Abstract
Temozolomide (TMZ) is widely used for treating glioblastoma (GBM), which can effectively inhibit the GBM growth for some months; however, it still could not prevent the invariable recurrence of GBM. The existence of glioma stem cells (GSCs) was considered to be a key factor. But TMZ has poor effects on GSCs. Recently, demethoxycurcumin (DMC) has been shown to display anti-tumor activities in malignant gliomas. However, its effects and the potential mechanisms on GSCs were still unclear. Our study showed that DMC was prior to TMZ on resulting in a significant increase in GSC apoptosis and a marked inhibition of cell growth in vitro. And combined treatment of DMC and TMZ showed more significant anti-GSC effects. Further research into the underlying mechanism demonstrated that this novel combinatorial regimen leads to changes of multiple cell signaling pathways including reactive oxygen species (ROS) production and caspase-3 signaling mitochondria-related apoptosis activation as well as inactivation of JAK/STAT3 signaling pathway. Taken together, our data demonstrate that the anti-GSC effects of DMC are better than TMZ, and combined treatment of DMC and TMZ has much stronger effects on GSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is the most malignant subtype of gliomas, which is aggressive, highly invasive, and neurologically destructive. The first choice is normally treated by surgery, followed by radiotherapy and chemotherapy. And the first-line combined chemotherapy consists of the temozolomide (TMZ). However, with a combination of surgery, chemotherapy, and radiotherapy, the median survival duration of patients with GBM was observed as only 2∼5 years in a large randomized trial [1, 2].
TMZ is a 3-methyl derivative of mitozolomide that leads to the formation of O6-methylguanine, which results in GBM cell apoptosis, autophagy, and proliferation inhibition [3]. However, glioma stem cells (GSCs) were reported to be not sensitive to TMZ, which could not effectively inhibit the proliferation and induce apoptosis of GSCs [4]. Although GSCs were only very small part cells in gliomas, they are crucial for glioma malignancy and are responsible for the therapy resistance and recurrence of GBM. GSCs have been demonstrated to possess high proliferation activity and survivability and present the characteristics with increasing neurosphere formation ability and tumorigenicity [5]. However, until now, there are no effective drugs for the clinical treatment of GSCs. Previous research by us revealed that TMZ had poor effects on the proliferation, invasion, and apoptosis of GSCs [6]. In considering glioblastoma cell TMZ resistance, we believe that seeking a break from GSCs may become a new direction for the TMZ treatment resistance of GBM.
In the present study, we isolated GSCs from three primary glioblastoma tissue cell cultures and found that demethoxycurcumin (DMC) might be a prospective drug for treating GSCs. DMC, one of three major components in curcuminoids including curcumin, DMC, and bisdemethoxycurcumin (BDMC) [7], has been reported to have the most potent anti-tumor activities on glioma cells among the three components [8]. However, its function on GSCs was still unknown. In this study, our data showed that DMC but not TMZ could effectively inhibit the growth of GSCs and induce parts of GSC apoptosis. Moreover, combined treatment of DMC and TMZ effectively conquered drug resistance in GSCs in vitro and induced more significant GSC apoptosis. Further research on the mechanism showed that DMC functioned synergistically with TMZ on cell proliferation inhibition and apoptosis accompanied by modulating multiple signaling pathways including reactive oxygen species (ROS) production, caspase-3 signaling mitochondria-related apoptosis activation as well as inactivation of JAK/STAT3 signaling pathway.
Materials and methods
Primary glioblastoma cell cultures
Human glioblastoma tissues were obtained after informed consent from adult patients diagnosed with WHO grade IV glioma. Primary glioblastoma cell cultures were obtained after mechanical dissociation according to the technique described by Darling [9]. The cells were subcultured in 2 % fetal calf serum (FCS) to prevent the growth of contaminating rodent fibroblasts for 1 week before in vitro analyses, after which cells were cultured in 10 % FCS and antibiotics. Glial origin was confirmed by morphology and staining with the anti-glial fibrillary acidic protein (GFAP) monoclonal antibody (mAb) clone 6F2 (Dako, Glostrup, Denmark). All of the experiments on these cells were performed before passage 5.
Magnetic cell separation of CD133-positive cells
Cells were dissociated and resuspended in phosphate-buffered saline (PBS) containing 0.5 % bovine serum albumin and 2 mmol/l ethylenediaminetetraacetic acid (EDTA). CD133/1 MicroBeads (Miltenyi Biotech) were used for magnetic labeling. Positive magnetic cell separation (MACS) was performed using a series of several MACS columns. Cells were stained with CD133/2-PE (Miltenyi Biotech) and analyzed on a BD FACSCalibur. Tumor cells converted to neurosphere cultures were then grown in stem cell-permissive DMEM/F12 medium supplemented with 20 ng/ml each of human recombinant epidermal growth factor, human recombinant basic fibroblast growth factor (both from R and D Systems), and human leukemia inhibitory factor (Chemicon), in addition to 2 % B27 (Life Technologies). These culture conditions enabled tumor cells to retain the molecular characteristics of the primary tumor, with only minor changes in differentiation, expression pattern, and genetic mutation profile.
Cell growth assay
To assess the effects of TMZ and/or DMC on the growth of CD133-positive GSCs, CD133-positive cells were plated at 5 × 103 cells per well in 96-well plates with six replicate wells at the indicated concentrations of TMZ and/or DMC. At certain time points (12, 24, 36, 48, and 72 h, respectively) after transfection, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to analyze the cell proliferation as described previously [10].
Cell cycle analysis
CD133-positive cells treated with TMZ and/or DMC were trypsinized and subsequently fixed with ice-cold ethanol (70 %) for at least 1 h. After extensive washing, the cells were suspended in Hank’s Balanced Salt Solution (HBSS) containing 50 μg/ml propidium iodide (PI) (Sigma-Aldrich) and 50 μg/ml of RNase A (Boehringer Mannheim, Indianapolis, IN), incubated for 1 h at room temperature, and analyzed by FACScan (Becton Dickinson, San Jose, CA). The cell cycle was analyzed by ModFit LT software [11].
Annexin V/PI apoptosis assays
After 24 and 48 h treatment with TMZ and/or DMC, the apoptosis ratio of CD133-positive cells was analyzed by Annexin V/FITC and PI double stain assay via using Annexin V FITC Apoptosis Detection Kit (BD Biosciences, San Diego, CA) according to the manufacturer’s instructions. Annexin V/FITC and propidium iodide double stain were used to evaluate the percentages of apoptosis. Annexin V− and PI− cells were used as controls. Annexin V+ and PI− cells were designated as apoptotic and Annexin V+ and PI+ cells displayed as necrotic. Tests were repeated in triplicate [12].
Caspase-3 activity assay
The activity of caspase-3 was determined using the Caspase-3 activity kit (Beyotime Institute of Biotechnology, Haimen, China). To evaluate the activity of caspase-3, cells were homogenized in 100 ml reaction buffer (1 % NP-40, 20 mM Tris–HCl (pH 7.5), 137 mM Nad, and 10 % glycerol) containing 10 ml caspase-3 substrate (Ac-DEVD-pNA) (2 mM) after all treatments. Lysates were incubated at 37 °C for 2 h. Samples were measured with an ELISA reader at an absorbance of 405 nm.
NF-κB transcription factor assay
After 24 and 48 h treatment with TMZ and/or DMC, the NF-κB transcription factor was assessed by the NoShift II NF-κB transcription factor assay kit (NOvagen, USA) and confocal microscopy [13].
Western blot analysis
To determine the levels of protein expression, soluble proteins were isolated with a lysis buffer (137 mM NaCl, 15 mM EGTA, 0.1 mM sodium orthovanadate, 15 mM MgCl2, 0.1 % Triton X-100, 25 mM MOPS, 100 μM phenylmethylsulfonyl fluoride, and 20 μM leupeptin, adjusted to pH 7.2). One-dimensional sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed with a corresponding gel concentration using the Laemmli discontinuous buffer system (Bio-Rad Laboratories, Richmond, CA). The electrophoresed proteins were transferred to a polyvinylidene difluoride membrane and subjected to immunoblot analysis with antibodies for c-Myc, CDC25A, Bcl-2, and Bcl-xL (used at a 1/200 dilution, Santa Cruz Biotechnology). The reaction was detected with enhanced chemiluminescence (Amersham Life Science, Arlington Heights, IL). The membranes were reblotted with a GAPDH antibody (1/2000, Santa Cruz Biotechnology) after washing to verify equal loading of the gel.
Quantification by ELISA detection
The Cell Apoptosis ELISA Detection Kit (Roche, Palo Alto, CA) was used to detect histone-DNA in GSCs with different treatments according to the manufacturer’s protocol. And the PathScan® Phospho-Stat3 (Tyr705) Sandwich ELISA kit was employed to detect p-STAT3 (Cell Signaling Technology Inc., Danvers, MA) according to the manufacturer’s advice. Briefly, after indicated treatments, cells were then lysed using ice-cold lysis buffer and bound to immobilized first detection antibody. Subsequently, the peroxidase-conjugated secondary antibody was added to each well. After addition of substrate for peroxidase, the spectrophotometric absorbance of the each sample was determined at 405 nM for histone-DNA detection and 450 nm for p-STAT3 detection.
ROS production detection
GSCs were loaded with 1 μM fluorescent dye dihydrorhodamine for 2 h before treatment, which reacts with ROS in cells and results in a change of fluorescence. After indicated treatments, GSCs were suspended in PBS on ice and fixed by 70 % ethyl alcohol in −20 °C. The changes in fluorescence were detected by FACS analysis.
Statistical analysis
All tests were performed using SPSS Graduate Pack 11.0 statistical software (SPSS, Chicago, IL). Descriptive statistics, including the mean and SE, in addition to one-way ANOVAs were used to determine significant differences. P < 0.05 was considered statistically significant.
Results
DMC or combination treatment of DMC and TMZ efficiently inhibits the proliferation of GSCs
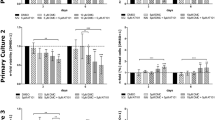
Here, we first tested the effects of DMC and TMZ co-administration on cell viability of GSCs. According to previous research by Gong et al., the GSC proliferation is minimally affected by low-dose TMZ [14]. Thus, to detect whether DMC can sensitize TMZ on its anti-GSC effects, we chose 100 μM and 200 μM TMZ for GSC treatment. As shown in Fig. 1a, low-dose TMZ as a single agent had minor effects on cell growth of GSCs-1 and GSCs-2 and had no effects on GSCs-3, which was conversely with a faint increase. Interestingly, 50 or 100 μM DMC as a single agent showed amazing anti-tumor effects on GSCs, which induced an inhibiting rate of cell growth about 50.2 and 77.7 % at 72 h, respectively (Fig. 1b). More interestingly, DMC plus TMZ caused more obvious synergistic effects. In total, 50 and 100 μM DMC inhibited GSCs-1 cell viability by about 53.1 and 79.3 % at 72 h, and 100 and 200 μM DMC inhibited cell viability by about 7.1 and 1.7 %, whereas the combination of these two caused a synergistic 73.1 % (50 μM DMC + 100 μM TMZ), 70.3 % (50 μM DMC + 200 μM TMZ), 93.3 % (100 μM DMC + 100 μM TMZ), and 95.3 % (100 μM DMC + 200 μM TMZ) loss of cell viability (Fig. 1c). The synergistic effects of DMC and TMZ co-administration were also seen in GSC lines, GSCs-2 and GSCs-3. These results suggest that co-administration of DMC and TMZ has potent inhibitory effects on cell proliferation of GSCs, especially the combined treatment of 100 μM DMC and 100 or 200 μM TMZ which almost totally inhibit the proliferation of GSCs.
Effects of co-administration of DMC and TMZ on cell proliferation of GSCs. The cell lines 1, 2, and 3 of GSCs were treated with an indicated concentration of TMZ (a), DMC (b), and co-administration (c). Cell viability was measured using MTT assay 24, 48, and 72 h after treatment as measured, and then the cell growth inhibition rates were further calculated
Effects of DMC and TMZ treatment on the cell cycle inhibition of GSCs
Cell growth and proliferation is dependent on their progression through the cell cycle including GSCs. Thus, the effects of DMC and TMZ treatment on proliferation inhibition of GSCs were always related with the change of cell cycle. In most glioma cells, TMZ induced cell cycle arrest at G2/M. However, the combined effects of DMC and TMZ treatment on GSC cell cycle were still unclear. To gain insights into the mechanism of the anti-proliferative activity of co-administration of DMC and TMZ, its effect on cell cycle distribution was determined via a flow cytometry assay. The cells were harvested at the indicated time point and analyzed to determine cell cycle distributions. As shown in Fig. 2a, a 48-h exposure of GSCs to 100 and 200 μM TMZ resulted in no significant accumulation of cell cycle in G0/G1 or G2/M phase, compared to the control groups (P > 0.05). However, DMC alone should have an obvious accumulation of cell cycle in G0/G1 phase. It was also observed that there was an increased percentage of sub-G1 phase form the concentration of 50 to 100 μM DMC, which implied that DMC could induce GSC apoptosis (Fig. 2b). Moreover, co-administration of DMC and TMZ showed a more obviously significant increase in sub-G1 apoptotic fraction accompanied by significant increase in G0/G1 phase, compared to either DMC or TMZ treatment alone (Fig. 2c).
Effects of DMC and TMZ treatment on the cell apoptosis of GSCs
Above results demonstrated that co-administration of DMC and TMZ significantly increased sub-G1 phrase in GSCs, which implied that such combined effects could induce GSC apoptosis. To determine whether DMC and/or TMZ led to GSC apoptosis, three different apoptosis assays were used including terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, histone-DNA ELISA, and Annexin V/PI [15]. All cell lines of GSCs were exposed to 50 μM DMC and 100 μM TMZ for 24 and 48 h. We demonstrated that DMC but not TMZ could induce GSC apoptosis. Co-administration of DMC and TMZ significantly increased the cell apoptosis, compared to DMC or TMZ alone by TUNEL staining and histone-DNA ELISA (Fig. 3a, b). In Annexin V-PI staining detection, 100 μM TMZ alone-treated cells showed no significant apoptotic cells, compared to the control (P > 0.05) (Fig. 3c). Apoptosis was obviously induced by 50 μM DMC compared with that in untreated controls and 100 μM TMZ group (P < 0.05). Apoptosis rate in respect to the control group were 24.53 ± 3.37 % (24 h) and 35.43 ± 6.73 % (48 h) (P < 0.05). Moreover, when cells were treated with co-administration of DMC and TMZ, the apoptosis effect was obviously increased, which reached 37.90 ± 6.17 % for 24 h and 49.93 ± 5.31 % for 48 h. Collectively, these data suggested DMC and TMZ combined treatment had notable effects on inducing GSC apoptosis.
Effects of DMC and TMZ treatment on reactive oxygen species production and caspase-3 signaling cascade activation
Sandur et al. reported that DMC could effectively inhibit the cell proliferation by increasing ROS production [16]. Lee et al. found that DMC could enhance the production of ROS, the release of mitochondrial cytochrome c (Cyt c), and the subsequent activation of caspase-3, which resulted in human renal carcinoma [17]. Oliva et al. also showed that low ROS production and tighter mitochondrial coupling are the important factors for the chemoresistance to TMZ in glioma [18]. Although no reports until now which demonstrate that TMZ or DMC has effects on the ROS production in GSCs, we still speculate increased production of ROS by DMC and TMZ are one of the key factors responsible for GSC apoptosis. In this study, we found that TMZ enhanced DMC-induced ROS production in GSCs (Fig. 4a). The antioxidant N-acetyl-l-cysteine (NAC) could eliminate ROS effectively according to the concentration [19]. The following treatment with antioxidant NAC, which blocked 50 μM DMC and 100 μM TMZ co-administration-induced ROS production (Fig. 4a), resulted in decreased cell growth inhibition rate (Fig. 4b) and inhibited GSC apoptosis (Fig. 4c) induced by DMC and TMZ combined treatment, suggesting that ROS production was important for GSC apoptosis after the co-administration. Previous study has shown that curcumin induces ROS production to activate caspase-3 signaling cascade, leading to a complex crosstalk between autophagy and apoptosis [20]. We then analyzed the Cyt c release and caspase-3 activation. As shown in Fig. 4, DMC was effective in inducing the release of Cyt c (Fig. 4d), and an increase in caspase-3 activity (Fig. 4e) was observed after 12 h treatment with DMC. The effect of TMZ alone on caspase-3 activation was slight; however, it was dramatically enhanced after combined treatment with DMC. Importantly, Z-DEVDfmk (short for ZD), known as a specific caspase-3 inhibitor, largely inhibited co-administration-induced GSC proliferation inhibition (Fig. 4f) and apoptosis (Fig. 4g), suggesting that caspase-3 activation and apoptosis might be critical for the function of co-administration on GSCs. These data suggest that ROS-dependent activation of caspase-3 is involved in GSC apoptosis induced by DMC and TMZ co-administration.
Effects of DMC and TMZ on ROS production, Cyt c release, and caspase-3 activity in GSCs. GSCs were treated for 6 and 12 h with 50 μM DMC, 100 μM TMZ, a combination of both, or a combination plus NAC. ROS production is shown in (a). GSCs were treated for 24 and 48 h with a combination of 50 μM DMC and 100 μM TMZ, or a combination plus NAC. The cell growth inhibition rate was analyzed by MTT assay (b), and the cell apoptosis was analyzed by a fluorogenic assay (c). Then, cells were treated for 12 h with 50 μM DMC, 100 μM TMZ, or a combination of both. Expression level of Cyt c was measured and quantified by Western blots (d). Caspase-3 activity was analyzed by a fluorogenic assay (e). Finally, GSCs were treated for 24 and 48 h with a combination of 50 μM DMC and 100 μM TMZ, or a combination plus Z-DEVDfmk. The cell growth inhibition rate was analyzed by MTT assay (f), and the cell apoptosis was analyzed by a fluorogenic assay (g)
Effects of DMC and TMZ treatment on the JAK/STAT3 pathway inactivation
JAK-STAT3 signaling pathway is a common pathway which plays important roles on cell proliferation, differentiation, and apoptosis. Many STAT3 target genes including c-Myc, CDC25A, Bcl-xL, and Bcl-2 are key components for regulating cell cycle progression. Thus, STAT3 activation is important to cell growth and apoptosis [21]. In order to verify the involvement of the JAK-STAT3 signaling pathway in GSCs, the expression of phosphorylated STAT3 (p-STAT3) was detected by ELISA after 2 h treatment with DMC and TMZ. We observed a dose-dependent reduction of p-STAT3 by DMC, but slight effects were induced by TMZ. Expression of p-STAT3 levels, in respect to control groups, declined to 31.33 ± 3.16 % after treatment with 50 μM DMC and to 4.43 ± 0.71 % after treatment with 100 μM DMC (Fig. 5a). And the co-administration of DMC and TMZ resulted in a more significant effect on the reduction of p-STAT3. Further, we examined the long-lasting inhibition effects of co-administration of DMC and TMZ on p-STAT3 of GSCs. As shown in Fig. 5b, p-STAT3 levels remain low for up to 24 h in cells co-treated with DMC and TMZ. To confirm the important role of p-STAT3 in DMC- and TMZ-induced cell apoptosis and proliferation inhibition, a novel selective inhibitor of p-STAT3 [22], Cucurbitacin I (JSI-124) purchased from Sigma-Aldrich (St. Louis, MO), was used to inhibit the p-STAT3 of GSCs for detecting effects of p-STAT3 downregulation on the cell proliferation and apoptosis of GSCs. Our results showed the phosphorylation of STAT3 could be almost completely inhibited by the specific p-STAT3 inhibitor JSI-124 (10 μM) (Fig. 5c). And downregulation of p-STAT3 induced obvious cell proliferation inhibition (Fig. 5d) and apoptosis increase (Fig. 5e). The results suggested that induction of apoptosis and inhibition of JAK/STAT3 signaling could be caused by co-administration of DMC and TMZ in human GSCs; in another way, the effect of TMZ on inhibition of JAK/STAT3 pathway is relatively weak in GSCs, while the effect of DMC was obviously superior to TMZ.
Effects of DMC and TMZ on the phosphorylated STAT3 levels in GSCs. a GSCs were treated with DMC (0, 50, and 100 μM) and TMZ (0, 100, and 200 μM) for 2 h, and then the p-STAT3 levels were determined by ELISA. b GSCs were co-treated with DMC and TMZ for 2 h, and then co-administration of DMC and TMZ were removed. The restoration of p-STAT3 levels in GSCs were determined by ELISA for 6, 12, and 24 h. c GSCs were incubated with or without different concentrations of JSI-124 for 6 h. Then, the p-STAT3 levels were measured by ELISA. The cell growth inhibition rate was analyzed by MTT assay (d), and the cell apoptosis was analyzed by a fluorogenic assay (e)
Effects of DMC and TMZ treatment on the downstream consequences of STAT3 proteins
The downstream consequences of STAT3 proteins including c-Myc, CDC25A, Bcl-xL, and Bcl-2 in GSCs were further determined by Western blotting after 24-h exposure to 50 μM DMC and 100 μM TMZ. Ji et al. reported that the activated STATs could effectively stimulate transcription of c-Myc and CDC25A genes resulted in activating G1 to S cell cycle progression [23]. As shown in Fig. 6a, our data showed that reduced expression of c-Myc and CDC25A was observed after 24 h of DMC (50 or 100 μM) or co-administration of DMC (50 or 100 μM) and TMZ (100 or 200 μM) treatment; however, no effects were observed when GSCs were given TMZ treatment alone (Fig. 6b).
Effects of DMC and TMZ on the STAT3 downstream proteins. GSCs were treated with DMC (50 or 100 μM) or co-administration of DMC (50 or 100 μM) and TMZ (100 or 200 μM) (a) or TMZ (100 or 200 μM) (b) for 24 h, and then the expression of cell cycle regulating c-Myc and CDC25A was analyzed by Western blot. And the following alterations of Bcl-2 and Bcl-xL expression were also analyzed by Western blot after treated with DMC (50 or 100 μM) (c), TMZ (100 or 200 μM) (d), and co-administration of DMC and TMZ (e)
Bcl-2 and Bcl-xL, blocking cell death and enhancing cell survival by inhibiting apoptosis, are two important proteins of the Bcl-2 family [24]. The high expression of Bcl-2 and Bcl-xL proteins contributed to the resistance of anticancer therapy on GBM [25]. To confirm that the effects of DMC and TMZ on the alterations of Bcl-2 and Bcl-xL expression, Western blot assay was used to measure the levels of Bcl-2 and Bcl-xL. In this study, we observed that there was a clear reduction in Bcl-2 and Bcl-xL levels after DMC treatment alone. The expression of Bcl-2 protein decreased 47 % vs. control group after 50 μM DMC treatment and 73 % vs. control group after 100 μM DMC treatment; while the expression of Bcl-xL protein decreased 57 % vs. control group after 50 μM DMC treatment and 77 % vs. control group in 100 μM DMC (Fig. 6c). Compared to control cells, a slight decrease in Bcl-xL expression (17 and 13 %) but not obvious Bcl-2 expression was also observed in GSCs exposed to TMZ (100 and 200 μM) alone (Fig. 6d). Moreover, co-administration of DMC and TMZ induced a more significant decrease on Bcl-2 and Bcl-xL expression, compared to DMC or TMZ treatment alone (Fig. 6e). The results clearly showed the involvement of the STAT3 pathway in the growth inhibition and apoptosis of GSCs by co-administration of DMC and TMZ.
Discussion
Until now, glioblastomas are incurable malignant tumors. The existence of a few GSCs in glioblastomas may be the important factor for difficult cure and recurrence. TMZ has been used as the first-line drugs in the clinic treatment for glioblastomas. However, it has been identified to have poor effects on GSCs. Therefore, there is an urgent need to enhance the chemosensitivity of TMZ on GSCs, which may result in prolonging the survival time for patients. In this study, we first reported that co-administration of DMC and TMZ had a stronger synergistic anti-tumor effect on GSCs, and in fact, DMC showed much stronger anti-GSC effects than TMZ in vitro.
Previous research has shown that DMC has anti-tumor activities in a variety of tumors. Shieh et al. reported that DMC has potent effects against breast cancers and greatly reduces the survival of triple-negative breast cancer cells [26]. Ni et al. found that when prostate cancer cells were treated with DMC, all the tumor characteristics including cell proliferation, migration, and invasion were effectively inhibited [27]. Hsu et al. pointed out that DMC could induce colon cancer cell apoptosis [28]. Huang et al. reported that DMC could effectively inhibit the cell growth and induce apoptosis of GBM 8401 cells [13]. Luthra et al. showed that DMC could induce Bcl-2 mediated G2/M arrest and apoptosis of GBM U87 cells [8]. However, until now, the function and mechanisms of DMC on GSCs are still unknown. In this study, our data showed that DMC could efficiently inhibit the cell growth and induce cell apoptosis in GSCs in vitro. And we identified that its effects on GSCs were more obvious than TMZ in vitro. In contrast, TMZ alone showed poor anti-tumor effects on GSCs, which almost had no effects on GSC proliferation and apoptosis. However, co-administration of DMC and TMZ demonstrated a stronger synergistic anti-tumor effect on GSCs, resulting in more obvious cell proliferation inhibition and increased apoptosis.
Cell cycle control plays a critical role on the regulation of tumor cell proliferation. Many cytotoxic agents arrest cell cycle at the G0/G1 or G2/M phase [29]. In the present study, our data from flow cytometry analysis showed either 50 or 100 μM DMC could induce a significant G0/G1 phase arrest, while 100 or 200 μM TMZ resulted in slight effects on cell cycle phase of GSCs. Further, we found that co-administration of DMC and TMZ-induced apoptosis was correlated with an increased production of ROS, the enhanced activity of caspase-3, and the over-activated JNK signaling. ROS has been implicated in diverse processes of various cancers, and generally the increase of ROS plays a crucial role in cell growth and apoptosis of cancer. Moreover, ROS has been considered as the most significant mutagens in stem cells, whose accumulation blocked self-renewal [30]. Recently, Lee et al. studies have demonstrated that induction of apoptosis by DMC in human renal carcinoma Caki cells was through ROS production [17]. Here, we identified DMC could also increase ROS production in GSCs. Previous research by Jiang et al. showed TMZ could remarkably increase ROS production to trigger a robust increase in cell apoptosis of glioblastoma cells [31]. In this study, no such obvious effects were observed by TMZ on GSCs. However, co-administration of DMC and TMZ induced more ROS production than either DMC or TMZ treatment alone, which implied that DMC and TMZ had a synergistic effect on ROS production in GSCs. Excessive production of ROS leads to oxidative stress. Several signaling pathways enhanced by oxidative stress are suggested to have important roles in the self-renewal ability of stem or cancer stem cells. It is now well established that the mitochondria signaling pathway plays a prominent role in apoptosis and increasing evidence supports that apoptosis and autophagy are physiological phenomena closely linked with oxidative stress [30]. ROS production leads to the release of the pro-apoptotic molecule Cyt c from the mitochondrial membrane results in an increased level of Cyt c in the cytoplasm and nucleus, which, in turn, triggers the effector caspase-3 [32]. Here, we used an antioxidant agent, NAC, to block the ROS production, and Cyt c release, which rescued GSCs from co-administration-induced cell apoptosis. Therefore, co-administration of DMC and TMZ-induced apoptosis was, at least in part, mediated through ROS-dependent activation of caspase-3.
JAK-STAT3 pathway is one of the key factors regulating the mitochondrial apoptosis pathway. JAKs could directly activate STAT3 by phosphorylation, which further regulate the genes involved in cell proliferation, differentiation, and apoptosis [33, 34]. In gliomas, Dauer et al. reported that the increased of the degree of malignancy is often accompanied by STAT3 activation [35]. Suppression of STAT3 activation could effectively inhibit the proliferation and induce apoptosis of glioma cells [36], while in contrast to tumor cells, normal cells are rarely affected by the STAT3 inactivation [37]. To date, there are no reports available on the role of JAK-STAT3 signaling pathway in DMC-induced apoptosis in GSCs. In this study, we demonstrate that when GSCs were exposed to 50 μM DMC for 12 and 24 h, the p-STAT3 expression began to significantly decline. And 100 μM TMZ alone did not affect the activation of STAT3; however, co-administration of DMC and TMZ showed a stronger inhibitory effect on p-STAT3 expression.
Downstream proteins of STAT3 have been shown to regulate proliferation and apoptosis in glioma cells. For example, c-Myc and cyclin D1 have been shown to mediate transcription of cell cycle [23], while Bcl-xL and Bcl-2 have been shown to suppress apoptosis [29]. c-Myc has been identified to play an important role in regulating the cell proliferation and survival of GSCs. Knockdown of c-Myc in GSCs reduced cell proliferation and increased cell apoptosis, while non-GSCs displayed limited dependence on c-Myc expression for survival and proliferation [38]. CDC25A is one of the cell cycle-regulated proteins, which controls cell cycle entry into the S phase. Activation of endogenous CDC25A occurs during the late G1 phase and increases in the S and G2 phases. Knockdown of CDC25A induced cell cycle arrest at G0/G1 phase [11]. The expression of Bcl-2 and Bcl-xL proteins is required for the maintenance of GSCs, knockdown of which effectively inhibited cell proliferation and induced apoptosis of GSCs [39]. Because of the fact that STAT3-downregulating genes are all critically involved in the development of GSC aggressiveness, we further identified the expression of c-Myc, CDC25A, Bcl-xL, and Bcl-2 following 24-h exposure to GSCs with co-administration of DMC and TMZ. Here, our results demonstrated that co-administration-induced effects on GSCs derived from human patients were correlated with a decreased expression of c-Myc, CDC25A, Bcl-xL, and Bcl-2. These data suggest that the JAK-STAT3 pathway plays an important role in co-administration of DMC and TMZ-induced apoptosis in GSCs.
In summary, we have investigated the anti-GSC efficacy of co-administration of DMC and TMZ, which inhibited GSC growth and induced apoptosis by targeting multiple signaling pathways. Co-administration of DMC and TMZ shows substantial promise for further development as a potential agent for treating GSCs.
References
Stupp R, Tonn JC, Brada M, Pentheroudakis G, ESMO Guidelines Working Group. High-grade malignant glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v190–3.
Clarke MJ, Mulligan EA, Grogan PT, Mladek AC, Carlson BL, Schroeder MA, et al. Effective sensitization of temozolomide by ABT-888 is lost with development of temozolomide resistance in glioblastoma xenograft lines. Mol Cancer Ther. 2009;8(2):407–14.
Athanassiou H, Synodinou M, Maragoudakis E, Paraskevaidis M, Verigos C, Misailidou D, et al. Randomized phase II study of temozolomide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23(10):2372–7.
Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6(6):425–36.
Braine J, Herpin F. Molecular hydrogen beyond the optical edge of an isolated spiral galaxy. Nature. 2004;432(7015):369–71.
Chen J, Fu X, Wan Y, Wang Z, Jiang D, Shi L. miR-125b inhibitor enhance the chemosensitivity of glioblastoma stem cells to temozolomide by targeting Bak1. Tumour Biol. 2014;35(7):6293–302.
Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumourand antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94(1):79–83.
Luthra PM, Kumar R, Prakash A. Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem Biophys Res Commun. 2009;384(4):420–5.
Darling JL. The in vitro biology of human brain tumors. In: Thomas DGT, editor. Neuro-oncology: primary malignant brain tumors. Baltimore: Johns Hopkins University Press; 1990. p. 1–25.
Shi L, Zhang J, Pan T, Zhou J, Gong W, Liu N, et al. MiR-125b is critical for the suppression of human U251 glioma stem cell proliferation. Brain Res. 2010;1312:120–6.
Shi L, Chen J, Wang YY, Sun G, Liu JN, Zhang JX, et al. Gossypin induces G2/M arrest in human malignant glioma U251 cells by the activation of Chk1/Cdc25C pathway. Cell Mol Neurobiol. 2012;32(2):289–96.
Zhang S, Wan Y, Pan T, Gu X, Qian C, Sun G, et al. MicroRNA-21 inhibitor sensitizes human glioblastoma U251 stem cells to chemotherapeutic drug temozolomide. J Mol Neurosci. 2012;47(2):346–56.
Huang TY, Hsu CW, Chang WC, Wang MY, Wu JF, Hsu YC. Demethoxycurcumin retards cell growth and induces apoptosis in human brain malignant glioma GBM 8401 cells. Evid Based Complement Alternat Med. 2012;2012:396573.
Gong X, Schwartz PH, Linskey ME, Bota DA. Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy. Neurology. 2011;76(13):1126–34.
Narayan P, Mentzer Jr RM, Lasley RD. Annexin V staining during reperfusion detects cardiomyocytes with unique properties. Am J Physiol Heart Circ Physiol. 2001;281(5):H1931–7.
Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28(8):1765–73.
Lee JW, Hong HM, Kwon DD, Pae HO, Jeong HJ. Dimethoxycurcumin, a structural analogue of curcumin, induces apoptosis in human renal carcinoma caki cells through the production of reactive oxygen species, the release of cytochrome C, and the activation of caspase-3. Korean J Urol. 2010;51(12):870–8.
Oliva CR, Moellering DR, Gillespie GY, Griguer CE. Acquisition of chemoresistance in gliomas is associated with increased mitochondrial coupling and decreased ROS production. PLoS ONE. 2011;6(9):e24665.
Yu M, Zheng Y, Sun HX, Yu DJ. Inhibitory effects of enalaprilat on rat cardiac fibroblast proliferation via ROS/P38MAPK/TGF-β1 signaling pathway. Molecules. 2012;17(3):2738–51.
Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11(3):495–510.
Barré B, Avril S, Coqueret O. Opposite regulation of Myc and p21waf1 transcription by STAT3 proteins. J Biol Chem. 2003;278(5):2990–6.
Zhong K, Song W, Wang Q, Wang C, Liu X, Chen D, et al. Murine myeloid dendritic cells that phagocytose apoptotic T cells inhibit the immune response via NO. PLoS ONE. 2012;7(11):e49378.
Ji JD, Kim HJ, Rho YH, Choi SJ, Lee YH, Cheon HJ, et al. Inhibition of IL-10-induced STAT3 activation by 15-deoxy-delta12,14-prostaglandin J2. Rheumatology (Oxford). 2005;44(8):983–8.
Bojes HK, Suresh PK, Mills EM, Spitz DR, Sim JE, Kehrer JP. Bcl-2 and Bcl-xL in peroxide-resistant A549 and U87MG cells. Toxicol Sci. 1998;42(2):109–16.
Terrano DT, Upreti M, Chambers TC. Cyclin-dependent kinase 1-mediated Bcl-xL/Bcl-2 phosphorylation acts as a functional link coupling mitotic arrest and apoptosis. Mol Cell Biol. 2010;30(3):640–56.
Shieh JM, Chen YC, Lin YC, Lin JN, Chen WC, Chen YY, et al. Demethoxycurcumin inhibits energy metabolic and oncogenic signaling pathways through AMPK activation in triple-negative breast cancer cells. J Agric Food Chem. 2013;61(26):6366–75.
Ni X, Zhang A, Zhao Z, Shen Y, Wang S. Demethoxycurcumin inhibits cell proliferation, migration and invasion in prostate cancer cells. Oncol Rep. 2012;28(1):85–90.
Hsu YC, Weng HC, Lin S, Chien YW. Curcuminoids-cellular uptake by human primary colon cancer cells as quantitated by a sensitive HPLC assay and its relation with the inhibition of proliferation and apoptosis. J Agric Food Chem. 2007;55(20):8213–22.
Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, et al. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66(9):4826–34.
Dayem AA, Choi HY, Kim JH, Cho SG. Role of oxidative stress in stem, cancer, and cancer stem cells. Cancers (Basel). 2010;2(2):859–84.
Jiang JY, Sun Y, Yuan Y. Mechanism of temozolomide-induced anti-tumor effects on glioblastoma cells in vitro is via ROS-dependent SIRT1 signaling pathway. Zhonghua Zhong Liu Za Zhi. 2012;34(10):734–8.
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13(9):1423–33.
Senft C, Polacin M, Priester M, Seifert V, Kögel D, Weissenberger J. The nontoxic natural compound curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer. 2010;10:491.
Shi L, Wan Y, Sun G, Zhang S, Wang Z, Zeng Y. miR-125b inhibitor may enhance the invasion-prevention activity of temozolomide in glioblastoma stem cells by targeting PIAS3. BioDrugs. 2014;28(1):41–54.
Dauer DJ, Ferraro B, Song L, Yu B, Mora L, Buettner R, et al. Stat3 regulates genes common to both wound healing and cancer. Oncogene. 2005;24(21):3397–408.
Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21(55):8404–13.
Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8(4):945–54.
Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, et al. c-Myc is required for maintenance of glioma cancer stem cells. PLoS ONE. 2008;3(11):e3769.
Zhou Z, Sun L, Wang Y, Wu Z, Geng J, Miu W, et al. Bone morphogenetic protein 4 inhibits cell proliferation and induces apoptosis in glioma stem cells. Cancer Biother Radiopharm. 2011;26(1):77–83.
Acknowledgments
This work was supported by the China Natural Science Foundation (81000963 and 81370062), Jiangsu Province’s 333 Talent Program (BRA2011046), Jiangsu Province “six personnel peak” funded projects (2013-WSN-145/028), Jiangsu Province’s Natural Science Foundation (BK2012670), Medical Research Foundation by Jiangsu Province Health Department (YG201301 and Z201318), the Clinical Technology Development of Jiangsu University (JLY20120053), the Kunshan Social Development Foundation (KS1006, KS1009), and the Suzhou Social Development Foundation (SYS201063). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
All authors have declared the sources of research funding for this manuscript and have no financial or other contractual agreements that might cause (or be perceived as causes of) conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lei Shi and Xifeng Fei contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shi, L., Fei, X. & Wang, Z. Demethoxycurcumin was prior to temozolomide on inhibiting proliferation and induced apoptosis of glioblastoma stem cells. Tumor Biol. 36, 7107–7119 (2015). https://doi.org/10.1007/s13277-015-3427-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3427-x