Abstract
The aim of this study is to investigate the effects of inhibiting Aurora-B on osteosarcoma (OS) cell malignant phenotype, phosphorylation of valosin-containing protein (VCP), and the activity of NF-κB signaling in vitro. The expressions of Aurora-B and p-VCP proteins were detected by immunohistochemistry in 24 OS tissues, and the relationship between Aurora-B and p-VCP was investigated. The results showed that there was a positive correlation between Aurora-B and p-VCP proteins. The expression of Aurora-B in human OS cell lines U2-OS and HOS cells was inhibited by specific short hairpin RNA (shRNA) lentivirus (AURKB-shRNA lentivirus, Lv-shAURKB) which targeted Aurora-B. The results showed that the phosphorylation of VCP, the activity of NF-κB signaling pathway and the malignant phenotype of OS cells were all suppressed by knockdown of Aurora-B. It indicated that the inhibition of Aurora-B alters OS cells malignant phenotype by downregulating phosphorylation of VCP and activating of the NF-κB signaling pathway in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma (OS), which has a high tendency to metastasize, is the most common human primary malignant bone tumor. The pulmonary metastasis is the leading cause of death in patients with extremities OS. Numerous studies showed that the 5-year survival rate of patients with metastatic diseases was less than 20 % [1–3]. Therefore, making clear the underlying molecular mechanisms of metastasis is necessary for the management of OS to improve the curative effect.
Aurora-B is located on chromosome 17p13.1, a region not typically amplified in human malignancies. Increasing evidences showed that Aurora-B is an important anti-tumor target [4, 5]. Recently, studies revealed that nuclear Aurora-B expression is strongly associated with metastasis in tumors [6–8]. However, whether Aurora-B is involved in OS development, progress, and the potential molecular mechanisms are still uncertain.
Matrix metalloproteinases (MMPs) are involved in the degradation of the basement membrane and epimatrix. Among them, MMP-2 and MMP-9 markedly correlate with tumor invasion [9]. The expressions of MMP-2 and MMP-9 are increased in OS cells that promote OS cells migration and invasion by degrading components of the basement membrane and epimatrix [10]. Substantial studies revealed that activation of the NF-κB gene, the upstream regulator of MMPs, promotes the tumor cell invasive and migratory abilities [11, 12]. In addition, the phosphorylation of valosin-containing protein (VCP) has been recognized as an important regulatory factor of NF-κB pathway in OS [13]. Our previous study also has showed that knockdown of VCP inhibited OS cell migration and invasion by regulating NF-κB signaling pathway [14].
In this study, we found that the inhibition of Aurora-B by short hairpin RNA (shRNA) resulted in decreased phosphorylation of VCP and activity of NF-κB signaling pathway. Meanwhile, the OS cells malignant phenotype was also impaired by knockdown of Aurora-B.
Materials and methods
Antibodies
Rabbit monoclonal Aurora-B, VCP, p-VCP, and IgG isotype control antibody were purchased from Abcam, and NF-κB (p65), MMP-2, MMP-9, and mouse monoclonal β-Actin were purchased from Cell Signaling Technology Inc.
Patient specimens
Twenty-four specimens from OS patients with pulmonary metastasis were collected before neoadjuvant chemotherapy in the Department of Orthopedic Surgery and the Department of Oncology, The First Affiliated Hospital of Nanchang University from 2005 to 2012. The tumor samples have been confirmed by musculoskeletal pathologists. The samples were fixed with 10 % formalin and embedded in paraffin and were then cut into 4-μm-thick sections. In all cases, informed consent was obtained from the relative departments and persons, and the study had the approval of the Ethics Committee of Nanchang University.
Immunohistochemical analysis
Histological sections cut at 4 μm were stained with hematoxylin and eosin (H and E) staining and detected by immunohistochemical analysis that was performed with S–P procedure. Briefly, antigen retrieval was performed by heating the deparaffinized rehydrated sections in 10-mm citrate buffer (pH 6.0) for 20 min, followed by blocking with 10 % goat serum. Then sections were incubated overnight at 4 °C with the primary antibody (rabbit anti-Aurora-B monoclonal antibody, Abcam) at a final dilution of 1:500. For negative controls, sections were incubated with rabbit IgG isotype control antibody (Abcam). After washing with rabbit IgG isotype control antibody for three times, sections were incubated with biotinylated secondary antibody for 40 min, followed by incubation with HRP-conjugated streptavidin for half an hour. Then the sections were chemiluminescence stained and counterstained using hematoxylin. Stained sections were evaluated and scored by two pathologic doctors in a blind manner without prior knowledge of the clinical pathological features of patients. According to the staining intensity by examining at least 500 cells in five representative areas, the expression level of Aurora-B was judged and the intensity scores were recorded as follows: none, 0; weak, 1; moderate, 2; and intense, 3. According to the percentage of tumor cells with positive expression of Aurora-B, the following percentage scores were recorded: 0 % (score 0), less than 10 % (score 1), 11 to 50 % (score 2), 51 to 80 % (score 3), and 81 to100% (score 4). The final score was averaged with the scores from the two pathologic doctors; these scores were calculated by adding the intensity score to the percentage score. The final score < 4 defined as (−), = 4 defined as (+), = 5 defined as (++), and more than 6 is divided into (+++).
Construction of AURKB-shRNA-expressing lentiviral vector
The shRNAs targeting Aurora-B messenger RNA (mRNA) were designed online (www.invitrogen.com/rnai): Lv-shRNA-AURKB, 5′-AGAGCTGCACATTTGACGA-3′; Lv-shRNA-CON, 5′-TTCTCCGAACGTGTCACGT-3′, was used as control. The lentivirus expression plasmid (pGC-Lv-AURKB or pGC-Lv-control vector), together with pHelper 1.0 and pHelper 2.0 plasmids that contained the imperative elements for virus packaging, were coinfected into 293 T cells with Lipofectamine 2000, according to the manufacturer’s instructions for the generation of AURKB-shRNA lentivirus (Lv-shAURKB) or control lentivirus (LV-shCON). Lentivirus was harvested at 48 h post-infection, centrifuged to get rid of cell debris, and then filtered through 0.45-μm cellulose acetate filters followed by ultracentrifugation.
Cell culture and transfection
The human OS cell line U2-OS and HOS was purchased from American Type Culture Collection (Manassas, VA) and routinely cultured in RPMI-1640 (HyClone) supplemented with 10 % fetal bovine serum (Sigma) in a humidified 37 °C incubator containing 5 % CO2. OS cells were grown to 30–40 % confluence and infected with Lv-shAURKB or Lv-shCON at MOI of 12.5(U2-OS) or 25(HOS). In order to determine the infection efficiency, cells expressing GFP protein were observed using fluorescence microscopy (ECLIPSE-TS-100, Nikon, Japan) 48 h after infection.
qRT-PCR
The total RNA was isolated from OS cells after inflected for 72 h with Trizol reagent (Invitrogen, USA). Reverse transcription was performed with 2 mg of the total RNA using PrimeScript RT reagent Kit (Takara, Co, Japan). Then each sample was analyzed by quantitative real-time PCR (qPCR) (Bio-Rad, Hercules, CA, USA) under the conditions described in the SYBR Premix Ex Tap II (Invitrogen, USA): 50 °C for 2 min, 95°Cfor 2 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s. Primer sequences information is summarized in Table 1. Relative expression was calculated using the 2−ΔΔCt method. All experiments were repeated six times over multiple days.
Western blotting assays
The total protein from the cells was extracted using RIPA lysis buffer containing 60 μg/ml PMSF. Protein concentrations were determined by BCA protein assay kit (Boster, China). The protein samples were denatured at 100 °C for 10 min and then preserved at −20 °C for later use. The proteins were separated by SDS-polyacrylamied gels and transblotted onto PVDF membranes. The PVDF membranes were blocked with 5 % skim milk for 1 h at room temperature and probed with primary antibodies (rabbit anti-Aurora-B IgG, 1:5000; rabbit anti-VCP, anti-p-VCP, anti-NF-κB (p65), anti-MMP-9, and anti-MMP-2 IgG, 1:1000; mouse anti-β-actin, 1:2000) overnight at 4 °C. After an incubation with the appropriate anti-rabbit, or anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5000; Boster, China) for 1.5 h at room temperature, immunoreactive bands were visualized by the chemiluminescence dissolvent (Thermo, USA) and exposure to the X-ray film (Kodak, USA). The determination of grayscale value was processed by Image J. All experiments were repeated six times over multiple days.
Cell proliferation assay
OS cells were seeded into five 96-well tissue culture plates (Nunclon™) at a density of 5 × 103 cells/mL in a volume of 200-μL culture media. Each day, OS cells in 96-well plates were detected for continuous 5 days. Each well was added with 20 μL of MTT reagent (0.5 mg/mL) and incubated at 37 °C for 4 h. Afterwards, the supernatant was sucked out, and the same volume of dimethyl sulfoxide (DMSO) was added to each well to dissolve the resulting formazan crystals at 37 °C for 20 min. The optical density values (OD value) were measured at 490-nm wave length using a plate reader (BioTek Company). All experiments were repeated six times over multiple days.
TUNEL assay
We used TUNEL assay to evaluate apoptotic activity by DNA breakage. Each group cells were smeared on slides and fixed with 4 % paraformaldehyde at room temperature for 30 min then preprocessed with freshly prepared permeabilization solution containing 0.1 % Triton X–100 and 0.1 % sodium citrate for 15 min for optimal proteolysis. Apoptosis was detected with an apoptosis detection kit (KeyGen, China). The TdT reaction was carried out for 1 h at 37 °C in a humidified chamber, and sections were stained with DAPI for nuclei. TUNEL-positive cells were counted in a random selection of ten fields and expressed as a percentage of normal nuclei under confocal laser microscopy. The rate of apoptosis was calculated by the following formula: number of apoptotic cells counted in the five views/total number of cells counted in the five views × 100 %. The cells apoptotic rate was obtained by counting five fields per area and represented as the average of six independent experiments done over multiple days.
Cell cycle analysis
Cells were harvested and washed with cold PBS and then fixed with 75 % ethanol at −20 °C overnight. The fixed cells were washed with cold PBS twice, and incubated in 500 μL DNA staining solution (including 200 μg/mL RNase A and 20 μg/mL propidium iodide staining solution) for 30 min at 37.0 °C. Finally, cells were analyzed by flow cytometry in the presence of the dye. All experiments were repeated six times over multiple days.
Transwell assays
Invasion of OS cells were measured by BD BioCoat™ BD Matrigel™ Invasion Chamber (BD Bioscience, NJ, USA) according to the manufacturer’s protocol. The medium in the lower chamber contained 5 % fetal calf serum as a source of chemoattractants. Cultures were rinsed with PBS and replaced with fresh quiescent medium alone or containing 10 % FBS, following which the cells were incubated at 37 °C for 24 h. Cells that passed through the Matrigel-coated membrane were stained with Diff-Quik (Sysmex, Kobe, Japan) and photographed. Cell migration was quantified by direct microscopic visualization and counting. The values for invasion were obtained by counting three fields per membrane and represented as the average of six independent experiments done over multiple days.
Wound healing assay
We assessed cell migration by determining the ability of the cells to move into a cellular space in a two-dimensional in vitro “wound healing assay.” In brief, cells were grown to confluence in 6-well tissue culture plastic dishes to a density of approximately 5 × 106 cells/well. The cells were denuded by dragging a rubber policeman (Fisher Scientific, Hampton, NH, USA) through the center of the plate. Cultures were rinsed with PBS and replaced with fresh quiescent medium alone or containing 10 % FBS, following which the cells were incubated at 37 °C for 24 h. Photographs were taken at 0 and 24 h, and the migrated distance was measured. The cells migration rate was obtained by counting three fields per area and represented as the average of six independent experiments done over multiple days.
Statistical analysis
The correlation of Aurora-B with p-VCP protein in OS tissues was evaluated using the Wilcoxon rank-sum test. All measurement data were presented as \( \overline{X} \) ± SD and analyzed by one-way ANOVA. A value of P < 0.05 was considered as a significant difference. All analysis was performed with SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Positive correlation between Aurora-B and p-VCP protein expression in OS tissues with pulmonary metastasis
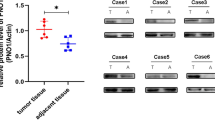
In order to investigate the relationship between Aurora-B and p-VCP in OS, the Aurora-B and p-VCP proteins in 24 OS samples from patients with pulmonary metastasis was detected by immunohistochemistry. Aurora-B and p-VCP protein was expressed in nucleus (Fig. 1), which was consistent with previous reports [15, 16]. The results revealed that the positive expression rates of Aurora-and p-VCP protein were 91.7 % (22/24) and 83.3 % (20/24) in OS tissues, respectively. The relationship was significant between Aurora-B and p-VCP protein expressions in OS tissues (Spearman’s rho, rs = 0.712).
Representative images of a HE staining and immunohistochemical staining of b Aurora-B or c p-VCP protein in OS. a Cells in osteosarcoma tissues with pulmonary metastasis were polygonal and short spindle, with large pleomorphic nuclei and abundant cytoplasm (×400). b Aurora-B protein was showed brownish-yellow particle deposition and expressed in the nucleus (×400). c p-VCP protein was shown as medium brown particle deposition and expressed in the nucleus (×400)
The specific shRNA lentivirus inhibited Aurora-B expression in OS cells
In order to investigate the effects of inhibiting Aurora-B in the subsequent experiments, the LV-shAURKB was used to suppress Aurora-B expression in U2-OS and HOS cells. The results of qRT-PCR and Western blot assays show that the Aurora-B mRNA and protein levels were significantly lower in cells infected with LV-shAURKB than in those infected with Lv-shCON or without infection (Fig. 2).
The specific shRNA lentivirus suppressed the expression of Aurora-B in OS cells. a The expression of Aurora-B protein in OS cells was measured by Western blot. It showed that the level of Aurora-B was significantly blocked by Lv-shAURKB. b, c The expression of Aurora-B mRNA was measured by qRT-PCR. It revealed that the Aurora-B mRNA level was inhibited by LV-shAURKB. Columns, mean (n = 6); bars, SD; *p < 0.05 vs Lv-shCON and CON groups
Silencing Aurora-B decrease phosphorylation of VCP in OS cells
In order to investigate the effect of inhibiting Aurora-B on VCP expression and activity, the U2-OS and HOS cells were transfected with LV-shAURKB or Lv-shCON for 8 h, then replaced by new nutrient medium and cultured for 72 h. The level of VCP mRNA was measured by qRT-PCR, and the proteins of VCP and p-VCP were detected by Western blot. The results showed that the expressions of VCP mRNA and protein did not inhibited by knockdown of Aurora-B. Interestingly, the p-VCP protein was significantly suppressed by silencing Aurora-B in OS cells (Fig. 3). It indicated that knockdown of Aurora-B decrease phosphorylation of VCP in OS cells.
The effect of inhibiting Aurora-B on decreasing phosphorylation of VCP and activating of NF-κB signaling pathway in OS cells was investigated by Western blot and qRT-PCR. a Inhibiting Aurora-B could decrease p-VCP, NF-κB (p65), MMP-2, and MMP-9 protein expression, which indicated that Aurora-B could downregulate the activity of VCP/NF-κB signaling. b, c The VCP mRNA was not inhibited by inhibiting Aurora-B. Columns, mean (n = 6); bars, SD; *p > 0.05 vs Lv-shCON and CON groups
Silencing Aurora-B downregulates NF-κB signaling activity in OS cells
To evaluate the effects of silencing Aurora-B on NF-κB signaling in OS cells, the NF-κB (p65), and MMP-2 and MMP-9 were measured using Western blot analysis in U2-OS and HOS cells. Results revealed that NF-κB (p65) and MMP-2 and MMP-9 protein expressions in cells transfected with Lv-shAURKB were significantly lower than the cells treated with Lv-shCON and untreated (Fig. 3a). It suggested that silencing Aurora-B could suppress NF-κB signaling activity in OS cells.
Inhibiting Aurora-B inhibits proliferation in OS cells
In order to investigate the effect of inhibiting Aurora-B on proliferation in U2-OS and HOS cells, the growth curve was measured by MTT assay. Results revealed the proliferation rate in cells transfected with Lv-shAURKB was obviously lower than cells infected with Lv-shCON and untreated (Fig. 4). It indicated that silencing Aurora-B could inhibit OS cells proliferation in vitro.
Silencing Aurora-B induces apoptosis and cell-cycle arrest in OS cells
To investigate the mechanisms that knockdown of Aurora-B inhibits the cell proliferation in OS cells, the effect of inhibiting Aurora-B on cell apoptosis and cell cycle were measured with TUNEL Kit and flow-cytometric analysis, respectively. The results revealed that the apoptotic rate in OS cells transfected with Lv-shAURKB was significantly higher than that in cells transfected with Lv-shCON and without transfection. And the cells in G2 phase were much more in cells infected Lv-shAURKB than that treated with Lv-shCON and untreated. It indicated that decreasing Aurora-B induces apoptosis and cell-cycle arrest in G2 phase in OS cells (Fig. 5).
The effect of inhibiting Aurora-B on cell apoptosis and cell-cycle in OS cells. a The apoptotic rate was significantly higher in cells transfected by Lv-shAURKB than those transfected by LV-shCON and un-transfected. b The cell numbers at G2 phase in cells infected with Lv-shAURKB was significantly more than those in cells infected with LV-shCON and without infection (*p < 0.05 vs LV-shCON and CON groups)
Knockdown of Aurora-B inhibits OS cells migration and invasion
To investigate the inhibitory effects of silencing Aurora-B on OS cells’ migratory and invasive ability, transwell and wound healing assays were performed. In transwell invasion assays, the number of invaded cells in cells infected by LV-shAURKB was significantly lower than those of cells infected by LV-shCON and without infection (P < 0.05) (Fig. 6a). The wound healing assays showed that the migrated rate of OS cells infected by Lv-shAURKB was significantly lower than in those cells infected by LV-shCON and without infection (Fig. 6b). These data indicated that inhibiting of Aurora-B could suppress the migratory and invasive abilities of OS cells in vitro.
Knockdown of Aurora-B inhibits OS cell migration and invasion in vitro. a The invaded cells were significantly lower in cells treated with Lv-shAURKB than those cells treated with LV-shCON and untreated. b The migration rate was significantly lower in cells infected with Lv-shAURKB than those cells infected with LV-shCON and without infection. It indicates the knockdown of Aurora-B could inhibit cell migratory and invasive ability of OS cells (*p < 0.05 vs LV-shCON and CON groups)
Discussion
In this study, we first found that knockdown of Aurora-B induced OS cell apoptosis and cell-cycle arrest and inhibited OS cells’ viability and migratory and invasive abilities in vitro. Besides, for the first time, we identified that silencing Aurora-B suppressed phosphorylation of VCP and the activity of NF-κB signaling pathway in OS.
Aurora kinases are serine/threonine kinases essential for cell cycle control and mitosis. Aurora-B, a member of Aurora kinases family, is located on the chromosome arms during prophase and at the centromeres during prometaphase and metaphase. Aurora-B subsequently localizes to the midbody during cytokinesis. Aurora-B was over-expressed and inhibition of Aurora-B could block cell proliferation and induce cell apoptosis, cell-cycle arrest in varieties tumor [17, 18]. Recently, numerous studies showed that the upregulated expression was associated with increased tumor cells metastasis, and downregulation of Aurora-B could inhibit cells invasion and migration in various tumors [19, 20]. These findings indicated that Aurora-B may be a promising molecular target for tumor management. However, the mechanism of inhibiting Aurora-B on cells malignant phenotype in OS remains to be fully elucidated. In present study, we found that knockdown of Aurora-B induced cell apoptosis, cell-cycle arrest at G2 phase and suppressed cell proliferation and migratory and invasive abilities in OS cells. Our findings suggested that knockdown of Aurora-B could alter OS cell malignant phenotype.
VCP acts as a molecular chaperone and is implicated in a large number of ATP-dependent cellular processes, such as membrane fusion, ubiquitin/proteasome-mediated proteolysis, endoplasmic reticulum -associated degradation, transcription activation, stress response, cell cycle regulation, and apoptosis [21, 22]. Recently, studies indicated that inhibiting the VCP-dependent degradation of polyubiquitinated IκBα could suppress NF-κB activation [23]. Activation of NF-κB was demonstrated to upregulate MMP-9 [24], and inhibition of NF-κB was identified to downregulate MMP-2 [25]. During the development of metastasis, cancer cells must degrade the components of the extracellular matrix. MMPs, particularly MMP-2 and MMP-9, are markedly associated with this process due to their capacity for degrading the extracellular matrix, promoting tumor invasion.
To investigate whether knockdown of Aurora-B could decrease the phosphorylation of VCP, we analyzed the relationship of Aurora-B and p-VCP protein expressions in OS tissues. The results revealed that there was a positive correlation between Aurora-B and p-VCP protein in OS tissues, suggesting that there may be an interaction between Aurora-B and phosphorylation of VCP. Furthermore, the effects of silencing Aurora-B on VCP expression were evaluated in OS cells. Our data showed that both VCP mRNA and protein levels were no significantly decreased in cells that silencing Aurora-B, when compared with those in normal OS cells. Interestingly, the p-VCP protein was significantly lower in Aurora-B knocked cells than in normal cells. It suggested that silencing Aurora-B decreased phosphorylation of VCP in OS cells. Furthermore, to investigate inhibiting Aurora-B led to decreasing of the NF-κB signaling pathway. NF-κB (p65) and MMP-2 and MMP-9 protein expression levels were detected by Western blot analysis. The results showed that the proteins of NF-κB (p65), MMP-2, and MMP-9 were significantly decreased in cells infected by Lv-shAURKB compared with those infected by LV-shCON and CON. It indicated that inhibiting Aurora-B downregulates NF-κB signaling pathway activity in OS cells.
In summary, the present study showed that silencing Aurora-B suppressed OS cell malignant phenotype. In addition, our results showed that inhibiting Aurora-B induced OS cell invasion and migration partly through decreasing phosphorylation of VCP and NF-κB signaling pathway. However, substantial data showed that tumor microenvironment played an important role in tumor development and metastasis. Therefore, it is necessary to perform further experiments in vivo to confirm whether silencing Aurora-B can be used as a new molecular strategy in the management of OS.
References
Mialou V, Philip T, Kalifa C, Perol D, Gentet JC, Marec-Berard P, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer. 2005;104(5):1100–9. doi:10.1002/cncr.21263.
Hegyi M, Semsei AF, Jakab Z, Antal I, Kiss J, Szendroi M, et al. Good prognosis of localized osteosarcoma in young patients treated with limb-salvage surgery and chemotherapy. Pediatr Blood Cancer. 2011;57(3):415–22. doi:10.1002/pbc.23172.
Stokkel MP, Linthorst MF, Borm JJ, Taminiau AH, Pauwels EK. A reassessment of bone scintigraphy and commonly tested pretreatment biochemical parameters in newly diagnosed osteosarcoma. J Cancer Res Clin Oncol. 2002;128(7):393–9. doi:10.1007/s00432-002-0350-5.
Bonet C, Giuliano S, Ohanna M, Bille K, Allegra M, Lacour JP, et al. Aurora B is regulated by the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway and is a valuable potential target in melanoma cells. J Biol Chem. 2012;287(35):29887–98. doi:10.1074/jbc.M112.371682.
Tsuno T, Natsume A, Katsumata S, Mizuno M, Fujita M, Osawa H, et al. Inhibition of Aurora-B function increases formation of multinucleated cells in p53 gene deficient cells and enhances anti-tumor effect of temozolomide in human glioma cells. J Neurooncol. 2007;83(3):249–58. doi:10.1007/s11060-007-9335-1.
Pohl A, Azuma M, Zhang W, Yang D, Ning Y, Winder T, et al. Pharmacogenetic profiling of Aurora kinase B is associated with overall survival in metastatic colorectal cancer. Pharmacogenomics J. 2011;11(2):93–9. doi:10.1038/tpj.2010.18.
Hetland TE, Nymoen DA, Holth A, Brusegard K, Florenes VA, Kaern J, et al. Aurora B expression in metastatic effusions from advanced-stage ovarian serous carcinoma is predictive of intrinsic chemotherapy resistance. Hum Pathol. 2013;44(5):777–85. doi:10.1016/j.humpath.2012.08.002.
Sanchez-Bailon MP, Calcabrini A, Gomez-Dominguez D, Morte B, Martin-Forero E, Gomez-Lopez G, et al. Src kinases catalytic activity regulates proliferation, migration and invasiveness of MDA-MB-231 breast cancer cells. Cell Signal. 2012;24(6):1276–86.
Li WW, Long GX, Liu DB, Mei Q, Wang JF, Hu GY, et al. Cyclooxygenase-2 inhibitor celecoxib suppresses invasion and migration of nasopharyngeal carcinoma cell lines through a decrease in matrix metalloproteinase-2 and -9 activity. Pharmazie. 2014;69(2):132–7.
Roomi MW, Kalinovsky T, Rath M, Niedzwiecki A. In vitro modulation of MMP-2 and MMP-9 in pediatric human sarcoma cell lines by cytokines, inducers and inhibitors. Int J Oncol. 2014;44(1):27–34. doi:10.3892/ijo.2013.2159.
Liao CL, Lin JH, Lien JC, Hsu SC, Chueh FS, Yu CC, et al. The crude extract of Corni fructus inhibits the migration and invasion of U-2 OS human osteosarcoma cells through the inhibition of matrix metalloproteinase-2/-9 by MAPK signaling. Environ Toxicol. 2013. doi:10.1002/tox.21894.
Tomonaga M, Hashimoto N, Tokunaga F, Onishi M, Myoui A, Yoshikawa H, et al. Activation of nuclear factor-kappa B by linear ubiquitin chain assembly complex contributes to lung metastasis of osteosarcoma cells. Int J Oncol. 2012;40(2):409–17. doi:10.3892/ijo.2011.1209.
Vandermoere F, El Yazidi-Belkoura I, Slomianny C, Demont Y, Bidaux G, Adriaenssens E, et al. The valosin-containing protein (VCP) is a target of Akt signaling required for cell survival. J Biol Chem. 2006;281(20):14307–13. doi:10.1074/jbc.M510003200.
Long XH, Zhang ZH, Liu ZL, Huang SH, Luo QF. Inhibiting valosin-containing protein suppresses osteosarcoma cell metastasis via AKT/nuclear factor of kappa B signaling pathway in vitro. Indian J Pathol Microbiol. 2013;56(3):190–5. doi:10.4103/0377-4929.120358.
Dobrynin G, Popp O, Romer T, Bremer S, Schmitz MH, Gerlich DW, et al. Cdc48/p97-Ufd1-Npl4 antagonizes Aurora B during chromosome segregation in HeLa cells. J Cell Sci. 2011;124(Pt 9):1571–80. doi:10.1242/jcs.069500.
Ioannou M, Kouvaras E, Stathakis E, Samara M, Koukoulis GK. Aurora B kinase in Hodgkin lymphoma: immunohistochemical pattern of expression in neoplastic Hodgkin and Reed-Sternberg cells. J Mol Histol. 2013. doi:10.1007/s10735-013-9561-0.
Long ZJ, Xu J, Yan M, Zhang JG, Guan Z, Xu DZ, et al. ZM 447439 inhibition of aurora kinase induces Hep2 cancer cell apoptosis in three-dimensional culture. Cell Cycle. 2008;7(10):1473–9.
Mehra R, Serebriiskii IG, Burtness B, Astsaturov I, Golemis EA. Aurora kinases in head and neck cancer. Lancet Oncol. 2013;14(10):e425–35. doi:10.1016/S1470-2045(13)70128-1.
Jha HC, Lu J, Saha A, Cai Q, Banerjee S, Prasad MA, et al. EBNA3C-mediated regulation of aurora kinase B contributes to Epstein-Barr virus-induced B-cell proliferation through modulation of the activities of the retinoblastoma protein and apoptotic caspases. J Virol. 2013;87(22):12121–38. doi:10.1128/JVI. 02379-13.
Marampon F, Gravina GL, Popov VM, Scarsella L, Festuccia C, La Verghetta ME, et al. Close correlation between MEK/ERK and Aurora-B signaling pathways in sustaining tumorigenic potential and radioresistance of gynecological cancer cell lines. Int J Oncol. 2014;44(1):285–94. doi:10.3892/ijo.2013.2167.
Wang Q, Song C, Li CC. Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol. 2004;146(1–2):44–57. doi:10.1016/j.jsb.2003.11.014.
Rumpf S, Lee SB, Jan LY, Jan YN. Neuronal remodeling and apoptosis require VCP-dependent degradation of the apoptosis inhibitor DIAP1. Development. 2011;138(6):1153–60. doi:10.1242/dev.062703.
Hotta K, Nashimoto A, Yasumura E, Suzuki M, Azuma M, Iizumi Y, et al. Vesnarinone suppresses TNFalpha mRNA expression by inhibiting valosin-containing protein. Mol Pharmacol. 2013;83(5):930–8. doi:10.1124/mol.112.081935.
Andela VB, Gordon AH, Zotalis G, Rosier RN, Goater JJ, Lewis GD, et al. NFkappaB: a pivotal transcription factor in prostate cancer metastasis to bone. Clin Orthop Relat Res. 2003;415 Suppl:S75–85. doi:10.1097/01.blo.0000093048.96273.aa.
Felx M, Guyot MC, Isler M, Turcotte RE, Doyon J, Khatib AM, et al. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin Sci (Lond). 2006;110(6):645–54. doi:10.1042/CS20050286.
Acknowledgments
The present study was supported by grants from the National Natural Science Foundation of China (no. 81260400), the Natural Science Foundation of Jiangxi Province (no. 20114BAB205093 and 20142BAB205056), and Jiangxi Province Education Department of Science and Technology (no. GJJ12097).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jian Ying He, Wei Hong Xi, and Liang Bo Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
He, J.Y., Xi, W.H., Zhu, L.B. et al. Knockdown of Aurora-B alters osteosarcoma cell malignant phenotype via decreasing phosphorylation of VCP and NF-κB signaling. Tumor Biol. 36, 3895–3902 (2015). https://doi.org/10.1007/s13277-014-3032-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-3032-4