Abstract
Galectin-1, a β-galactoside-binding protein implicated in cancer cell immune privilege, was highly expressed in activated pancreatic stellate cells (PSCs). This study was designed to investigate the relationship between PSC-derived galectin-1 and tumor immunity in pancreatic cancer. Isolated PSCs were identified as normal pancreas cells (hNPSCs) or pancreatic cancer cells (hCaPSCs) by immunohistochemical staining for α-SMA and vimentin, and galectin-1 expression was evaluated by Western blotting and quantitative RT-PCR. Apoptosis, caspase activity, and cytokine production (IL-6, IL-10, TNF-β, and IFN-γ) of T cells co-cultured with PSCs were evaluated, and immunohistochemical staining of galectin-1 was correlated with CD3 and clinicopathological variables in 66 pancreatic cancer and 10 normal pancreatic tissue samples. hCaPSCs exhibited higher galectin-1 expression than did hNPSCs, and hCaPSCs induced higher levels of apoptosis in T cells following co-culture. hCaPSCs activated caspase-9 and caspase-3 in the mitochondrial apoptotic pathway and stimulated secretion of Th2 cytokines (IL-6 and IL-10) but decreased secretion of Th1 cytokines (TNF-β and IFN-γ), compared with hNPSCs. Immunohistochemical staining indicated that galectin-1 and CD3 were more highly expressed in pancreatic cancer tissue. Galectin-1 expression was highest in poorly differentiated pancreatic cancer cells and lowest in well-differentiated pancreatic cancer cells and was associated with tumor size, lymph node metastasis, differentiation, and UICC stage. However, CD3 expression showed the opposite trend and was highest in well-differentiated pancreatic cancer cells and was associated with tumor differentiation and UICC stage. High expression of galectin-1 was associated with short survival, as was low expression of CD3. hCaPSC-derived galectin-1 enhanced apoptosis and anergy of T cells in pancreatic cancer, which contributes to the immune escape of pancreatic cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is an aggressive malignancy with the worst prognosis of all human cancers, usually due to widespread metastatic disease at diagnosis or soon after invasive operations [1, 2]. Over the past 30 years, most research has focused on genes and signal pathways that regulate pancreatic cancer cell proliferation and apoptosis or cell death, and unfortunately the survival of pancreatic cancer patients has not yet improved substantially [2, 3]. However, most of these studies have largely ignored the fact that the desmoplastic and hypoxia tumor microenvironment plays a critical role for the onset, growth, and development of pancreatic cancer cells. The desmoplastic tumor microenvironment, consisting of large amounts of extracellular matrix proteins, pancreatic stellate cells, inflammatory cells, and cytokines, is now known to promote the progress of pancreatic cancer and contributes to the low survival rate [4, 5]. Recent research suggests that the desmoplastic microenvironment creates a “fortress-like” fibrosis barrier that blocks the delivery of chemotherapeutic agents and promotes the malignant behavior of cancer cells [6]. Furthermore, this feature suppresses the infiltration of immune cells and/or their function inside tumors, in part via secretion of immunosuppressive cytokines, and consequently promotes the immune escape of tumor cells [7, 8]. Therefore, breaking this “desmoplastic fortress” represents a promising strategy for improving the delivery and efficacy of systemic chemotherapies and opens novel immune therapeutic avenues for treating pancreatic cancer [5]. However, the mechanisms underlying the interaction between the stromal microenvironment and tumor cells and the contribution this makes to tumor immune privilege remain poorly understood in pancreatic cancer [5, 9].
Galectin-1 has emerged as a promising cancer target, with the function of modulating cell proliferation, adhesion, and migration, all of which are linked to essential biological processes in tumor cells [10–13]. Galectin-1 also regulates the complex intercommunication and interaction between tumor cells and the components of the tumor microenvironment [14, 15]. By inducing the apoptosis of activated T cells [16, 17] and inhibiting T cell proliferation [18], endogenous galectin-1 has been considered to play an important role in tumor immune privilege. Targeted inhibition of galectin-1 expression in tumor cells results in heightened T cell-mediated rejection [19]. Recently, evidence has shown that galectin-1 is strongly expressed in activated pancreatic stellate cells (PSCs), mesenchymal cells specific to the pancreas which collaborate with cancer cells during initiation and progression of pancreatic cancer [20–22]. Activated PSCs exhibit a myofibroblast-like phenotype and secrete excessive amounts of cytokines and extracellular matrix proteins that comprise the fibrous microenvironment that is characteristic of pancreatic cancer [4, 5, 23]. PSCs possess functional heterogeneity and influence the progression of pancreatic cancer [24]. However, the role of PSC-associated galectin-1 in the pancreatic cancer microenvironment has not been well studied.
In our previous study, galectin-1 was knocked down in primary PSCs isolated from normal pancreatic tissues using small interfering RNA (siRNA) or overexpressed using recombinant lentiviruses, and PSCs were also co-cultured with T cells. The results suggested that overexpression of galectin-1 in PSCs induced T cell apoptosis and Th2 cytokine secretion, which may regulate PSC-dependent immunoprivilege in the pancreatic cancer microenvironment [25]. In the current study, we isolated PSCs from fresh pancreatic cancer tissues (hCaPSCs) and normal healthy pancreas tissues (hNPSCs) and co-cultured them with peripheral T cells. Apoptosis of T cells and the levels of Th1 cytokines (TNF-β and IFN-γ), Th2 cytokines (IL-6, IL-10), and caspase activity were evaluated. Finally, the relationship between galectin-1 and CD3 expression in pancreatic cancer tissues was investigated and correlated with the clinicopathological characteristics of this disease.
Materials and methods
Patients and pancreatic tissues
Information on patients, pancreatic tissues, and ethics statements have been previously described [15]. The clinicopathological characteristics of patients are described in Table 1. Survival was measured from the time of pancreatic resection until death, and follow-up data was available for all patients.
Cells and culture conditions
Collection of human PSCs and culture conditions were performed as previously described [15, 26, 27]. The PSC cell type was confirmed by immunohistochemical staining for α-smooth muscle actin (α-SMA) and vimentin and by morphological analysis (stellate-like or spindle-shaped cells) [28]. Separation of T lymphoblasts was performed as previously described [17, 25]. Briefly, peripheral blood mononuclear cells from healthy donors were isolated using a Ficoll gradient and separated on a nylon fiber column (Wako Pure Chemical Industries, Ltd., Osaka, Japan) according to the manufacturer’s instructions. Activation was for 72 h with 5 μg/mL PHA in RPMI medium containing 10% FCS, followed by culturing with 20 ng/mL IL-2. IL-2 was removed 24 h before the apoptosis assays.
Quantitative reverse transcription-PCR
The detailed experimental procedures of total RNA extraction from cultured cells and the quantitative reverse transcription-polymerase chain reaction (qRT-PCR) were performed as previously described [15, 25]. Gene-specific primers for human galectin-1 and β-actin (Table 2) were purchased from Invitrogen.
Immunohistochemical staining and evaluation
Immunohistochemical staining was performed as previously described [15, 25]. The primary antibodies were incubated as follows: mouse monoclonal anti-galectin-1 (sc-166618; 1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-CD3 (sc-52382; 1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-vimentin (sc-6260; 1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and anti-α-SMA (MA1-37027;1:200; Thermo, Fremont, CA, USA). The results of immunohistochemical staining were interpreted by two experienced pathologists, and the mean density of staining was calculated using Image-Pro Plus 6.0 software (Image-Pro, Bethesda, MD, USA). Galectin-1 and CD3 staining intensity was scored semiquantitatively as follows: no positive cells = 0 (negative); 1–29% positive = 1 (weak) and >30 % positive = 2 (strong).
Western blotting
Western blotting was performed as previously described [15, 25, 29].
Enzyme-linked immunosorbent assay
PSC and T cell co-culture supernatants were harvested and stored at −80 °C. The levels of IL-6, IL-10, TNF-β and IFN-γ were determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Boster, Wuhan, China). The ELISA detection sensitivity was ≥15.6 pg/mL for IL-6, IL-10, TNF-β, and IFN-γ.
Flow cytometry analysis
T cells were obtained from co-culture supernatants, suspended in PBS containing 1% fetal bovine serum at a density of 1 × 105 cells per 100 μL, and incubated with 20 μL anti-CD3-FITC (BD Pharmingen, Franklin Lakes, NJ, USA) at room temperature in the dark for 20 min. Cells were centrifuged for 4 min at 1500 rpm, resuspended in 250 μL 1 % FCS containing 0.1 % NaN3 (FACS buffer), added with 5 μL annexin V-APC (Bender MedSystems, San Diego, CA, USA), mixed gently, and incubated for 15 min at room temperature in the dark. Labeled cells were analyzed with a BD FACSCalibur flow cytometer (Beckman Coulter, Fullerton, CA, USA).
Measurement of caspase activity
The activity of caspase-3, -8, and -9 was measured with a caspase activity kit according to the manufacturer’s instructions (Boster, Wuhan, China), using substrate peptides Ac-DEVD-pNA, Ac-IETD-pNA, and Ac-LEHD-pNA, respectively. Briefly, cells were lysed and supernatants mixed with buffer containing caspase substrate peptides that included a p-nitroanilide (pNA) leaving group. Release of the pNA group was quantified by measuring the absorbance at 405 nm with an ELISA reader (Bio-Rad instrument Group, Hercules, CA, USA). All experiments were carried out in triplicate. Caspase activity was expressed as a percentage compared to the control.
Statistical analysis
Values are expressed as mean ± standard deviation. One-way ANOVA and t tests were used to compare differences between groups. The χ 2 and Fisher exact tests were used to analyze the correlations between galectin-1 and CD3 expression and clinicopathological characteristics. Univariate survival analysis was performed according to the Kaplan-Meier method; differences in survival curves were assessed with the log-rank test. Multivariate analysis was performed using Cox’s proportional hazard model. P values were two-sided, and values of P ≤ 0.05 were considered statistically significant. All statistical analyses were performed using SPSS 13.0 software.
Results
Analysis of galectin-1 expression in human PSCs
We established in vitro cultures of PSCs using specimens from patients with pancreatic cancer and bile duct cancer; four PSC cultures (hCaPSCs) were isolated from four fresh surgical specimens of human pancreatic cancer, and four PSC cultures (hNPSCs) were isolated from four fresh surgical normal pancreas specimens from patients with human bile duct cancer. The identity of these PSCs was confirmed by immunohistochemical staining for α-SMA and vimentin (Fig. 1a, b). From a morphological point of view, no difference was seen between hCaPSCs and hNPSCs. Following 10 days of culturing, total cell lysates and RNA were extracted from both cell types. Western blots demonstrated the expression of a 14.0-kDa galectin-1 protein, corresponding to the size of pro-galectin-1, in activated human PSCs, and the bands were stronger in hCaPSCs than in hNPSCs (Fig. 1c). Quantitative RT-PCR showed that the amount of galectin-1 mRNA was greater in hCaPSCs than in hNPSCs (P = 0.026) (Fig. 1d). The results indicated heterogeneity between hCaPSCs and hNPSCs, which may have been caused by pancreatic cancer cells in vivo.
Identification of PSCs and expression of galectin-1 in different PSCs. Expression of α-smooth muscle actin (α-SMA) (a) and vimentin (b), activation markers in isolated PSCs, was confirmed using cell immunohistochemistry. Higher galectin-1 expression was observed in hCaPSCs than in hNPSCs using Western blotting (c) and quantitative RT-PCR (d). *P < 0.05 vs. hNPSCs. hNPSCs, PSCs isolated from healthy pancreatic tissue; hCaPSCs, PSCs isolated from cancerous pancreatic tissue
High galectin-1 expression in PSCs induced apoptosis of T cells by activating caspase-9 and caspase-3
In order to investigate any difference in the capacity of hNPSCs and hCaPSCs to induce apoptosis in T cells, we co-cultured activated T cells with hCaPSCs and hNPSCs. After 24 h, the T cell population was analyzed by two-color flow cytometry to detect annexin-V and CD3+ cells. There was significantly more apoptosis of CD3+ T cells in hCaPSC co-cultures than in hNPSC co-cultures (P < 0.05) (Fig. 2a). We then examined the activities of caspase-3, -9, and -8, which are involved in initiating the mitochondria-mediated apoptotic pathway. As shown in Fig. 2, caspase-9 activity in T cells was enhanced significantly after the first 6 h of co-culture with hCaPSCs and after the first 12 h of co-culture with hNPSCs; similarly, caspase-3 activity in T cells was enhanced significantly after 12 and 18 h of co-culture with hCaPSCs and hNPSCs, respectively. Caspase-8 activity was significantly elevated only in T cells co-cultured with hCaPSCs at the 24-h time point. These effects could be inhibited by β-lactose, a competitive inhibitor of galectin-1, demonstrating the specificity of the endogenous galectin-1 effect in hCaPSCs. The results indicate that high galectin-1 expression in PSCs induced the apoptosis of T cells mainly by initiating caspase-9 and caspase-3 activities in the mitochondria-mediated apoptotic pathway.
Effect of PSC-derived galectin-1 on T cell apoptosis and caspase activity. a Effect of hCaPSCs and hNPSCs on CD3+ T cell apoptosis in the co-culture system. **P < 0.01 vs. complete medium (CM); # P < 0.05 vs. CM. Detection of apoptosis in T cells by measuring caspase-9 activity (b), caspase-3 activity (c), and caspase-8 activity (d) in the co-culture system. All observed effects were inhibited by the addition of 50 mM β-lactose, a competitive inhibitor of galectin-1. *P < 0.05 vs. hCaPSCs + β-lactose; **P < 0.01 vs. hCaPSCx + β-lactose; # P < 0.05 vs. hNPSCs + β-lactose; ## P < 0.01 vs. hNPSCs + β-lactose
Galectin-1 expression in PSCs promoted anergy of T cells
We then asked whether endogenous galectin-1 in PSCs affected the secretion of cytokines by T cells by assessing the levels of Th1/Th2 cytokines in supernatants from the co-cultures of activated T cells and hPSCs. Supernatants from T cells co-cultured with hCaPSCs contained higher levels of Th2 cytokines (IL-6 and IL-10) but lower levels of Th1 cytokines (TNF-β and IFN-γ) than those from T cells co-cultured with hNPSCs or naive T cells (P < 05, P < 0.01, respectively), and β-lactose specifically blocked this effect (Fig. 3). hNPSCs also induced an increase in secretion of Th2 cytokines (IL-6 and IL-10) (P < 0.01) and a reduction in secretion of Th1 cytokines (TNF-β and IFN-γ) (P < 0.01, P < 0.05) compared to naive T cells (Fig. 3). The results indicate that endogenous galectin-1 in PSCs promotes anergy of T cells by decreasing Th1-type cytokine production and enhancing Th2-type cytokine production in the microenvironment of pancreatic cancer.
Effect of PSC-derived galectin-1 on Th1/Th2 cytokine secretion by T cells. Co-culture with hCaPSCs and hNPSCs significantly reduced a TNF-β and b IFN-γ secretion by T cells (*P < 0.05, **P < 0.01 vs. CM) and significantly increased c IL-6 and d IL-10 secretion by T cells in comparison to control naive T cells cultured in complete medium only (CM) (**P < 0.01 vs. CM). These effects were all significantly greater with hCaPSCs than with hNPSCs (# P < 0.05) and were all blocked by 50 mM β-lactose (P > 0.05 vs. CM)
Correlation between PSC-derived galectin-1 expression, CD3 expression, and clinicopathological characteristics in pancreatic cancer
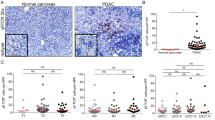
To evaluate the correlation between PSC-derived galectin-1, CD3 expression, and the clinicopathological factors of pancreatic cancer, galectin-1 and CD3 expression were evaluated using immunohistochemistry. Galectin-1 was expressed in the stromal cells surrounding the cancer cells, and CD3 was mainly expressed in the mesenchyme around the cancer cells, especially in well-differentiated pancreatic cancer tissues (Fig. 1). Strong galectin-1 expression in stromal cells was found in 40% (4/10) of well differentiated, 66.7 % (16/24) of moderately differentiated, and 84.4 % (27/32) of poorly differentiated pancreatic cancer tissue samples, and these differences were significant (P = 0.021), while strong CD3 expression was found in 80 % (8/10) of well differentiated, 54.2 % (13/24) of moderately differentiated, and 28.1 % (9/32) of poorly differentiated pancreatic cancer cells (P = 0.009) (Table 2). These results indicate that as galectin-1 staining intensity gradually increased, CD3 staining was highest in well-differentiated cells and lowest in poorly differentiated cells. This phenomenon was also apparent with UICC stage. In addition, strong galectin-1 staining was also associated with tumor size (P = 0.007) and lymph node metastasis (P = 0.019). Correlation analysis showed that CD3 expression was inversely proportional to galectin-1 expression in pancreatic cancer tissues (Table 3). Staining of galectin-1 and CD3 was found to be weak or negative in normal pancreatic tissue samples. These results suggest that PSC-derived galectin-1 expression is associated with tumor progression, including nodal metastasis and local invasion of cancer cells, and with immune privilege by decreasing the number of T cells.
Strong galectin-1 expression and weak CD3 expression is associated with shorter patient survival times
Strong galectin-1 expression and weak CD3 expression were associated with shorter patient survival times (Fig. 4). The median survival times for patients with strong and weak expression of galectin-1 were 14.1 and 24.8 months, respectively. The median survival times for patients with strong and weak expression of CD3 were 19.0 and 14.1 months, respectively. Next, we performed a multivariate survival analysis based on the Cox proportional hazard model on all parameters found to be significant by univariate analysis, including strong galectin-1 staining, pT3/pT4, pN1, UICC stages III/IV, and G3 tumor. Strong galectin-1 expression was an independent marker of poor prognosis in pancreatic cancer patients, with a relative risk of 4.676 (Table 4).
Characterization of galectin-1 and CD3 expression in healthy and cancerous pancreatic tissue. Expression of (a1) galectin-1 and (b1) CD3 was limited in normal pancreatic tissue. (a2–4) Galectin-1 was expressed in stromal cells of pancreatic cancer tissues, and expression levels increased from well (a2), moderately (a3), to poorly (a4) differentiated pancreatic cancer. (b2–4) CD3 expression in pancreatic cancer tissues decreased from well (b2), moderately (b3), to poorly (b4) differentiated pancreatic cancer. c Quantification of the mean density of galectin-1 expression in normal pancreas and pancreatic cancer tissues. *P < 0.05 vs. well, **P < 0.01 vs. well, # P < 0.05 vs. moderately. d Quantification of the mean density of CD3 expression in normal pancreas and pancreatic cancer tissues. *P < 0.05 vs. well, **P < 0.01 vs. well, # P < 0.01 vs. moderately. NP, normal pancreas; well, well-differentiated pancreatic cancer; moderately, moderately differentiated pancreatic cancer; poorly, poorly differentiated pancreatic cancer. Original magnification: ×200. e–f Kaplan-Meier survival curve for 66 pancreatic cancer patients analyzed by e galectin-1 and f CD3 staining intensity
Discussion
Despite strong infiltration of pancreatic cancer tissue with potentially tumor cytotoxic T lymphocytes (CTLs), the host immune system still fails to effectively eliminate these malignant cells [30, 31]. It is becoming increasingly evident that the pancreatic cancer microenvironment may have novel characteristics, among which is an intrinsic resistance to CTL infiltration that might enable cancer cells to evade immune surveillance [32, 33]. An understanding of the molecular mechanisms involved in the microenvironment of pancreatic cancer that enable pancreatic carcinoma cells to evade CTL-mediated killing and lead to a survival advantage is a prerequisite for the design of targeted immune therapies. A large body of evidence indicates that galectin-1 induces apoptosis in activated T cells, suggesting a role for galectin-1 in the mechanism of tumor immune privilege [17, 34]. Recent studies have also shown that activated PSCs in the pancreatic cancer microenvironment express increased levels of galectin-1, which in turn activate the quiescent PSCs [22, 35]. For these reasons, endogenous galectin-1 expressed by activated PSCs may qualify as a potential molecular target for immune therapy in pancreatic cancer. However, the detailed mechanisms involved and the functional effects on T cell immunity of endogenous galectin-1 secretion by activated PSCs are unclear at present.
Our previous study indicated that high expression of galectin-1 in primary cultured PSCs following gene transfection induced T cell apoptosis and Th2 cytokine secretion [25]. To evaluate the actual functional profile of PSCs in pancreatic cancer, we isolated and cultured primary PSCs from healthy and cancerous pancreas tissues and demonstrated that both galectin-1 mRNA and protein levels were higher in hCaPSCs than in hNPSCs. When PSCs were co-cultured with primary T lymphocytes, hCaPSCs induced higher levels of CD3+ T cell apoptosis, caspase-9 and caspase-3 activities and secretion of Th2 cytokines than did hNPSCs. Furthermore, these effects could all be inhibited by β-lactose, a competitive inhibitor of galectin-1. Taken together, these results support the view that high expression of galectin-1 in hCaPSCs may play a key role in the regulation of immune homeostasis in the microenvironment of pancreatic cancers.
An increasing body of evidence suggests that immune mediators and modulators released from different cells in the tumor microenvironment dictate in which direction the balance between tumor immunosuppression and antitumor immunity is tipped [36, 37]. Galectin-1, a member of the Galectin family that include β-galactoside-binding sites, plays a critical role in regulating growth and apoptosis of activated peripheral T lymphocytes and infiltration of tumor T cells [38, 39]. Consequently, galectin-1 contributes to the immunological balance not only in normal physiological situations but also in the pathological process of tumor development. Recently, galectin-1 emerged as a primary regulator of PSC-associated immunosuppressive function in the microenvironment of pancreatic cancer [25, 40]. PSC-derived galectin-1 has been implicated in helping pancreatic cancer cells escape from systematic immunosurveillance [19], with the potential to inhibit T cell proliferation [41], influence the persistence of T cell subsets [42], and induce apoptosis of infiltrative tumor T cells [17, 43]. The apoptotic processes can be classified into two simplified pathways: (1) the “caspase first” activation of the initiator caspase-8 and subsequent activation of the effector caspase-3; (2) the “mitochondria first” pathway in which mitochondria are targeted before caspase-9 activation [44]. Our present results indicated that hCaPSC-derived galectin-1 not only triggered the activation of caspase-9, with caspase 3 as an effector, but also the death receptor initiator caspase-8, albeit in a delayed fashion. Alternatively, caspase-8 may have been activated by a secondary feedback mechanism following activation of the caspase-9 and caspase-3 cascades, which may also be required for the induction of T cell apoptosis by hCaPSC-derived galectin-1. It is therefore likely that hCaPSC-induced apoptosis involving galectin-1 is mainly mediated by the “mitochondrion first” pathway, though it may also be followed by the “caspase first” apoptotic pathway. Moreover, our findings also demonstrate that endogenous galectin-1 secreted by activated hCaPSCs affects the function of T cell subsets by promoting the secretion of the cytokine Th2. This consequently alters the Th1/Th2 balance, suggesting a role for hCaPSC-derived galectin-1 in immune deviation.
There is also the question of why the isolated hCaPSCs and hNPSCs are functionally heterogeneous with respect to the expression of galectin-1 and consequently their effect on T cells, leading to immune privilege. Increasing evidence has indicated that PSCs interact with cancer cells to promote the progression of pancreatic cancer [45]. Pancreatic cancer cells secrete mitogenic and fibrogenic stimulants, such as transforming growth factor beta (1), platelet-derived growth factor (PDGF), and galectin-1, all of which may promote the activation of quiescent PSCs [22, 35]. PSCs in turn secrete various factors, including PDGF, galectin-1, stromal-derived factor 1, epidermal growth factor, and fibroblast growth factor, that play important roles in tumor growth, invasion, metastasis, and resistance to chemotherapy [20–22, 46]. The isolated hCaPSCs may therefore have been endued with the characteristic of high galectin-1 expression in the in vivo microenvironment of pancreatic cancer.
To further verify the immunosuppressive function of endogenous galectin-1 in pancreatic cancer, we collected data on galectin-1 expression in 66 pancreatic cancer tissue samples and 10 normal pancreas tissue samples. The results indicated that galectin-1 expression gradually increased with the degree of malignancy, while the reverse was true for CD3 expression. A previous study showed that the expression pattern of galectin-1 in pancreatic cancer tissues plays a role in the desmoplastic reaction occurring around the cancer cells [47]. Similarly, our study also showed that fibroblasts localized in the stromal region surrounding the pancreatic cancer cells, and which have been proven to be the activated PSCs [24, 48], express galectin-1 abundantly, which may form a fibrotic barrier prohibiting the infiltration of T cells [49]. In line with a recent study demonstrating that PSCs have functional heterogeneity that promotes the progression of pancreatic cancer [24], our results also suggest that hCaPSC-derived galectin-1 is associated with tumor progression, including nodal metastasis and local invasion of cancer cells. Furthermore, galectin-1 appeared to be correlated with immune privilege via the induction of T cell apoptosis and anergy. Strong galectin-1 expression is therefore an independent marker of poor prognosis in pancreatic cancer patients.
Based on the data presented in this study, we propose a mechanism for the induction of immune privilege by hCaPSC-derived galectin-1 in the microenvironment of pancreatic cancer (Fig. 5). PSCs become activated in the pancreatic cancer tissue and express large quantities of galectin-1 that binds to an as yet unidentified receptor on T cells. This triggers two cellular responses: endogenous galectin-1 affects the Th1/Th2 balance by mediating Th1 cell apoptosis and stimulating Th2 cytokine secretion; simultaneously, endogenous galectin-1 induces mitochondria-associated T cell apoptosis followed by caspase activation (caspase-9 as initiator caspase and caspase-3 as effector caspase). Caspase-8 might be activated via a feedback mechanism after activation of the caspase cascade, but this step is delayed and may not be required for galectin-1-induced T cell apoptosis.
Proposed mechanism of T cell apoptosis induced by hCaPSC-derived galectin-1. PSCs are activated in pancreatic cancer tissue and strongly express galectin-1, which binds to an as yet unidentified receptor on T cells, triggering the following cellular responses: (1) endogenous galectin-1 affects the Th1/Th2 balance by mediating Th1-cell apoptosis and stimulating Th2 cytokine secretion; (2) endogenous galectin-1 induces mitochondria-associated apoptosis of T cells followed by caspase activation (caspase-9 as initiator caspase and caspase-3 as effector caspase). Caspase-8 might be activated by a feedback mechanism after activation of the caspase cascade, but this step is delayed and may not be required for galectin-1-induced T cell apoptosis. PSC pancreatic stellate cells, PCC pancreatic cancer cells
In conclusion, hCaPSC-derived galectin-1 in the microenvironment of pancreatic cancer promotes evasion of immune surveillance by the cancer cells by inducing apoptosis and anergy of T cells. PSCs and galectin-1 will therefore be of potential value for selective therapeutic targeting in the treatment of pancreatic cancer.
References
Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–13.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217.
Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, et al. Pancreatic cancer microenvironment. Int J Cancer. 2007;121:699–705.
Neesse A, Michl P, Frese KK, Feig C, Cook N, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–8.
Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61.
Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–30.
Ganss R, Hanahan D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 1998;58:4673–81.
Guturu P, Shah V, Urrutia R. Interplay of tumor microenvironment cell types with parenchymal cells in pancreatic cancer development and therapeutic implications. J Gastrointest Cancer. 2009;40:1–9.
Salatino M, Croci DO, Bianco GA, Ilarregui JM, Toscano MA, et al. Galectin-1 as a potential therapeutic target in autoimmune disorders and cancer. Expert Opin Biol Ther. 2008;8:45–57.
Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–76.
Rabinovich GA. Galectin-1 as a potential cancer target. Br J Cancer. 2005;92:1188–92.
Cimmino F, Schulte JH, Zollo M, Koster J, Versteeg R, et al. Galectin-1 is a major effector of TrkB-mediated neuroblastoma aggressiveness. Oncogene. 2009;28:2015–23.
Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, et al. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci U S A. 2007;104:13134–9.
Tang D, Zhang J, Yuan Z, Gao J, Wang S, et al. Pancreatic satellite cells derived galectin-1 increase the progression and less survival of pancreatic ductal adenocarcinoma. PLoS ONE. 2014;9(3):e90476.
He J, Baum LG. Presentation of galectin-1 by extracellular matrix triggers T cell death. J Biol Chem. 2004;279:4705–12.
Kovacs-Solyom F, Blasko A, Fajka-Boja R, Katona RL, Vegh L, et al. Mechanism of tumor cell-induced T-cell apoptosis mediated by galectin-1. Immunol Lett. 2010;127:108–18.
Motran CC, Molinder KM, Liu SD, Poirier F, Miceli MC. Galectin-1 functions as a Th2 cytokine that selectively induces Th1 apoptosis and promotes Th2 function. Eur J Immunol. 2008;38:3015–27.
Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; a potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–51.
Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085–93.
Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–96.
Masamune A, Satoh M, Hirabayashi J, Kasai K, Satoh K, et al. Galectin-1 induces chemokine production and proliferation in pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G729–736.
Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101:887–907.
Ikenaga N, Ohuchida K, Mizumoto K, Cui L, Kayashima T, et al. (2010) CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology 139: 1041-1051, 1051 e1041-1048.
Tang D, Yuan Z, Xue X, Lu Z, Zhang Y, et al. High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int J Cancer. 2012;130:2337–48.
Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–32.
Ohuchida K, Mizumoto K, Murakami M, Qian LW, Sato N, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64:3215–22.
Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–26.
Masamune A, Kikuta K, Watanabe T, Satoh K, Satoh A, et al. Pancreatic stellate cells express Toll-like receptors. J Gastroenterol. 2008;43:352–62.
Park HD, Lee Y, Oh YK, Jung JG, Park YW, et al. Pancreatic adenocarcinoma upregulated factor promotes metastasis by regulating TLR/CXCR4 activation. Oncogene. 2011;30:201–11.
Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802.
Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–83.
Plate JM, Harris JE. Immunobiotherapy directed against mutated and aberrantly expressed gene products in pancreas cancer. J Cell Biochem. 2005;94:1069–77.
Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–9.
Fitzner B, Walzel H, Sparmann G, Emmrich J, Liebe S, et al. Galectin-1 is an inductor of pancreatic stellate cell activation. Cell Signal. 2005;17:1240–7.
Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83.
Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50.
Yang RY, Liu FT. Galectins in cell growth and apoptosis. Cell Mol Life Sci. 2003;60:267–76.
Liu FT. Regulatory roles of galectins in the immune response. Int Arch Allergy Immunol. 2005;136:385–400.
Tang D, Wang D, Yuan Z, Xue X, Zhang Y, et al. Persistent activation of pancreatic stellate cells creates a microenvironment favorable for the malignant behavior of pancreatic ductal adenocarcinoma. Int J Cancer. 2013;132:993–1003.
Blaser C, Kaufmann M, Muller C, Zimmermann C, Wells V, et al. Beta-galactoside-binding protein secreted by activated T cells inhibits antigen-induced proliferation of T cells. Eur J Immunol. 1998;28:2311–9.
Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–34.
Hahn HP, Pang M, He J, Hernandez JD, Yang RY, et al. Galectin-1 induces nuclear translocation of endonuclease G in caspase- and cytochrome c-independent T cell death. Cell Death Differ. 2004;11:1277–86.
Ion G, Fajka-Boja R, Kovacs F, Szebeni G, Gombos I, et al. Acid sphingomyelinase mediated release of ceramide is essential to trigger the mitochondrial pathway of apoptosis by galectin-1. Cell Signal. 2006;18:1887–96.
Duner S, Lopatko Lindman J, Ansari D, Gundewar C, Andersson R. Pancreatic cancer: the role of pancreatic stellate cells in tumor progression. Pancreatology. 2011;10:673–81.
Kikuta K, Masamune A, Watanabe T, Ariga H, Itoh H, et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403:380–4.
Berberat PO, Friess H, Wang L, Zhu Z, Bley T, et al. Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J Histochem Cytochem. 2001;49:539–49.
Li L, Bimmler D, Graf R, Zhou S, Sun Z, et al. PSP/reg inhibits cultured pancreatic stellate cell and regulates MMP/TIMP ratio. Eur J Clin Invest. 2011;41:151–8.
Montiel JL, Monsivais-Urenda A, Figueroa-Vega N, Moctezuma JF, Burgos-Vargas R, et al. Anti-CD43 and anti-galectin-1 autoantibodies in patients with systemic lupus erythematosus. Scand J Rheumatol. 2010;39:50–7.
Acknowledgments
The authors wish to thank the staff of the Virology Laboratory at the Medical College of YangZhou University for their assistance. This work was supported by grants from the Postdoctoral Science Foundation of China (No. 2013M530243), the Social Development of Science and Technology Research Projects of Yangzhou City (No. 2012123), the Jiangsu Province Natural Science Foundation of China (BK20140495), and the Six Big Talent Peak Projects of Jiangsu Province (2014-WSW-078).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dong Tang and Jun Gao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tang, D., Gao, J., Wang, S. et al. Apoptosis and anergy of T cell induced by pancreatic stellate cells-derived galectin-1 in pancreatic cancer. Tumor Biol. 36, 5617–5626 (2015). https://doi.org/10.1007/s13277-015-3233-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3233-5