Abstract
Prognosis in patients with lung cancer is poor. Neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinase-9 (MMP-9) are proteins involved in the invasion and metastases of cancer. The objective of this study is to determine if there is a relationship between tumor expression of NGAL and MMP-9 in lung adenocarcinoma patients with prognosis and overall survival. Retrospective analysis was made of patients with lung adenocarcinoma treated at Medica Sur Hospital between 2005 and 2013. Tumor tissue was analyzed for NGAL and MMP-9 expression by immunohistochemistry. We identified 41 patients. Mean overexpression in tumoral tissue of NGAL was 70 % and 30 % for MMP-9. Univariate analysis revealed that prognostic factors associated with overall survival (OS) were NGAL expression and stage at diagnosis. Median OS for NGAL expression <70 % was 45.7 months (95 % CI; 15.2–76.2) and for patients with ≥70 % 4.6 months (95 % CI; 0.5–18.8; P < 0.0001), and for stage at diagnosis (stages I and II mean not reached), stage III mean OS 15.57 months (95 % CI; 9.8–21.2) and stage IV 9.6 months (95 % CI; 0.8–18.4. P = 0.002). No differences in OS were found for expression of MMP-9. Multivariate analysis revealed significance for OS in NGAL expression (HR 5.01 [95 % CI; 1.68–14.93] P = 0.004) and stage at diagnosis (HR 2.05 [95 % CI 1.30–3.22] P = 0.002). Tumoral tissue expression of NGAL ≥70 % confers a worse prognosis compared to those who did not. NGAL is an independent prognostic factor of stage at diagnosis.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Lung cancer is an aggressive disease. In 2012, it was estimated that 1,825,000 patients around the world were diagnosed with lung carcinoma, and mortality rates were as high as 1,590,000 deaths that year. In México, in that same year it had an incidence of 8,439 new cases (5,471 for men and 2,968 for women) and 7,608 casualties in both sexes representing the first cause of death from all malignancies [1]. As today, the most important factor for the prognosis of patients is the stage at diagnosis, with a worse outcome for those who present with a more advanced disease [2].
Despite new treatments, survival has been low even in the earliest stages (IA with a median overall survival of 60 months) and even lower in advanced stages (median survival of 6 months in stage IV) [2]. Due the impact of AJCC staging system for patient staging, it is also used to decide the most appropriate treatment. Generally, stages I and II benefit from local treatment with surgical resection and in some specific cases the use of adjuvant chemotherapy. Stage III patients need more aggressive treatment, even combining three modes of therapeutics: surgery, chemotherapy, and/or radiotherapy. Stage IV benefits from the palliative use of chemotherapy or molecular targeted therapy [3]. This staging system and its use for treatment only reflect the anatomical extent of the disease without a correlation with progression and/or overall survival of patients. Even in early stages, the prognosis can be more aggressive than expected and reflected with a rapid progression of regional disease or distant metastases [4], hence the importance for identifying the subset of individuals that are within this risk group.
Neutrophil gelatinase-associated lipocalin (NGAL) is a 25-kDa protein which is stored in the granules of human neutrophils. Overexpression of NGAL in human tumors and its impact in the prognosis and clinical outcomes has led to contradictory results. It has been described a worst stage at diagnosis and a short progression-free and overall survival in breast, esophagus, stomach, pancreas, and colon cancer [5–7] and a better prognosis in anaplastic thyroid and ovarian cancer [8].
One of the possible explanations of the negative prognosis of patients with malignant tumors that overexpress NGAL is the ability of this protein to positively modulate the activity of the matrix metalloproteinase-9 (MMP-9) enzyme. MMP-9 degrades the basement membranes and extracellular matrix, thus enabling angiogenesis, invasion, and metastasis of malignant cells [9]. There is a lack of prognostic information and clinical outcomes of NGAL and MMP-9 overexpression in lung adenocarcinoma. In this study, we investigated the NGAL and MMP-9 prognostic value in lung adenocarcinoma.
Material and methods
Patients
This retrospective study was approved by the institutional review board of our hospital. Tumoral tissue samples were obtained from patients with a diagnosis of non-small cell lung carcinoma (NSCLC) (stages I to IV according to TNM Staging System, 7th edition), with histological confirmation of adenocarcinoma, who attended the outpatient Oncology Clinic from Médica Sur Hospital from year 2005 to 2013. We excluded patients with non-adenocarcinoma histology and with previous chemotherapy or radiation therapy. Clinical data of all patients were obtained retrospectively, and it included clinical history, histopathology diagnosis, and smoking history. Never smoker was defined as having a lifetime exposure of <100 cigarettes [10]; the tobacco smoking index was calculated by multiplying the number of cigarette packs consumed per day by the number of years spent smoking [11].
Tissue procurement and expression of NGAL or MMP-9 by immunohistochemistry
Selecting the procedure for obtaining a biopsy from all patients depended on patient and tumor characteristics. Tumor specimens were collected at the time of diagnosis using computed tomography (CT)-guided tru-cut, lobectomy, or by bronchoscopy and were analyzed by the pathology department for their histologic diagnosis, who were blinded to the clinical outcomes. Immunostains were performed using the standard avidin-biotin-peroxidase method. Samples were later embedded in paraffin until processing. Antibodies were used with appropriate controls (Lipocalin-2 antibodies [NGAL-GTX63306] Clone: EPR5084 Brand: GeneTex® Dilution 1:100. Anti-metalloproteinase-9 [MMP-9-GTX100458] Clone: NC1 Brand: GeneTex® Dilution 1:200) then permanently mounted in resin for microscopy, and coverslips were placed for evaluation by an expert pathologist. To register the positivity of the immunoreactions, a qualitative and quantitative system determined by direct light microscopy observation was used. Tissue used as control to evaluate the positivity of NGAL-2 was the intense staining (3+) in the cytoplasm of the glandular cells of the endocervix. The tissue used as control to evaluate the reactivity of MMP-9 was a strongly positive reaction in alveolar macrophage cytoplasm (3+). A scale of 1+ (for weak staining) and for up to a 3+ (intense staining) for both antibodies in the cytoplasm of tumor cells was used. Likewise, in each case, the percentage of neoplastic cells that showed weak to strong positive staining was determined. Immunohistochemical staining results were delivered to researchers in a single-blinded way by the pathology department.
Regimen of treatment
Patients were treated according to the international guidelines for the treatment of lung cancer [3]. If they were suitable for adjuvant or palliative chemotherapy (CT), all patients received platinum-based CT as the first line of treatment. CT regimens included paclitaxel plus cisplatin or carboplatin and pemetrexed plus cisplatin or carboplatin. Second-line treatment choice was determined by the oncologist’s criteria.
Outcome measurement
All patients underwent CT or positron emission tomography (PET-CT) scans and were evaluated according to RECIST 1.1 at the baseline (before each line of treatment) and afterwards, every 2 to 3 cycles of the treatment in case of palliative treatment. Progression-free survival (PFS) was defined as the time from the start of the treatment until disease progression or last visit. Overall survival (OS) was defined as the time from histologic diagnosis until death or last follow-up visit.
Statistical analysis
Continuous variables were summarized as arithmetic means, medians, and standard deviations (SDs) for descriptive purposes, and categorical variables consisted of percentages and their respective 95 % confidence intervals (95 % CI). Inferential comparisons were made using the Student’s t test or the Mann-Whitney U test, according to the data distribution (normal or abnormal) determined by the Kolmogorov-Smirnov test. The χ 2 test or Fisher’s exact test were used for assessing the statistical significance of categorical variables, determined as being P < 0.05 when using a two-tailed test. Statistically significant and borderline significant variables (P < 0.1) were included in a multivariate logistic regression analysis. OS was analyzed with the Kaplan-Meier method, whereas comparisons among the subgroups were analyzed using the log rank or Breslow tests, if the two survival curves crossed. For survival curve analysis, all the variables were dichotomized (for age, we utilized the median). Adjustment for potential confounders was performed by using a multivariate Cox regression model, and hazard ratios (HR) were calculated along with their corresponding 95 % CIs as a measure of association. Statistical significance was determined as P < 0.05 using a two-tailed test. SPSS software version 19 (IBM) was used for all statistical analyses.
Results
Patient population
It was possible to identify a total of 41 patients who met the inclusion criteria within the time frame 2005–2013. The median age was 61.4 ± 10.1 years, 53.7 % were women and 46.3 % men; 75.6 % of patients had a smoking history.
The most common types of invasive adenocarcinoma were as follows: the predominant type was the mixed type (70.7 %), followed by lepidic (14.6 %). The grade of differentiation was moderately differentiated in the majority of patients (53.7 %), followed by poorly differentiated (39.1 %) and well differentiated (7.3 %). Vascular permeation was present in 68.3 % and perineural invasion in 9.8 % of all patients.
The stages at diagnosis were stage I (19.5 %), II (12.2 %), III (19.5 %), and IV (48.8 %). All patients stage II through IV received platinum-based cytotoxic chemotherapy treatment (adjuvant or palliative). The median follow-up of all patients was 25.6 ± 32.2 months. For most patients, the EGFR mutation status was unknown (n = 36, 87.80 %). Patient general characteristics are listed in Table 1.
Expression and intensity of NGAL and MMP-9 by immunohistochemistry
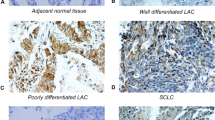
Of the 41 patients, 39 expressed NGAL in tumor tissue (95.12 %) (Fig. 1) and 31 patients (75.60 %) expressed MMP-9 (Fig. 2). All patients negative for NGAL immunohistochemistry were also negative for MMP-9. Most patients had a stain intensity of 3+ for NGAL (32 patients, 78.04 %) and 2+ for MMP-9 (15 patients, 35.68 %). Mean NGAL expression was 70 and 30 % for MMP-9. (Table 2) We found a relationship between the percentage expression of NGAL related to percentage of expression of MMP-9. For patients with NGAL <70 % (n = 19), the mean expression of MMP-9 was 22.3 % ± 27. For those with NGAL expression ≥70 % (n = 22), the mean expression of MMP-9 was 52.1 % ± 39 (P = 0.007, R = 0.48) (Fig. 3).
Progression-free survival
The median PFS for stages I–II was not reached. Median PFS for stages III–IV was 10.8 months (5.9–15.7). In univariate and multivariate analyses, no variables directly influenced PFS. A suggestive trend for better PFS with NGAL expression <70 % was 13.1 months (95 % CI 8.3–18) and for patients with NGAL expression ≥70 % was 7.8 months (95 % CI, 5.3–10.2, P = 0.062). For MMP-9, non-significant difference in PFS was shown: MMP-9 expression <30 % was 13.5 months (95 % CI 12.4–14.7) and for those with expression ≥30 % was 7.8 months (95 % CI, 1.7–13.9) (Table 3).
Response to chemotherapy
Only 30 patients were evaluated for RECIST with an objective response of 40 % (CR + PR 12 patients). There were no statistically significant differences regarding the objective responses in patients treated with CT related to sex, differentiation, vascular permeation, perineural invasion, and percentage of expression of MMP-9. We found a suggestive trend for worse response to chemotherapy with NGAL expression ≥70 % (no overall response in 11/15 (73.3 %) P = 0.065) (Table 4). The most common schemes used were paclitaxel and carboplatin (CBP) (55.4 %) and Pemetrexed + CBP (15.2 %) (Table 1).
Overall survival
The median OS was 17.0 months (range 4.7–29.2 months). In univariate analysis, only two variables directly influenced OS, stage at diagnosis and NGAL expression. Stages I and II (median OS not reached), stage III (median 15.57 months [95 % CI 9.8–21.2]), and stage IV (median 9.6 months [95 % 0.8–18.4 months], P = 0.002). OS with NGAL expression <70 % was 45.7 months (95 % CI 15.2–76.2) and for patients with NGAL expression ≥70 % was 4.6 months (95 % CI, 0.5–18.8; P < 0.0001) (Fig. 4). We found no relationship between OS and NGAL stain intensity in tumoral cells.
Median OS for patients with MMP-9 expression <30 % was 36 months (95 % CI 1.92–70.1) and for those with expression ≥30 % was 15 months (CI 95 %, 7.3–22.7). No statistical difference between groups was found (P = 0.568) (Fig. 5). No differences in OS were either found for MMP-9 stain intensity in tumoral cells, gender, smoking status, vascular permeation, or degree of differentiation.
In the multivariate analysis, stage at diagnosis (HR 2.05 [CI 95 %; 1.30–3.22] P < 0.002) and NGAL expression ≥70 % (HR 5.01 [CI 95 %; 1.68–14.93] P < 0.004) were statistically significant for OS (Table 5).
Discussion
For lung cancer, there are conflicting data regarding the prognosis of histopathological variables (lymphovascular invasion, adenocarcinoma vs. epidermoid histology), with controversial results, and they are only described for resectable disease, with no information for metastatic patients [12–15]. Another variable with no definite results is the degree of tumor differentiation in patients with operable disease (worst prognosis in undifferentiated tumors) [14, 15]. Vascular invasion was described as a negative prognostic factor for patients with T1–2 N0 tumors. In a series of 746 patients, microscopic vascular permeation was identified in 257 individuals (34 %) and conferring them a worse 5-year OS compared to those who did not (65 vs. 55 %, respectively) [16], hence the importance of identifying new histological prognostic markers in order to use them in conjunction with the TNM system and allow a better selection of treatment for each patient.
NGAL has been known for many years for its role in the innate immune system. It also receives the names of lipocalin-2, siderocalin, uterocalin, and 24p3 [17]. With more than 50 members, it belongs to the lipocalin superfamily. They all have similar three-dimensional structure in a single eight-stranded anti-parallel b-barrel surrounding a central pocket [18]. NGAL has an ability to bind and transport small hydrophobic substances such as fatty acids, prostaglandins, arachidonic acid, retinoids, and hormones [19].
NGAL’s principal mechanism of action is to capture the extracellular iron particles (also known as siderophores) and transport them to the inner cell after interacting with the specific membrane receptor 24p3R. This causes an increase in the iron cytoplasmic levels [20]. By activating an iron depletion strategy, this protein participates in the iron-dependent enzymatic defensive systems [21]. Through the regulation of iron-responsive genes, which are important in the differentiation of primordial cells, NGAL seems to participate in the proliferation, differentiation, and development of human tumors, by favoring iron uptake from extracellular space within the malignant cells [9]. Various in vitro experimental models demonstrated that factors known to promote proliferation of malignant cells such as hepatocyte growth factor, the neu-transforming factor, the phorbol ester PMA, retinoic acid, poliomavirus infection, and even glucocorticoids can induce the synthesis and release of NGAL. This has suggested that its overexpression in tumoral tissue is a negative prognostic factor and associated with shorter overall and disease-free survival in several neoplasms [22, 23].
NGAL was originally identified as a protein covalently associated with 92-kDa gelatinase/MMP-9 from human-activated neutrophils (for hence its name, being the MMP-9 a gelatinase). Indeed, by forming the NGAL/MMP-9 dimeric complex, NGAL can protect MMP-9 from its auto and proteolytic degradation and consequently results in a higher gelatinolytic action of MMP-9 on extracellular matrix [24]. This would trigger an enhancement of the enzymatic activity of MMP-9 and explain the tumoral invasiveness and diffusion associated with NGAL overexpression [25, 26]. The overexpression in tumoral tissue of NGAL/MMP-9 complex as a worse prognostic factor has been identified in bladder, brain, breast, esophageal, gastric, and hematological malignancies [24].
In our study, the expression of NGAL was prognostic for overall survival in all patients and not only limited to those with operable disease. We are aware that one limitation of our study is the vast heterogeneity of the population and that we included local, locally advanced, and metastatic disease. Most of the patients had advanced disease at diagnosis: stage III (n = 8; 19.5 %) and stage IV (n = 20; 48.8 %), and tissue for those patients was obtained from core needle biopsies. On the other hand, stages I and II were present in only 13 patients (31.7 %), and tissue was obtained from therapeutic lobectomies performed at our institution that influenced in the prognosis of the local disease. However, in the multivariate analysis, a ≥70 % expression of NGAL in tumoral tissue correlated with a less favorable prognosis, and it was independent of the stage at diagnosis. To our knowledge, only one study has evaluated the role of NGAL expression as a marker of resistance to EGFR-TKIs in cell lines of lung cancer [27], but there are no reports that have assessed the prognostic role of NGAL in patients with NSCLC and limited to the subset of lung adenocarcinoma and neither with the expression of both NGAL and MMP-9.
The prognostic value of NGAL, independently of MMP-9 expression, has been previously reported in patients with stage I colon adenocarcinoma [7]. NGAL expression and its poor prognosis have been evaluated in breast, esophagus, and stomach cancer [5–7, 22, 28]. There is inconclusive or conflicting data in patients with renal [29] and pancreatic carcinoma [30] and a less unfavorable prognosis in anaplastic thyroid and ovarian cancer [8]. Our results may suggest that identifying patients with a worse prognosis (overexpression of NGAL ≥70 % in tumoral tissue) regardless of stage at diagnosis could help the physician selecting an aggressive treatment approach and a stricter follow-up. On the other hand, patients who do not could avoid overtreatment and have a loose surveillance.
Furthermore, it has been found that NGAL results as a physiological regulator of cell proliferation, and it is active in the process of growth and differentiation of tissues since the embryonic stage. In fact, it was found that binding to the 24p3R receptor, NGAL regulates cell proliferation by a mechanism of depletion and increase of the intracellular iron, which induces cell to apoptosis or survival, respectively [31]. In our series, we assume that NGAL overexpression of 95.12 % is secondary to an increased iron need for NSCLC cancerogenesis [32].
MMP-9 and its expression as an unfavorable prognostic factor have been extensively studied in NSCLC [33–41] besides being a possible therapeutic target in this group of patients [42–44]. The lack of correlation between MMP-9 and overall survival in our study might reflect the relative insensitivity of Northern blot analyses used in the previously mentioned studies that, compared to immunohistochemistry, cannot precisely discriminate between stromal and neoplastic cell protein expression.
NGAL also has been described in pre-clinical models as a novel mechanism of NSCLC resistance to the small molecule tyrosine-kinase inhibitor erlotinib. In a recent study of mice NSCLC cell lines, NGAL overexpression in erlotinib-sensitive cells augmented apoptosis resistance. This was mediated by NGAL-dependent modulation of the pro-apoptotic protein Bcl-2-like protein 11 (BIM). This might contribute to the TKI resistance in some adenocarcinoma lung cancer patients by overcoming the apoptosis pathway in malignant cells [27]. In an Asian population study, BIM deletion polymorphism predicted shorter PFS to EGFR-TKIs and OS in advanced NSCLC [45].
Our results indicate that NGAL expression is associated with a worse OS prognosis compared to patients who did not, independently of stage at diagnosis. On the other hand, we found no prognostic significance for the expression of MMP-9 and OS or any correlation with the expression of NGAL. This suggests that NGAL may be independent of the regulation of MMP-9. This has not been documented in NSCLC, but observed in cell lines of colon adenocarcinoma. In this study, in contrast to the positive relationship between the two markers in colon cancer previously published [46], it was observed that the expression of NGAL promotes invasion and the metastatic potential of neoplastic cells by a mechanism that is iron dependent, thus decreasing the inter-cellular-mediated E-cadherin adhesion [47]. This suggests a novel therapeutic strategy with iron-chelating drugs for the inhibition of NGAL, used as anti-neoplastic drugs. By reducing iron stores and transporters such as NGAL protein, these drugs inhibit cell proliferation. This has already been investigated in some animal models with promising results [48, 49].
Conclusion
In this group of patients with lung adenocarcinoma, overexpression of NGAL in tumor tissue greater than or equal to 70 % confers a worse prognosis compared to those who did not, and it is independent of the stage at diagnosis. Expression of MMP-9 did not confer any significant prognostic value nor correlation of this marker with NGAL, suggesting an independent mechanism of NGAL in lung adenocarcinoma for invasion and metastases. The prognostic value of NGAL is currently being validated in a larger series of patients and in a multi-institutional form.
Abbreviations
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- MMP-9:
-
Matrix metalloproteinase–9
References
GLOBOCAN. International Agency for Research on Cancer. Cancer incidence and mortality worldwide in 2012. Globocan cancer fact sheets: lung cancer 2014. http://globocan.iarc.fr/. Accessed 17 June 2012.
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol Off Publ Int Assoc Stud Lung Cancer. 2007;2(8):706–14. doi:10.1097/JTO.0b013e31812f3c1a.
National Comprehensive Cancer Network. Occult primary (version 1.2015). 2014. http://www.nccn.org/professionals/physician_gls/pdf/occult.pdf. Accessed 28 Oct 2014.
Nesbitt JC, Putnam Jr JB, Walsh GL, Roth JA, Mountain CF. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg. 1995;60(2):466–72.
Wenners AS, Mehta K, Loibl S, Park H, Mueller B, Arnold N, et al. Neutrophil gelatinase-associated lipocalin (NGAL) predicts response to neoadjuvant chemotherapy and clinical outcome in primary human breast cancer. PLoS One. 2012;7(10):e45826. doi:10.1371/journal.pone.0045826.
Kaur S, Chakraborty S, Baine MJ, Mallya K, Smith LM, Sasson A, et al. Potentials of plasma NGAL and MIC-1 as biomarker(s) in the diagnosis of lethal pancreatic cancer. PLoS One. 2013;8(2):e55171. doi:10.1371/journal.pone.0055171.
Barresi V, Reggiani-Bonetti L, Di Gregorio C, Vitarelli E, Ponz De Leon M, Barresi G. Neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinase-9 (MMP-9) prognostic value in stage I colorectal carcinoma. Pathol Res Pract. 2011;207(8):479–86. doi:10.1016/j.prp.2011.05.012.
Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826(1):129–69. doi:10.1016/j.bbcan.2012.03.008.
Fernandez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11(15):5390–5. doi:10.1158/1078-0432.CCR-04-2391.
Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer. 2007;7(10):778–90. doi:10.1038/nrc2190.
Villalba Caloca J, Martínez Heredero R. Frecuencia del carcinoma broncopulmonar en pacientes fumadores y no fumadores diagnosticados en el Instituto Nacional de Enfermedades Respiratorias en el año 2001. Rev Inst Nac Enferm Respiratorias. 2004;17:27–34.
Martini N, Bains MS, Burt ME, Zakowski MF, McCormack P, Rusch VW, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109(1):120–9.
Postoperative T1 N0 non-small cell lung cancer. Squamous versus nonsquamous recurrences. The Lung Cancer Study Group. J Thorac Cardiovasc Surg. 1987;94(3):349–54.
Harpole Jr DH, Herndon 2nd JE, Young Jr WG, Wolfe WG, Sabiston Jr DC. Stage I nonsmall cell lung cancer. A multivariate analysis of treatment methods and patterns of recurrence. Cancer. 1995;76(5):787–96.
Lipford 3rd EH, Eggleston JC, Lillemoe KD, Sears DL, Moore GW, Baker RR. Prognostic factors in surgically resected limited-stage, nonsmall cell carcinoma of the lung. Am J Surg Pathol. 1984;8(5):357–65.
Ruffini E, Asioli S, Filosso PL, Buffoni L, Bruna MC, Mossetti C, et al. Significance of the presence of microscopic vascular invasion after complete resection of Stage I-II pT1-T2N0 non-small cell lung cancer and its relation with T-size categories: did the 2009 7th edition of the TNM staging system miss something? J Thorac Oncol Off Publ Int Assoc Stud Lung Cancer. 2011;6(2):319–26. doi:10.1097/JTO.0b013e3182011f70.
Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45(1):17–23. doi:10.1006/geno.1997.4896.
Bratt T. Lipocalins and cancer. Biochim Biophys Acta. 2000;1482(1–2):318–26.
Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318(Pt 1):1–14.
Goetz DH, Willie ST, Armen RS, Bratt T, Borregaard N, Strong RK. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry. 2000;39(8):1935–41.
Zhao H, Konishi A, Fujita Y, Yagi M, Ohata K, Aoshi T, et al. Lipocalin 2 bolsters innate and adaptive immune responses to blood-stage malaria infection by reinforcing host iron metabolism. Cell Host Microbe. 2012;12(5):705–16. doi:10.1016/j.chom.2012.10.010.
Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat. 2008;108(3):389–97. doi:10.1007/s10549-007-9619-3.
Shinriki S, Jono H, Ueda M, Obayashi K, Nakamura T, Ota K, et al. Stromal expression of neutrophil gelatinase-associated lipocalin correlates with poor differentiation and adverse prognosis in oral squamous cell carcinoma. Histopathology. 2014;64(3):356–64. doi:10.1111/his.12293.
Candido S, Maestro R, Polesel J, Catania A, Maira F, Signorelli SS, et al. Roles of neutrophil gelatinase-associated lipocalin (NGAL) in human cancer. Oncotarget. 2014;5(6):1576–94.
Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169(4):681–91. doi:10.1083/jcb.200409115.
Bolignano D, Donato V, Lacquaniti A, Fazio MR, Bono C, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: a new protein enters the scene. Cancer Lett. 2010;288(1):10–6. doi:10.1016/j.canlet.2009.05.027.
Krysan K, Cui X, Gardner BK, Reckamp KL, Wang X, Hong L, et al. Elevated neutrophil gelatinase-associated lipocalin contributes to erlotinib resistance in non-small cell lung cancer. Am J Transl Res. 2013;5(5):481–96.
Provatopoulou X, Gounaris A, Kalogera E, Zagouri F, Flessas I, Goussetis E, et al. Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer disease. BMC Cancer. 2009;9:390. doi:10.1186/1471-2407-9-390.
Dic A. Evaluation of neutrophil gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9 (MMP-9) and their complex MMP-9/NGAL in sera and urine of patients with kidney tumors. Oncol Lett. 2013;5(5):1677–81. doi:10.3892/ol.2013.1252.
Tong Z, Kunnumakkara AB, Wang H, Matsuo Y, Diagaradjane P, Harikumar KB, et al. Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008;68(15):6100–8. doi:10.1158/0008-5472.can-08-0540.
Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276(40):37258–65. doi:10.1074/jbc.M106089200.
Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100(1):9–16. doi:10.1111/j.1349-7006.2008.01001.x.
Cao L, Yang H, Hu C. The expression and its clinical significance of MMP-2 and MMP-9 in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi = Chin J Lung Cancer. 2003;6(6):484–7. doi:10.3779/j.issn. 1009-3419.2003.06.18.
Iniesta P, Moran A, De Juan C, Gomez A, Hernando F, Garcia-Aranda C, et al. Biological and clinical significance of MMP-2, MMP-9, TIMP-1 and TIMP-2 in non-small cell lung cancer. Oncol Rep. 2007;17(1):217–23.
Liu Z, Xu S, Xiao N, Song C, Zhang H, Li F. Overexpression of IL-8 and MMP-9 confer high malignant phenotype in patients with non-small cell lung cancer. Zhongguo Fei Ai Za Zhi = Chin J Lung Cancer. 2010;13(8):795–802. doi:10.3779/j.issn. 1009-3419.2010.08.09.
Ramanujum R, Lin YL, Liu JK, He S. Regulatory expression of MMP-8/MMP-9 and inhibition of proliferation, migration and invasion in human lung cancer A549 cells in the presence of HGF variants. Kaohsiung J Med Sci. 2013;29(10):530–9. doi:10.1016/j.kjms.2013.01.011.
Rollin J, Regina S, Vourc'h P, Iochmann S, Blechet C, Reverdiau P, et al. Influence of MMP-2 and MMP-9 promoter polymorphisms on gene expression and clinical outcome of non-small cell lung cancer. Lung Cancer. 2007;56(2):273–80. doi:10.1016/j.lungcan.2006.11.021.
Roomi MW, Monterrey JC, Kalinovsky T, Niedzwiecki A, Rath M. Modulation of MMP-2 and MMP-9 by cytokines, mitogens and inhibitors in lung cancer and malignant mesothelioma cell lines. Oncol Rep. 2009;22(6):1283–91.
Schveigert D, Cicenas S, Bruzas S, Samalavicius NE, Gudleviciene Z, Didziapetriene J. The value of MMP-9 for breast and non-small cell lung cancer patients’ survival. Adv Med Sci. 2013;58(1):73–82. doi:10.2478/v10039-012-0066-y.
Wang JL, Wu DW, Cheng ZZ, Han WZ, Xu SW, Sun NN. Expression of High Mobility Group Box - B1 (HMGB-1) and Matrix Metalloproteinase-9 (MMP-9) in Non-small Cell Lung Cancer (NSCLC). Asian Pac J Cancer Prev APJCP. 2014;15(12):4865–9.
Zheng S, Chang Y, Hodges KB, Sun Y, Ma X, Xue Y, et al. Expression of KISS1 and MMP-9 in non-small cell lung cancer and their relations to metastasis and survival. Anticancer Res. 2010;30(3):713–8.
O'Sullivan S, Medina C, Ledwidge M, Radomski MW, Gilmer JF. Nitric oxide-matrix metaloproteinase-9 interactions: biological and pharmacological significance—NO and MMP-9 interactions. Biochim Biophys Acta. 2014;1843(3):603–17. doi:10.1016/j.bbamcr.2013.12.006.
Chang CK, Hung WC, Chang HC. The Kazal motifs of RECK protein inhibit MMP-9 secretion and activity and reduce metastasis of lung cancer cells in vitro and in vivo. J Cell Mol Med. 2008;12(6B):2781–9. doi:10.1111/j.1582-4934.2008.00215.x.
Zheng R, Qin X, Li W, Kang J. Effect of Src tyrosine kinase inhibition on secretion of MMP-2 and MMP-9 by non-small cell lung cancer cells. Zhongguo Fei Ai Za Zhi = Chin J Lung Cancer. 2011;14(1):13–7. doi:10.3779/j.issn. 1009-3419.2011.01.03.
Lee JH, Lin YL, Hsu WH, Chen HY, Chang YC, Yu CJ, et al. Bcl-2-like protein 11 deletion polymorphism predicts survival in advanced non-small-cell lung cancer. J Thorac Oncol Off Publ Int Assoc Stud Lung Cancer. 2014;9(9):1385–92. doi:10.1097/jto.0000000000000238.
Zhang XF, Zhang Y, Zhang XH, Zhou SM, Yang GG, Wang OC, et al. Clinical significance of Neutrophil gelatinase-associated lipocalin(NGAL) expression in primary rectal cancer. BMC Cancer. 2009;9:134. doi:10.1186/1471-2407-9-134.
Hu L, Hittelman W, Lu T, Ji P, Arlinghaus R, Shmulevich I, et al. NGAL decreases E-cadherin-mediated cell-cell adhesion and increases cell motility and invasion through Rac1 in colon carcinoma cells. Lab Investig J Tech Methods Pathol. 2009;89(5):531–48. doi:10.1038/labinvest.2009.17.
Buss JL, Greene BT, Turner J, Torti FM, Torti SV. Iron chelators in cancer chemotherapy. Curr Top Med Chem. 2004;4(15):1623–35.
Jones DT, Trowbridge IS, Harris AL. Effects of transferrin receptor blockade on cancer cell proliferation and hypoxia-inducible factor function and their differential regulation by ascorbate. Cancer Res. 2006;66(5):2749–56. doi:10.1158/0008-5472.can-05-3857.
Conflicts of interest
None
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional committee. For this type of study, formal consent is not required.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz-Morales, J.M., Dorantes-Heredia, R., Arrieta, O. et al. Neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinase-9 (MMP-9) prognostic value in lung adenocarcinoma. Tumor Biol. 36, 3601–3610 (2015). https://doi.org/10.1007/s13277-014-2997-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2997-3