ-producing tumor-infiltrating CD8+ T cells under unstimulated condition ex vivo. c Percentages of IFN-γ-producing tumor-infiltrating CD8+ T cells after being stimulated for 6 h with plate-bound anti-CD3/CD28 antibodies. Bar represents mean. Kruskal-Wallis one-way ANOVA and Dunn’s test. **P < 0.01. ***P < 0.001. N.S. not significant
We also examined the proliferation capacity of tumor-infiltrating CD8+ T cells by CFSE staining. We found that in stage III patients, the total intratumoral CD8+ T cells proliferated less than those from stage II patients when stimulated through TCR. Moreover, we also examined the proliferation of IFN-γ-producing cells and found that IFN-γ-producing CD8+ T cells from stage III patients had a significant reduction in proliferation capacity than those from stage I and stage II patients. When examining the proliferation of CD8+ T cells within each stage, we found that in stage I and stage II, the proliferating CFSElo cells were enriched in the IFN-γ+ compartment, while in stage III, no such enrichment was seen (Fig. 3b). Rather, the proliferating CFSElo cells were enriched in the CD4+CD25+FoxP3+ T cell compartment in stage III, compared to those in stage I and stage II (Fig. 3c).
Proliferation of tumor-infiltrating CD8+ T cells and CD4+CD25+FoxP3+ T cells in resected tumor. a Representative gating strategy for CFSElo cells in CD8+ T cells, IFN-γ+CD8+ T cells, and CD4+CD25+FoxP3+ T cells. Data were from one stage III patient. Cells were treated with CFSE and stimulated with anti-CD3/CD28 for 72 h before surface and intracellular staining. b Percentage of CFSElo cells in total or IFN-γ-secreting CD8+ T cells in all three stages. c Percentage of CFSElo cells in CD4+CD25+FoxP3+ T cells in all three stages. Bar represents mean. Kruskal-Wallis one-way ANOVA and Dunn’s test. *P < 0.05. **P < 0.01. ***P < 0.001
Intratumoral CD4+CD25+ T cells could suppress IFN-γ production from tumor-infiltrating CD8+ T cells ex vivo
Having observed the reduction in the frequency, IFN-γ secretion, and proliferation of tumor-infiltrating CD8+ T cells and the increase in the frequency of CD4+CD25+FoxP3+ T cells in stage III breast cancer patients, we wondered whether CD4+CD25+FoxP3+ T cells in breast cancer tumors have regulatory capacity in suppressing tumor-infiltrating CD8+ T cell responses. We first examined the correlation between CD8+ T cell responses and the frequencies of CD4+CD25+FoxP3+ T cells. We found that in stage III subjects, the frequency of tumor-infiltrating CD8+ T cells and the percentage of IFN-γ-producing CD8+ T cells were negatively correlated with the frequency of tumor-infiltrating CD4+CD25+FoxP3+ T cells (Fig. 4a). No significant correlation in stages I and II was observed.
The association between CD4+CD25+FoxP3+ T cells and CD8+ T cell inflammation in all three stages. a Correlation between the percentages of tumor-infiltrating CD4+CD25+FoxP3+ T cells and the frequency, IFN-γ expression, and proliferation of tumor-infiltrating CD8+ T cells in all three stages. Pearson correlation test. If P < 0.05, a standard line of interpolation is drawn. b IFN-γ expression in tumor-infiltrating CD8+ T cells after incubation with either autologous whole CD4+ T cells or autologous CD4+CD25+-depleted T cells at 1:1 ratio for 72 h. Cells were then stimulated by anti-CD3/CD28 antibodies and IFN-γ production measured by flow cytometry. Wilcoxon matched-pairs signed-rank test. *P < 0.05
CD4+CD25+FoxP3+ regulatory T cells were described in numerous studies to have suppressive effects on antitumor responses [22, 23]. To examine whether the tumor-infiltrating CD4+CD25+FoxP3+ T cells could suppress IFN-γ production from CD8+ T cells ex vivo, we cocultured purified tumor-infiltrating CD8+ T cells with either whole CD4+ T cells or CD4+CD25+-depleted T cells at 1:1 ratio. The IFN-γ production from tumor-infiltrating CD8+ T cells after TCR stimulation was then measured. The number of tumor-infiltrating T cells isolated from smaller tumors, especially those in earlier stages, was limited in quantity. Therefore, we were only able to perform this experiment with samples from four stage II patients and eight stage III patients. We found that in stage III patients, the tumor-infiltrating CD8+ T cells cocultured with tumor-infiltrating CD4+CD25+-depleted T cells had significantly higher levels of IFN-γ production after TCR stimulation (Fig. 4b). The same trend is observed in four out of four stage II patients.
Together, these data demonstrated that the frequency of tumor-infiltrating CD4+CD25+FoxP3+ T cells was negatively correlated with the frequency and IFN-γ production of tumor-infiltrating CD8+ T cells. In addition, intratumoral CD4+CD25+ T cells were able to suppress IFN-γ production from autologous tumor-infiltrating CD8+ T cells ex vivo.
Discussion
Understanding the functions and interactions of different tumor-infiltrating lymphocytes is crucial for the development of antitumor immunotherapies. Inflammation seems to promote tumorigenesis under certain conditions, while in other situations, infiltration of lymphocytes were shown to suppress tumor growth [24, 25]. This highlights the fact that efficacy of immune responses in tumor suppression are highly dependent on the specific types of cancer and the time of immune activation. In this study, we focused on breast cancer and examined the role of intratumoral lymphocytes in different tumor stages. First, we observed that similar to that in other cancers, resected tumor from breast cancer patients of all stages had significantly reduced numbers of tumor-infiltrating CD8+ T cells compared to peripheral blood. This reduction was only observed on CD8+ T cells but not on CD4+CD25+FoxP3+ T cells. We also observed an upregulation of intratumoral CD4+CD25+FoxP3+ T cells in stage III breast cancer patients. These data suggested a more tolerogenic immune profile in stage III cancer. When we examined the IFN-γ production, we found that the IFN-γ production as well as proliferation by tumor-infiltrating CD8+ T cells after TCR stimulation was reduced in stage III subjects compared to stage I and stage II patients, suggesting that tumor-infiltrating CD8+ T cells in stage III patients were more refractory to TCR stimulation. The tumor-infiltrating CD4+CD25+FoxP3+ T cells, on the other hand, were proliferating more robustly in stage III cancer patients, which may help explain the upregulation of CD4+CD25+FoxP3+ T cells in stage III tumors. Together, these data demonstrated that the ex vivo tumor microenvironment tended to be more suppressive in stage III tumors than stage I and stage II tumors.
CD4+CD25+FoxP3+ surface expression is frequently used as markers for regulatory T cells [26, 27]. We found that the frequency of intratumoral CD4+CD25+FoxP3+ T cells was negatively correlated with the frequency of intratumoral CD8+ T cells and their IFN-γ production in stage III cancer. These correlations may simply reflect the tendency that an overall tolerogenic immune profile tends to be enriched in regulatory T cells and deficient in effector CD8+ T cells. To assess whether the intratumoral CD4+CD25+FoxP3+ T cells in effect possessed regulatory activity to intratumoral CD8+ T cells, we cocultured CD8+ T cells from resected tumors with whole CD4+ T cells or with CD4+CD25+-depleted T cells and found that CD8+ T cells cocultured with CD4+CD25+-depleted T cells had increased IFN-γ production, suggesting that intratumoral CD4+CD25+ T cells can suppress intratumoral CD8+ T cell function ex vivo. Previously, regulatory T cells was found to suppress tumor-specific CD8+ T cell and CD4+ T cell effector functions and was thought to account for potential failures in antitumor immunotherapy [26, 28, 29]. These results further demonstrated the importance of diagnosing and treating breast cancer in earlier stages.
Some remaining issues need to be addressed in future experiments. First, we noticed that the CD8+ T cell proliferation after TCR stimulation is enriched in IFN-γ-producing cells in stage I and stage II but not in stage III. The effector function of non-IFN-γ-producing CFSElo CD8+ T cells and their impact on tumorigenesis need to be studied. Second, since stage III tumors in general are larger in size (Table 1), the lack of CD8+ T cell activation and proliferation might be due to a state of hypoxia observed in larger tumors [30], but the precise mechanism of higher immune suppression in stage III requires further study. Third, tumor-infiltrating lymphocytes were known to modulate tumor microenvironment and promote or inhibit local immune activations [5]. The impact of tumor-infiltrating T cells in shaping the local inflammation in different stages of cancer needs to be examined.
References
Fan L, Strasser-Weippl K, Li J-J, St Louis J, Finkelstein DM, Yu K-D, et al. Breast cancer in China. Lancet Oncol. 2014;15:e279–89.
Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–49.
Gloeckler Ries LA. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2003;8:541–52.
DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212.
Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22.
Ascierto ML, De Giorgi V, Liu Q, Bedognetti D, Spivey TL, Murtas D, et al. An immunologic portrait of cancer. J Transl Med. 2011;9:146.
Mahmoud SMA, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AHS, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55.
Mahmoud S, Lee A, Ellis I, Green A. CD8+ T lymphocytes infiltrating breast cancer: a promising new prognostic marker? Oncoimmunology. 2012;1:363–4.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4.
Galon J, Franck P, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205.
Bos R, Marquardt KL, Cheung J, Sherman LA. Functional differences between low- and high-affinity CD8(+) T cells in the tumor environment. Oncoimmunology. 2012;1:1239–47.
Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–77.
Lindley S, Dayan CM, Bishop A, Roep BO, Peatman M, Tree TIM. Defective suppressor function in CD4+CD25+ T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9.
Horwitz DA. Regulatory T, cells in systemic lupus erythematosus: past, present and future. Arthritis Res Ther. 2008;10:227.
Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9.
Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85.
Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–30.
Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–67.
Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, et al. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–78.
Watanabe MAE, Oda JMM, Amarante MK, Cesar Voltarelli J. Regulatory T cells and breast cancer: implications for immunopathogenesis. Cancer Metastasis Rev. 2010;29:569–79.
Knutson KL, Dang Y, Lu H, Lukas J, Almand B, Gad E, et al. IL-2 immunotoxin therapy modulates tumor-associated regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in neu-transgenic mice. J Immunol. 2006;177:84–91.
Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21:3–10.
Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfl V, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical utcome. Cancer Res. 2009;69:2000–9.
Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–83.
Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28:4531–8.
Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–74.
Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64.
Chen M-L, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–24.
Nishikawa H, Jäger E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–11.
Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–47.
Acknowledgments
We thank Dr. Zhan at BGC Biotechnology Research Center for assisting in data analysis.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Shiguang Zhu and Jun Lin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, S., Lin, J., Qiao, G. et al. Differential regulation and function of tumor-infiltrating T cells in different stages of breast cancer patients. Tumor Biol. 36, 7907–7913 (2015). https://doi.org/10.1007/s13277-015-3507-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3507-y