Abstract
Cystatin SN (cystatin 1, CST1) is a member of the cystatin superfamily that inhibits the proteolytic activity of cysteine proteases. CST1 is a tumor biomarker that provides useful information for the diagnosis of esophageal, gastric, and colorectal carcinomas. However, the significance of CST1 in pancreatic cancer is unknown. The aim of this study was to assess whether CST1 is a potential biomarker for early diagnosis of malignant pancreatic neoplasms. Microarray analysis of mRNA extracted from pancreatic cancer and pancreatic normal tissues was performed. Bioinformatics revealed that CST1 was one of the highest expressed genes on the array in pancreatic cancer, compared with normal tissue. In addition, the upregulation of CST1 in pancreatic cancer and several pancreatic cancer cell lines was confirmed using real-time PCR (RT-PCR), immunohistochemistry, and Western blotting. Next, CST1 knockdown using siRNA reduced the expression of the proliferation-related proteins p-AKT and PCNA significantly, as well as colony formation and xenograft development in vitro. Consistent with this, CST1 mRNA overexpression was correlated closely with malignancy-associated proteins such as PCNA, cyclin D1, cyclin A2, and cyclin E in pancreatic cancer cell lines. In conclusion, our data suggest that CST1 might contribute to the proliferation of pancreatic cancer cells and could be a potential biomarker for the early detection of pancreatic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer (PC) is an aggressive disease with an extremely poor prognosis and a 5-year survival rate of <5 % [1, 2]. It is the sixth leading cause of malignancy-related death in China [3]. The characteristics of PC including its rapid progression, proximity to major vessels, and non-specific vague symptoms result in high malignancy and mortality rates [1]. Although considerable effort has been made to investigate the mechanisms involved in pancreatic carcinogenesis and identify novel markers for its early detection [4, 5], additional work is needed. Therefore, the identification of novel biomarkers for PC is urgent.
Cysteine proteases, such as cathepsins and papain, are proteolytic enzymes that are expressed widely in tissues and have numerous functions, including inflammatory tissue destruction, modulation of the immune response, tissue remodeling, and inducing the migration of monocytes and cancer cells [6–8]. Previous clinical studies revealed that the upregulation of cysteine proteases is involved in various malignant tumors and that tumor growth and invasion can be reduced by inhibiting cysteine proteases. The cystatin superfamily comprises proteins that inhibit the proteolytic activity of cysteine proteases specifically [9]. Cystatin SN is a secretory peptide encoded by CST1 gene and belongs to the type 2 cystatin superfamily, together with cystatins C, D, S, SA, SN, M, and F [10–12]. Previous studies reported that most of the type 2 cystatins are closely related to tumor invasion and metastasis [13–16]. The upregulation of cystatin SN contributes to cell proliferation and the inhibition of cathepsin in gastric cancer [13]. Cystatin SN was also identified as a novel tumor biomarker for colorectal cancer [14, 15] and is an independent predictor of 5-year survival in patients with surgically resected esophageal squamous cell carcinoma [16]. However, the relationship between CST1 and PC remains unclear. Based on previous reports, we hypothesize that CST1 may play an important role in the initiation and development of PC and that it could be a potential indicator for the early diagnosis of PC.
In the current study, we used microarrays to reveal that CST1 was upregulated in PC compared with normal tissues. We then evaluated CST1 mRNA and protein expression in PC and normal tissues using real-time PCR (RT-PCR), immunohistochemistry, and Western blotting. We also investigated the expression of CST1 in PC and liver, colorectal, and gastric cancer cell lines. Importantly, knocking down its expression in vitro resulted in reduced colony formation, delayed xenograft development, and decreased expression of proliferation-related proteins. Therefore, the results of the current study suggest that CST1 contributes to PC cell proliferation and that it could be a useful biomarker for the early detection of PC.
Materials and methods
Tissue, serum samples
PC tissues from six patients were obtained, and six pancreatic normal control tissue specimens were isolated from patients with abdominal trauma in the Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). And venous blood samples were obtained. Clinical diagnoses were made based on the developmental history of disease and pathological evidence. Specimens were frozen immediately and stored at −80 °C until analysis using microarrays and real-time PCR. The acquisition of tissue samples was approved by the Institutional Review Board at The Third Affiliated Hospital of Sun Yat-sen University, and written informed consent was received from all patients.
Cells
Human PC cell lines, including PANC-1, Bxpc-3, and SW1990, were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Bxpc-3 and SW1990 cells were cultured in RPMI 1640 medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 10 % heat-inactivated fetal bovine serum (FBS), whereas PANC-1 were cultured in DMEM supplemented with 10 % FBS. Cells were cultured in a humidified incubator at 37 °C with 5 % CO2.
RNA extraction and RT-PCR
Total RNA was isolated from PC tissues, matched pancreatic tissues, and cell lines using the RNAgents Total RNA Isolation System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. First-strand complementary DNA (cDNA) was synthesized using Superscript Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The cDNA was diluted 1:10 using diethyl pyrocarbonate-treated water. Real-time PCR was performed using specific primers and the QuantiTect SYBR Green PCR Kit (Roche, Basel, Switzerland) on an Applied Biosystems 7500 real-time PCR machine (Applied Biosystems, Foster City, CA, USA). The primers used were as follows: CST1 (sense, 5′-GGTACAGCGTGCCCTTCA-3′, and antisense, 5′-TTGGGCTGGGACTTGGTA-3′, 171-bp product), PCNA (sense, 5′-CCTGCTGGGATATTAGCTCCA-3′, and antisense, 5′-CAGCGGTAGGTGTCGAAGC-3′, 109-bp product), cyclin A2 (sense, 5′-CGCTGGCGGTACTGAAGTC-3′, and antisense, 5′-GAGGAACGGTGACATGCAT-3′, 120-bp product), cyclin D1 (sense, 5′-GCTGCGAAGTGGAAACCATC-3′, and antisense, 5′-CCTCCTTCTGCACACATTTGAA-3′, 135-bp product), cyclin E (sense, 5′-CCTGCGCGAGAAGGAACTG-3′, and antisense, 5′-CGTTGTAGCGATCCATGAAGTG-3′, 173-bp product), and β-actin (sense, 5′-GTCTTCCCCTCCATCGTG-3′, and antisense, 5′-AGGGTGAGGATGCCTCTCTT-3′, 113-bp product). CST1 expression was normalized to that of β-actin. PCR products were analyzed using agarose gel electrophoresis.
Immunohistochemistry
Formalin-fixed tissues were embedded in paraffin and sectioned. Next, 4-mm sections were incubated with monoclonal antibodies against CST1 (1:100, Sigma, St Louis, MO, USA) overnight at 4 °C followed by incubation with anti-rabbit secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). 3,3′-Diaminobenzidine was used as the chromogen. The sections were then stained using hematoxylin and mounted. CST1 expression was analyzed qualitatively in a blinded fashion, i.e., without knowledge of the clinical and pathological information.
Western blotting
Total protein extracts from tissues and cell lines were purified and analyzed by Western blotting. They were separated on 13 % gradient SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were then blocked with 5 % skimmed milk in PBS for 2 h and incubated with primary antibodies against CST1 (Sigma) and β-actin (Santa Cruz) followed by anti-rabbit secondary antibodies.
Colony formation assay
To assess clonogenicity, PANC-1 cells were transfected with CST1-small interfering RNA (siRNA) (GenePharma, Shanghai, China) and NC control for 48 h and then seeded in six-well plates at a density of 200 cells/well. They were washed with PBS, fixed in methanol for 15 min, and stained with 0.5 % crystal violet for 15 min. The plates were then photographed, and the colonies were counted.
MTT assay
PANC-1 cells were seeded at a density of 5 × 103 cells per well in 96-well plates. Each well contained 100 ml of RPMI-1640 medium supplemented with 10 % FBS, and the cells were cultured for 24 h at 37 °C. They were then incubated for 48 h with 100 nM CST1- and NC-siRNA. After incubation with the complexes, the media were replaced, and 10 μl MTT solution (5 mg/ml) in PBS was added to each well. After the cells were further incubated for an additional 4 h, the MTT-containing medium was removed, and 110 μl dimethylsulfoxide (DMSO) was added to each well. The cells were then agitated gently for 5 min, and the absorbance of each well was recorded at 490 nm using an Infinite F200 Multimode plate reader (Tecan, Crailsheim, Germany). All experiments were performed in triplicate, and data are presented as the means ± standard deviations.
Xerograft assays in nude mice
Six- to eight-week-old male BALB/c athymic nude mice were purchased from Sun Yat-Sen University (Guangzhou, China). All animal experiments were approved by the Institutional Animal and Use Committee at the Third Affiliated Hospital of Sun Yat-Sen University. Two clones from PANC-1 cells transfected with CST1-siRNA and control NC-siRNA were used to form xenografts. For the xenografts, cells were collected by centrifugation, washed twice in PBS, and counted. Then, 3 × 106 cells were resuspended in 0.1 ml PBS and injected s.c. into nude mice (six mice per group). The volume of the tumors was monitored twice weekly.
ELISA analysis
Five serum samples of PC patients and five samples of healthy controls were prepared. CST1 protein expression was detected using human CST1 ELISA kit (CUSABIO, Wuhan, China) following the manufacturer’s protocol. The absorbance at 450 nm was measured by a microplate reader system (Towa Labo, Tokyo, Japan).
Statistical analysis
SPSS 18.0 software was used for all statistical analyses. Paired t tests were used to compare CST1 expression between PC and matched non-cancer tissues. Unpaired t tests were used to compare CST1 expression between cell lines. Differences were considered to be statistically significant if the probability of the difference occurring by chance was <5 in 100 (P < 0.05).
Results
Upregulation of CST1 in PC patients
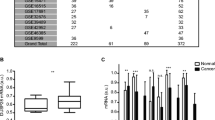
To identify a novel tumor marker for PC, the RNA expression profiles of three PC tissues and three normal tissues were compared using GeneChip microarrays. The data revealed that CST1 was upregulated significantly in PC tissues compared with normal pancreatic tissues (Fig. 1). Therefore, CST1 might be useful for the early diagnosis of PC.
CST1 mRNA and protein expression in patients
To confirm whether CST1 was upregulated in PC tissues, we next analyzed six PC tissues and six normal pancreatic tissues using RT-PCR. Consistent with the microarray data, CST1 mRNA expression was increased significantly by ~10-fold in cancer compared with normal tissues (Fig. 2a, b). In addition, Western blotting revealed that CST1 protein expression was also highly expressed in PC tissues but not in normal tissues (Fig. 2c). Immunohistochemistry confirmed that CST1 protein was upregulated in tumor tissues (Fig. 2d) but not in normal tissues. These results suggest that CST1 is highly expressed in PC tissues.
Upregulation of CST1 mRNA and protein expression in human pancreatic cancer tissues. Tissues from six patients with pancreatic cancer and six with other non-pancreatic cancer were prepared. Whole RNA was extracted, and PCR analysis was performed. Each cDNA generated from non-tumorous (N) and tumorous (T) tissues was used to amplify CST1. a The expression level of CST1 was calculated as the relative ratio of CST1 to β-actin in each sample, *P < 0.05. b PCR products were analyzed using agarose gel electrophoresis and EtBr staining. c Three tumorous and three non-tumorous tissues were analyzed using Western blotting. d Tissues from patients with pancreatic cancer were stained with anti-CST1 antibody. CST1 expression was significantly increased in tumorous tissues
CST1 mRNA and protein expression in cancer cell lines
We further investigated the effect of CST1 overexpression in PC cells. Its mRNA and protein expression was analyzed in nine different digestive cancer cell lines, including PC (PANC-1, Bxpc-3, and SW1990), liver cancer (HepG2 and Hep3B), colon cancer (HCT116, HT-29, and SW480), and gastric cancer cell lines (SCG-7901). As shown in Fig. 3a, CST1 was expressed differentially and at high levels in PC, colon cancer, and gastric cancer cell lines. However, CST1 was not expressed in liver cancer cell lines. CST1 expression was significantly higher in PC cell lines compared with the other cell lines (P < 0.05). Consistent with this, CST1 protein was expressed at high levels in PC but not in the other cell lines (Fig. 3b). This suggests that CST1 overexpression might play a specific role in PC but not in other digestive malignant diseases.
CST1 overexpression in human pancreatic cancer cell lines. Human pancreatic cancer cell lines (BXPC-3, PANC-1, SW1990), colorectal cancer cell lines (HCT116, SW480), gastric cancer cell lines SGC7901, liver cancer cell lines (Hep3B, HepG2), and gastric cancer cell line (SGC7901) were used. Total RNA was extracted and PCR analysis was performed. cDNA generated from each cell line was used as template for CST1 amplification. a The expression level of CST1 was calculated as the relative ratio of CST1 to β-actin in each sample.*, #, $P < 0.05 versus Bxpc-3, and HepG2 and Hep3B had no CST1 expression. b CST1 expression of different cell lines was analyzed by Western blotting
CST1 has an important effect on cell proliferation
Three siRNAs were purchased and assessed for their ability to downregulate CST1 protein; the siRNA that knocked down CST1 most efficiently was used in subsequent experiments (Fig. 4f). Colony formation experiments were performed using PANC-1 cells transfected with CST1-siRNA and NC-siRNA, and the data revealed that the mean colony number was decreased significantly in the CST1-siRNA group compared with the control (Fig. 4a, d). Moreover, the cell viability of PANC-1 cells was reduced significantly after transfection with CST1-siRNA (Fig. 4c).
CST1 expression contributes to human pancreatic cancer cell proliferation. PANC-1 cell was transfected with CST1-siRNA or the negative control. a Images of colony formation in PANC-1 cell transfected with NC or CST1-siRNA. b PANC-1 cell transfected with NC and CST1-siRNA, respectively, was injected to nude mice. Tumor volumes were measured. c MTT assay and cell viability was detected in PANC-1 cell transfected with NC and CST1-siRNA. d Colony number of colony formation test. e Tumor size of xenograft experiment. f CST1-siRNA significantly knocks down CST1 expression in PANC-1 cell line by Western blotting. Expression of proliferation-related protein p-AKT and PCNA by Western blotting indicated that CST1 may contribute to cell proliferation
Xenograft assays in nude mice showed that the tumor size was significantly smaller in the CST1-siRNA group 1 week after 3 × 106 cells transfected with CST1-siRNA or control vector had been transplanted on the surface of hip skin in nude mice (Fig. 4b, e). Next, the mechanism behind the reduced proliferation of PANC cells transfected with CST1-siRNA was assessed. The expression of PCNA and p-AKT, which both indicate cell proliferation, was analyzed (Fig. 4f), revealing that the expression of both markers was inhibited markedly in PANC-1 cell lines transfected with CST1-siRNA. In addition, the expression of malignancy-associated proteins, such as PCNA, cyclin D1, cyclin A2, and cyclin E, was decreased significantly in PANC-1 cells transfected with CST1-siRNA compared with control vector (Fig. 5). Therefore, these data suggest that CST1 might contribute to the proliferation of PC cells.
CST1 overexpression is closely associated with proliferative proteins in pancreatic cancer cell line. PANC-1 cell was transfected with CST1-siRNA or the negative control. Total RNA was extracted and PCR analysis was performed. cDNA generated from NC and CST1-siRNA cell line was used as template for a PCNA, b cyclin A2, c cyclin D1, and d cyclin E amplification. The expression level of each was calculated as the relative ratio of CST1 to β-actin, *P < 0.05
Expression of CST1 in serum
We detected CST1 expression in serum using ELISA kit. A representative calibration curve based on CST1 concentration of 0.156–10 ng/ml is shown in Fig. 6a. The results showed that CST1 can be detected in serum of pancreatic cancer patients. And the data revealed that CST1 expression in serum elevated in patients compared to healthy controls, and the differences were statistically significant (P < 0.05, Fig. 6b). These results revealed that CST1 can be a potential biomarker for clinical detection of PC.
Expression of CST1 in serum. Serum samples from five patients with pancreatic cancer and five healthy controls were prepared. Calibration curve of the ELISA system for CST1. Calibration values using purified CST1 (in ng/ml): 0.0, 0.156, 0.312, 0.625, 1.25, 2.5, 5.0, and 10. a The equation for the line is y = 0.296x + 0.089; r 2 = 0.998. b Serum samples from PC patients (T) and healthy controls (N) were analyzed using ELISA. *P < 0.05
Discussion
The CST superfamily inhibits the proteolytic activity of cysteine proteases by forming tight but reversible complexes [9, 17]. The type 2 cystatin superfamily includes CST1, CST2, CST3, CST4, CST5, CSTP1, CSTP2, and CST1 (CST SN). In addition, CST2 (CST SA) and CST4 (CST S) are known as S-type cystatins. Type 2 cystatins share >70 % sequence similarity, whereas S-type cystatins exhibit ~90 % homology with one another [10, 18, 19]. S-type cystatins have been detected in seminal plasma, tears, and tracheobronchial fluid, but not in other fluids [20, 21]. Many studies have revealed that cystatins play roles in tumor invasion and metastasis. For example, CST3 (CST C) was associated with invasion and metastasis in thyroid carcinomas [23] and colorectal tumors [24]. In addition, CST6 (CST M) and CST7 (CST F) were expressed differentially in metastatic mammary epithelial cells and metastatic squamous carcinomas [25–27].
Cystatin 1 encoding cystatin SN is a member of type 2 cystatins, and it is expressed in the submandibular gland, gall bladder, and uterus, but not in the pancreas according to the studies [22]. Little is known about the role of CST1 in cancer, but upregulation of cystatins is associated with tumor invasion and metastasis. Several studies have assessed the role of CST1 in gastric cancer, colorectal cancer, and esophageal squamous cell carcinoma. CST1 is strongly upregulated in gastric cancers and its overexpression is involved in cell proliferation [13]; CST1 expression elevated in colorectal cancer and its upregulation contributes to colorectal tumorigenesis by neutralizing the inhibitory effect of CST3 on cathepsin B’s proteolytic activity [14, 15]. Specifically, CST1 is overexpressed in these cancers and affects their survival [13–16]. In our study, we focused on the relationship between CST1 and PC and provided robust evidence to support the potential of CST1 as a biomarker for PC for the first time.
PC is one of the most malignant cancers because of its delayed diagnosis and ineffective treatments. The molecular mechanisms that underlie the development of PC remain unclear. Although several studies have investigated the mechanisms behind PC carcinogenesis, early diagnosis biomarkers, and novel therapeutic strategies, the overall 5-year survival rate remains at <5 % [1, 2]. Therefore, establishing new biomarkers for PC is urgent. The most commonly used serum biomarkers for PC are CA19-9, CA242, and carcinoembryonic antigen (CEA). CA19-9 is considered to be the most useful biomarker for PC, with a sensitivity of ~80 % and a specificity of ~75–80 %, and its serum levels correlate positively with the stages of PC [28, 29]. The sensitivity of CA242 for detecting PC is 41–75 %, and the specificity is 85–95 % [30, 31]. CEA is widely used as a tumor marker because of its high expression in many cancers. However, its sensitivity and specificity as a tumor marker for PC are both low, and its levels are also increased in other gastrointestinal tumors and some benign diseases. Therefore, CEA has only limited use for the early detection of PC. The low sensitivity and specificity of serum markers alone cannot meet the clinical requirements for PC. Therefore, multi-marker detection systems are now used to improve sensitivity and specificity. In addition, researchers have been seeking novel tumor biomarkers for improved early diagnosis of PC.
In the current study, we used microarrays to compare gene expression in PC and control tissues and revealed that the S-type cystatin genes (CST SN, CST SA, and CST A) were the most highly and significantly expressed of the 22,000 genes present on the array (Fig. 1). From the results, we hypothesized that CST1 can be a biomarker for pancreatic cancer. To verify our hypothesis, we found that CST1 mRNA and protein were highly expressed in PC tissues compared with non-cancer controls (Fig. 2). Moreover, CST1 was expressed at much higher levels in PC cell lines compared with liver or gastric cancer cell lines (Fig. 3). The use of CST1-siRNA to suppress CST1 in PANC-1 cells followed by colony formation assays, xenografts, and the MTT assay suggested that CST1 might be related to cell proliferation (Fig. 4). Consistent with this, CST1 expression upregulated the expression of malignancy-associated proteins, such as PCNA, cyclin D1, cyclin A, and cyclin E in PANC-1 cells (Fig. 5). Therefore, we speculate that CST1 might contribute to cell proliferation and that it could be used as a potential biomarker for PC. If we want to use the expression level of CST1 for early detection of PC, it would be necessary that it can be detected in serum, then we detected CST1 protein expression in serum by ELISA, the results showed that it can be detected in serum, and its expression in serum elevated in patients compared to healthy controls, and the differences were statistically significant (P < 0.05, Fig. 6). It may raise the accuracy of diagnosis by combining with other commonly used biomarkers such as CA19-9, CA242, and CEA. Therefore, further studies are necessary to validate this marker for clinical application.
This study shows that the upregulation of CST1 in PC contributes to cell proliferation, and it may be a potential biomarker for early detection of PC.
References
Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Guo X, Cui Z. Current diagnosis and treatment of pancreatic cancer in China. Pancreas. 2005;31:13–22.
Costello E, Greenhalf W, Neoptolemos JP. New biomarkers and targets in pancreatic cancer and their application to treatment. Nat Rev Gastroenterol Hepatol. 2012;9:435–44.
Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol. 2013;107:15–22.
Lah TT, Babnik J, Schiffmann E, Turk V, Skaleric U. Cysteine proteinases and inhibitors in inflammation: their role in periodontal disease. J Periodontol. 1993;64:485–91.
Travis J, Potempa J. Bacterial proteinases as targets for the development of second-generation antibiotics. Biochim Biophys Acta. 2000;1477:35–50.
Koblinski JE, Ahram M, Sloane BF. Unraveling the role of proteases in cancer. Clin Chim Acta. 2000;291:113–35.
Barrett AJ. The cystatins: a diverse superfamily of cysteine peptidase inhibitors. Biomed Biochim Acta. 1986;45:1363–74.
Dickinson DP, Zhao Y, Thiesse M, Siciliano MJ. Direct mapping of seven genes encoding human type 2 cystatins to a single site located at 20p11.2. Genomics. 1994;24:172–5.
Turk B, Turk V, Turk D. Structural and functional aspects of papain-like cysteine proteinases and their protein inhibitors. Biol Chem. 1997;378:141–50.
Dickinson DP, Thiesse M, Hicks MJ. Expression of type 2 cystatin genes CST1-CST5 in adult human tissues and the developing submandibular gland. DNA Cell Biol. 2002;21:47–65.
Choi EH, Kim JT, Kim JH, Kim SY, Song EY, Kim JW, et al. Upregulation of the cysteine protease inhibitor, cystatin SN, contributes to cell proliferation and cathepsin inhibition in gastric cancer. Clin Chim Acta. 2009;406:45–51.
Yoneda K, Lida H, Endo H, Hosono K, Akiyama K, Takahashi H, et al. Identification of cystatin SN as a novel tumor marker for colorectal cancer. Int J Oncol. 2009;35:33–40.
Kim JT, Lee SJ, Kang MA, Park JE, Kim BY, Yoon DY, et al. Cystatin SN neutralizes the inhibitory effect of cystatin C on cathepsin B activity. Cell Death Dis. 2013;19(4):e974.
Chen YF, Ma G, Cao X, Luo RZ, He LR, He JH, et al. Overexpression of Cystatin SN positively affects survival of patients with surgically resected esophageal squamous cell carcinoma. BMC Surg. 2013;13:15.
Lindahl P, Abrahamson M, Bjork I. Interaction of recombinant human cystatin C with the cysteine proteinases papain and actinidin. Biochem J. 1992;281:49–55.
Isemura S, Saitoh E, Sanada K, Minakata K. Identification of full-sized forms of salivary (S-type) cystatins (cystatin SN, cystatin SA, cystatin S, and two phosphorylated forms of cystatin S) in human whole saliva and determination of phosphorylation sites of cystatin S. J Biochem. 1991;110:648–54.
Thiesse M, Millar SJ, Dickinson DP. The human type 2 cystatin gene family consists of eight to nine members, with at least seven genes clustered at a single locus on human chromosome 20. DNA Cell Biol. 1994;13:97–116.
Henskens YM, Veerman EC, Nieuw Amerongen AV. Cystatins in health and diseases. Biol Chem Hoppe Seyler. 1996;37:71–86.
Buttle DJ, Burnett D, Abrahamson M. Levels of neutrophil elastase and cathepsin B activities, and cystatins in human sputum: relationship to inflammation. Scand J Clin Lab Invest. 1990;50:509–16.
Dickinson DP, Thiesse M, Dempsey LD, Millar SJ. Genomic cloning, physical mapping, and expression of human type 2 cystatin genes. Crit Rev Oral Biol Med. 1993;4:573–80.
Burka T, van der Noen H, Patil S. Cysteine proteinase inhibitor in cultured human medullary thyroid carcinoma cells. Lab Invest. 1992;66:691–700.
Kos J, Krasovec M, Cimerman N, Nielsen HJ, Christenser IJ, Brunner N. Cysteine proteinase inhibitors stefin A, stefin B, and cystatin C in sera from patients with colorectal cancer: relation to prognosis. Clin Cancer Res. 2000;6:505–11.
Sotiropoulou G, Anisowicz A, Sager R. Identification, cloning, and characterization of cystatin M, a novel cysteine proteinase inhibitor, down-regulated in breast cancer. J Biol Chem. 1997;272:903–10.
Haider AS, Peters SB, Kaporis H, Cardinale I, Fei J, Ott J, et al. Genomic analysis defines a cancer-specific gene expression signature for human squamous cell carcinoma and distinguishes malignant hyperproliferation from benign hyperplasia. J Invest Dermatol. 2006;126:869–81.
Vigneswaran N, Wu J, Zacharias W. Upregulation of cystatin M during the progression of oropharyngeal squamous cell carcinoma from primary tumor to metastasis. Oral Oncol. 2003;39:559–68.
Rosty C, Goggins M. Early detection of pancreatic carcinoma. Hematol Oncol Clin Am. 2002;16:37–52.
Kokhanenko NI, Ignashov AM, Varga EV, Polkanova MS, Aleshina LA, Kimbarovskaia AA, et al. Role of the tumor markers CA 19-9 and carcinoembryonic antigen (CEA) in diagnosis, treatment and prognosis of pancreatic cancer. Vopr Onkol. 2001;47:294–7.
Haglund C, Lundin J, Kuusela P, Roberts PJ. CA 242, a new tumour marker for pancreatic cancer: a comparison with CA 19-9, CA 50 and CEA. Br J Cancer. 1994;70:487–92.
Ventrucci M, Ubalducci GM, Cipolla A, Panella MA, Ligabue A. Serum CA 242: the search for a valid marker of pancreatic cancer. Clin Chem Lab Med. 1998;36:179–84.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, J., Liu, HL., Liu, ZH. et al. Identification of cystatin SN as a novel biomarker for pancreatic cancer. Tumor Biol. 36, 3903–3910 (2015). https://doi.org/10.1007/s13277-014-3033-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-3033-3