Abstract
Homeobox genes, a superfamily of evolutionarily conserved developmental genes, function as critical master regulatory factors in controlling body plan specification and cell fate determination. Recently, a substantial body of evidence indicates that the aberrant Homeobox (HOX) genes also play key roles in the development of cancers. Many reports have shown not only that HOX gene expression is upregulated or downregulated in many cancers but also that the expression of specific HOX genes tends to differ based on tissue type. Homeobox A5 (HOXA5) is a master regulator of the morphogenesis and cell differentiation, and its expression is also downregulated in many cancers mediated by DNA methylation. However, its biological role and clinical significance in nonsmall cell lung cancer (NSCLC) development and progression are not well documented. In this study, we found that expression levels of HOXA5 were significantly decreased in NSCLC tissues compared with adjacent normal tissues. Its expression level was significantly correlated with tumor–node–metastasis (TNM) stages, tumor size, and lymph node metastasis. Moreover, patients with lower levels of HOXA5 expression had a relatively poor prognosis. Furthermore, ectopic overexpression of HOXA5 could inhibit cell proliferation and invasion, while knockdown HOXA5 by siRNA promoted cell proliferation in NSCLC cells partly via regulating p21 expression. Our findings present that decreased HOXA5 could be identified as a poor prognostic biomarker in NSCLC and regulate cell proliferation and invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonsmall cell lung cancer (NSCLC), including adenocarcinoma and squamous cell carcinoma, is the predominant form of lung cancer and accounts for the majority of lung cancer associated deaths worldwide [1]. Despite recent advances in clinical and experimental oncology, the prognosis of NSCLC remains poor with a 5-year overall survival rate of around 11 % [2]. Thus, a detailed understanding of the mechanisms and molecular pathways underlying NSCLC development and progression is essential for improving diagnosis, prevention, and treatment of this disease. Recently, there is growing evidence indicating that homeobox (HOX) genes play important roles in tumorigenesis, which may be involved in NSCLC pathogenesis, providing new insights into the biology of this disease [3, 4].
The HOX genes constitute a family of transcription factors that determine cellular identity during embryonic development and contribute to a variety of biological pathways including homeostasis, cell differentiation, and the maintenance of organ functioning in adult tissue [5–7]. To date, a number of evidence show that misregulated expression of many HOX genes has been observed in a wide variety of malignancies and their expression in cancers tends to differ based on tissue type and tumor site [8–10]. For example, HOXC6 expression plays a critical role in apoptosis during prostate cancer, and silencing of HOXC6 expression in prostate cancer cells decreased proliferation rates [11]. Moreover, HOXB7 expression is significantly upregulated in colorectal cancer, as a valuable marker of CRC prognosis, and contributes to proliferation and tumorigenesis via upregulation of cyclin D1 and downregulation of p21Kip1 [12]. Recently, HOXA family genes were found to be downregulated in primary NSCLC tissues compared to normal lung tissues, while immunocytochemical analysis showed higher expression of HOXB3, HOXB4, and HOXC6 in lung carcinoma tissues [13, 14].
Homeobox A5 (HOXA5) expression has been shown to be regulated by RA and participate in the developmental regulation of the lung. Isabel Mandeville et al. observed impaired postnatal lung development in HOXA5−/− mice [15]. Similarly, Alan Packer et al. reported that HOXA5 is likely to be involved in development and patterning of the mouse lung [16]. Recently, several studies reported that HOXA5 plays important roles in breast tumorigenesis by directly regulating the expression of p53, Twist, and hMLH1 [17–19]. Moreover, accumulating evidence suggests that HOXA5 gene is frequently inactivated by aberrant promoter methylation and functions as a tumor suppressor in the development of multiple cancers including NSCLC [20, 21]. However, the biological role and clinical significance of HOXA5 in NSCLC development and progression are still not well documented and should be further investigated.
In this study, we investigate HOXA5 expression in NSCLC tissues and adjacent normal tissues by immunohistochemistry and qPCR. Moreover, the effect of HOXA5 on NSCLC cell proliferation, migration, and invasion was assayed by ectopic expression or knockdown of HOXA5 expression in NSCLC cells. Furthermore, we found that HOXA5 contributes to NSCLC cell proliferation partly via regulating p21 expression. Our findings present that decreased HOXA5 expression could be identified as a poor prognostic biomarker in NSCLC.
Materials and methods
Immunohistochemical analysis
To determine the expression levels of HOXA5 in NSCLC tissues, paraffin-embedded, formalin-fixed tissues were immunostained for HOXA5. The immunohistochemical staining was performed on an automated staining system (TechMate 500, DakoCytomation) with a rabbit anti-HOXA5 antibody (1:50, Santa Cruz) for 30 min. For immunohistochemical measurement of HOXA5 expression, the signal was amplified and visualized with diaminobenzidine chromogen, followed by counterstaining with hematoxylin. For HOXA5, an immunohistochemistry (IHC) score of 2+ or more was defined as positive, and IHC scores of 0 and 1+ were defined as negative.
Tissue collection
We obtained 28 paired NSCLC and adjacent nontumor lung tissues from patients who underwent surgery at Jiangsu Province Hospital between 2010 and 2011 and were diagnosed with NSCLC based on histopathological evaluation. Clinicopathological characteristics, including tumor–node–metastasis (TNM) staging, were recorded. No local or systemic treatment was conducted in these patients before surgery. All collected tissue samples were immediately snap-frozen in liquid nitrogen and stored at −80 °C until required. Our study was approved by the Research Ethics Committee of Nanjing Medical University, China. Written informed consent was obtained from all patients.
Cell lines
Four NSCLC adenocarcinoma cell lines (A549, SPCA1, PC9, and H1975) and one NSCLC squamous carcinomas cell line (SK-MES-1) were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). A549 and H1975 cells were cultured in RPMI 1640; 16HBE, PC9, SK-MES-1, and SPCA1 cells were cultured in DMEM (GIBCO-BRL) supplemented with 10 % fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C/5 % CO2.
Treatment of NSCLC cells with 5-aza-CdR
A549 and SPCA1 cells (2.5 × 105) were seeded into six-well culture plate on day 0 and exposed to 0, 2, or 5 μM 5-aza-2-deoxycytidine (5-aza-CdR) (Sigma-Aldrich)for day 1 to day 3. Thereafter, the cells treated with 5-aza-CdR were harvested on day 3 and used for detection of HOXA5 expression.
RNA extraction and qPCR assays
Total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA (500 ng) was reverse transcribed in a final volume of 10 μl using random primers under standard conditions for the PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). We used the SYBR Premix Ex Taq (TaKaRa, Dalian, China) to determine HOXA5, p21, and E2F1 expression levels, following the manufacturer’s instructions. Results were normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The specific primers are shown in Supplementary Table 1. The qPCR assays were conducted on an ABI 7500, and data were collected with this instrument. Our qPCR results were analyzed and expressed relative to threshold cycle (CT) values and then converted to fold changes.
Cell transfection
Plasmid vectors (pCDNA-HOXA5 and pCDNA3.1) for transfection were prepared using DNA Midiprep or Midiprep kits (Qiagen, Hilden, Germany) and transfected into SPCA1 or A549 cells. The si-HOXA5 or si-NC was transfected into SPCA1 or A549 cells. A549 and SPCA1 cells were grown on six-well plates to confluency and transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. At 48 h post-transfection, cells were harvested for qPCR or Western blot analysis.
Cell viability assays
Cell viability was monitored using a Cell Proliferation Reagent Kit I (MTT) (Roche Applied Science). The A549 or SPCA1 cells transfected with si-HOXA5 (3000 cells/well) and A549 or SPCA1 cells transfected with pCDNA-HOXA5 were grown in 96-well plates. Cell viability was assessed every 24 h following the manufacturer’s protocol. All experiments were performed in quadruplicate. For each treatment group, wells were assessed in triplicate. For the colony formation assay, a total of 500 SPCA1 or A549 cells transfected with pCDNA-HOXA5 or si-HOXA5 were placed in a fresh six-well plate and maintained in media containing 10 % FBS, replacing the medium every 4 days. After 14 days, the cells were fixed with methanol and stained with 0.1 % crystal violet (Sigma). Visible colonies were manually counted. Triplicate wells were measured in each treatment group.
Cell migration and invasion assays
For the migration assays, at 48 h post-transfection, 5 × 104 cells in serum-free media were placed into the upper chamber of an insert (8-μm pore size; Millipore). For the invasion assays, 1 × 105 cells in serum-free medium were placed into the upper chamber of an insert coated with Matrigel (Sigma-Aldrich). Medium containing 10 % FBS was added to the lower chamber. After incubation for 24 h, the cells remaining on the upper membrane were removed with cotton wool. Cells that had migrated or invaded through the membrane were stained with methanol and 0.1 % crystal violet, imaged, and counted using an IX71 inverted microscope (Olympus, Tokyo, Japan). Experiments were independently repeated three times.
Tumor formation assay in a nude mouse model
Female athymic BALB/c nude mice (4 weeks old) were maintained under pathogen-free conditions and manipulated according to protocols approved by the Shanghai Medical Experimental Animal Care Commission. SPCA1 cells were stably transfected with pCDNA-HOXA5 and empty vector and harvested from six-well cell culture plates, washed with phosphate-buffered saline (PBS), and resuspended at a concentration of 1 × 108 cells/ml. A total of 100 μL of suspended cells was subcutaneously injected into a single side of the posterior flank of each mouse. Tumor growth was examined every 3 days, and tumor volumes were calculated using the equation V = 0.5 × D × d2 (V, volume; D, longitudinal diameter; d, latitudinal diameter). At 18 days post-injection, mice were euthanized, and the subcutaneous growth of each tumor was examined. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Nanjing Medical University.
Western blot assay and antibodies
Cells protein lysates were separated by 10 % sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (SDS-PAGE), transferred to 0.22-μm NC membranes (Sigma), and incubated with specific antibodies. ECL chromogenic substrate was used to be quantified by densitometry (Quantity One software; Bio-Rad). GAPDH antibody was used as control, and anti-p21 (1:1000) was purchased from Cell Signaling Technology, Inc (CST).
Statistical analysis
Student’s t test (two-tailed), one-way ANOVA, and the Mann–Whitney U test were used to analyze data, along with SPSS 16.0 (IBM, IL, USA). P values of less than 0.05 were considered statistically significant.
Results
HOXA5 expression is downregulated in NSCLC tissues
As HOXA5 gene has been reported to be inactivated in many cancers, we firstly investigated the expression level of HOXA5 in NSCLC and compared normal tissues by using the data in GEO datasets. The results showed that HOXA5 messenger RNA (mRNA) levels were significantly downregulated in NSCLC tissues in GSE5364, GSE10072, GSE19188, GSE43458, GSE7670, GSE18842, GSE19804, and GSE32864 (Fig. 1a, b). In addition, we analyze the prognostic value of HOXA5 in NSCLC patient by using KM plotter [22], and the results showed that decreased HOXA5 expression was correlated with poor prognosis (Fig. 1c).
Analysis of HOXA5 expression profile in NSCLC tissues. a, b HOXA5 expression levels in NSCLC tissues were analyzed by using data from GEO datasets, and HOXA5 expression was significantly downregulated in NSCLC tissues compared with normal tissues (GSE5364, GSE10072, GSE19188, GSE43458, GSE7670, GSE18842, GSE19804, and GSE32864). c Patients with lower levels of HOXA5 expression showed reduced survival times compared to patients with higher levels of HOXA5 expression
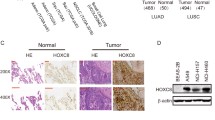
To further determine whether HOXA5 expression is decreased in NSCLC tissues, we detect the HOXA5 mRNA and protein levels in NSCLC tissues and normal tissues by qPCR and immunohistochemistical staining assays. The results of immunohistochemistical staining assays also indicated that the HOXA5 protein levels are also decreased in 128 paired NSCLC tissues compared with normal tissues (Fig. 2a). Moreover, the results showed that HOXA5 mRNA expression is significantly downregulated in 28 paired NSCLC tissues compared with normal tissues (Fig. 2b). These data indicated that the HOXA5 expression is downregulated in NSCLC tissues and may contribute to NSCLC development.
Relative HOXA5 expression in NSCLC tissues and its clinical significance. a Relative expression of HOXA5 in NSCLC tissues (n = 128) in comparison with corresponding nontumor normal tissues (n = 128). HOXA5 protein levels were examined by immunohistochemistry. b HOXA5 expression was examined by qPCR and normalized to GAPDH expression (shown as ΔCT). c Patients with lower levels of HOXA5 expression showed reduced survival times compared to patients with higher levels of HOXA5 expression (log rank P < 0.001). *P < 0.05; **P < 0.01
The relationship between HOXA5 expression and clinicopathological features in patients with NSCLC
According to the median ratio of relative HOXA5 expression in tumor tissues, the 128 NSCLC patients were classified into two groups: relative high-HOXA5 group (n = 64, HOXA5 expression ratio ≥ median ratio) and relative low-HOXA5 group (n = 64, HOXA5 expression ratio ≤ median ratio). Furthermore, correlation analysis of HOXA5 expression with clinical pathological features of NSCLC patients revealed a significant association between decreased HOXA5 expression and advanced pathological stage, lymph nodes metastasis, and NSCLC tumor size. However, HOXA5 expression was not correlated with histological subtype, patient age, or gender (Table 1).
Kaplan–Meier survival analysis and log-rank tests using patient postoperative survival were performed to further evaluate the correlation between HOXA5 expression and NSCLC patient prognosis. The Kaplan–Meier survival curve showed that 3 years of overall survival in patients with low HOXA5 expression is shorter than that in patients with high HOXA5 expression. With respect to progression-free survival (PFS), survival in patients with low HOXA5 expression is also shorter than patients with high HOXA5 expression (Fig. 2c). These findings support the hypothesis that downregulated HOXA5 plays a key role in NSCLC development and progression.
Effect of DNA methylation on HOXA5 expression in NSCLC cells
We next detected the expression of HOXA5 in five human NSCLC cell lines, including both adenocarcinoma and squamous carcinoma subtypes. Of these, all cells expressed lower levels of HOXA5 compared with the normal bronchial epithelial cell line 16HBE (Fig. 3a). Furthermore, the expression of HOXA5 was frequently downregulated in NSCLC, and hypermethylation of HOXA5 promoter has been reported to be involved in HOXA5 transcriptional inactivation. Following treatment of SPCA1 and A549 cells with DNA-demethylating agent (5-aza-CdR), we found that HOXA5 expression was significantly increased in 5-aza-CdR-treated cells compared with control (Fig. 3b). To assess the role of HOXA5 in NSCLC, SPCA1 and A549 cells were transfected with pCDNA-HOXA5 or si-HOXA5. The results showed that HOXA5 expression was significantly upregulated or downregulated in SPCA1 and A549 cells after transfected with pCDNA-HOXA5 or si-HOXA5 (Fig. 3c, d).
Modulation of HOXA5 expression in NSCLC cells. a Results from qPCR demonstrate HOXA5 expression level in NSCLC cell lines. b qPCR analyses of HOXA5 expression level following treatment of SPCA1 and A549 cells with 5-aza-CdR. c qPCR analyses of HOXA5 expression level following treatment of SPCA1 and A549 cells with pCDNA-HOXA5 or empty vector. d qPCR analyses of HOXA5 expression level following treatment of SPCA1 and A549 cells with si-HOXA5 or si-NC.*P < 0.05;**P < 0.01
Overexpression of HOXA5 impaired NSCLC cell proliferation
To assess the role of HOXA5 in NSCLC, we investigated the effect of upregulation of HOXA5 on cell proliferation. MTT assays revealed that cell growth was inhibited in SPCA1 and A549 cells transfected with pCDNA-HOXA5 compared with controls (Fig. 4a, b). Similarly, the results of colony formation assays revealed that clonogenic survival was decreased following enhanced HOXA5 expression in SPCA1 and A549 cells (Fig. 4c, d).
Overexpression of HOXA5 impairs NSCLC cell proliferation in vitro. SPCA1 and A549 cells were transfected with pCDNA-HOXA5 or empty vector, respectively. a, b MTT assay was performed to determine the proliferation of pCDNA-HOXA5-transfected SPCA1 and A549 cells. c, d Clone formation assays were performed to investigate the clone formation ability of pCDNA-HOXA5-transfected SPCA1 and A549 cells. Data represent the mean ± SD. from three independent experiments. *P < 0.05;**P < 0.01
Knockdown of HOXA5 promotes NSCLC cell viability in vitro
To further investigate the role of HOXA5 downregulation in NSCLC cell viability, si-HOXA5 was transfected into SPCA1 and A549 cells. MTT assays revealed that cell viability was enhanced in SPCA1 and A549 cells transfected with si-HOXA5 compared with controls (Fig. 5a, b). Moreover, clonogenic survival was increased following downregulation of HOXA5 expression in SPCA1 and A549 cells compared with control (Fig. 5c).
HOXA5 inhibits NSCLC cell proliferation in vivo. SPCA1 and A549 cells were transfected with si-HOXA5 or si-NC, respectively. a, b MTT assay was performed to determine the proliferation of si-HOXA5-transfected SPCA1 and A549 cells. c, d Clone formation assays were performed to investigate the clone formation ability of si-HOXA5-transfected SPCA1 and A549 cells. e The tumor volume was calculated once every 3 days after injection of SPCA1 cells stably transfected with pCDNA-HOXA5 or empty vector. Bars indicate SD. f Tumor weights are represented as means of tumor weights ± SD. g Tumors developed from pCDNA-HOXA5-transfected SPCA1 cells showed lower Ki67 protein levels than tumors developed from control cells. Upper: H & E staining; Lower: immunostaining. *P < 0.05, **P < 0.01; NS not significant
HOXA5 inhibits tumorigenesis of NSCLC cells in vivo
To explore whether HOXA5 affects tumorigenesis, pCDNA-HOXA5 and empty-vector stably transfected SPCA1 cells were inoculated into female nude mice. Twenty days after injection, the tumors formed in pCDNA-HOXA5 group were substantially smaller than those in the empty vector group (Fig. 5d). Moreover, the mean tumor weight at the end of the experiment was markedly lower in the pCDNA-HOXA5 group (0.3 ± 0.04 g) compared to the control group (0.66 ± 0.07 g) (Fig. 5e). Immunostaining was used to analyze Ki67 protein expression in resected tumor tissues. Ki67 levels in tumors formed from control cells (empty vector) exhibited decreased positivity for Ki67 than in tumors from pCDNA-HOXA5-transfected SPCA1 cells (Fig. 5f). These results indicate that overexpression of HOXA5 could inhibit tumor growth in vivo.
Upregulation of HOXA5 inhibits cell migration and invasion
Cell invasion is a significant aspect of cancer progression and involves the migration of tumor cells into contiguous tissues and the dissolution of extracellular matrix proteins. To investigate whether HOXA5 had a direct functional role in cell invasion in NSCLC, we evaluated cancer cell invasion through transwell Matrigel assay. As shown in Fig. 6, overexpression of HOXA5 impeded the migration of SPCA1 and A549 cells compared with control; invasion of SPCA1 and A549 cells was reduced following upregulation of HOXA5. These data indicate that HOXA5 could impair the migratory and invasive phenotype of NSCLC cells.
Overexpression of HOXA5 inhibits NSCLC cell migration and invasion. SPCA1 and A549 cells were transfected with pCDNA-HOXA5 or empty vector, respectively. a–d Transwell assays were performed to investigate the migratory and invasive ability of pCDNA-HOXA5-transfected SPCA1 and A549 cells. Data represent the mean ± SD from three independent experiments. *P < 0.05;**P < 0.01
HOXA5 affects the levels of p21 expression
To explore the molecular mechanisms by which HOXA5 contributes to the phenotypes of NSCLC cells, we investigated potential targets involved in tumor proliferation. Therefore, we sought to determine whether there was any interaction between E2F1, p21, and HOXA5. As expected, clear changes in the levels of p21 mRNA and protein were observed that correlated with the upregulation of HOXA5 in A549 and SPCA1 cells (Fig. 7a, c). However, overexpression of HOXA5 could not affect p53 expression. These data indicate that HOXA5 may influence the proliferation of NSCLC cells partly by altering p21 expression, which is independent of p53.
HOXA5 affects p21 expression levels. a p21 and E2F1 mRNA levels were examined by qPCR in pCDNA-HOXA5-transfected SPCA1 and A549 cells. b p53 mRNA levels were examined by qPCR in pCDNA-HOXA5-transfected SPCA1 and A549 cells. c Western blot analysis of p21 in pCDNA-HOXA5-transfected SPCA1 and A549 cells and control cells. GAPDH protein was used as an internal control. *P < 0.05
Discussion
Recently, more and more evidence indicates the functional significance of loss of expression or overexpression of distinct sets of HOX genes in the hallmarks of cancers. For example, HOXB7 was been found to have tumor-promoting properties in multiple different types of cancers [23, 24]. Moreover, L. Plowright reported that a number of HOXA genes are strongly expressed in normal tissue but are downregulated in primary NSCLC tumors, including HOXA3 and HOXA5. Conversely, many HOXC and HOXD genes are upregulated in the primary NSCLC tumors, including HOXC4, HOXC8, HOXC13, HOXD8, and HOXD10 [14]. In this study, we analyzed the data from GEO datasets and found that HOXA5 expression was significantly downregulated in NSCLC tissues. Moreover, we detect the HOXA5 mRNA and protein levels in NSCLC tissues and showed that its expression was similarly decreased. Meanwhile, decreased HOXA5 expression was correlated with NSCLC patients’ clinicopathological features, poor prognosis, and short survival time. These data indicate that HOXA5 downregulation may play an important role in NSCLC development and progression.
Inactivation of HOXA5 transcription or lost of HOXA5 expression had also been found in other cancers, and DNA methylation was one of the major factors that contribute to decreased HOXA5 expression. For example, downregulation of HOXA5 by DNA methylation could be related to short-term prognosis in AML patients [25]. Epigenetic inactivation of HOXA5 gene was also found in clear cell renal cell carcinoma by large-scale high-throughput DNA methylation profiling technique [20]. Moreover, HOXA5 expression is lost in more than 60 % of breast carcinomas, and overexpression of HOXA5 resulted in cell death through a p53-dependent apoptotic pathway or mediated by caspase-2 and caspase-8 [17, 26]. In this study, we found that HOXA5 expression could be regulated by DNA methylation, and overexpression of HOXA5 impaired NSCLC cell proliferation and invasion, while knockdown of HOXA5 expression promoted NSCLC cell growth. These data demonstrated that decreased HOXA5 expression contributes to NSCLC cell proliferation; however, its potential targets are not well known.
As previously reported, HOXA5 could interact with Twist to regulate p53 expression, and HOXA5 also can directly bind to p53 promoter region to promote p53 transcription. P21 is an important p53 downstream target gene, and it plays a key role in p53-mediated cell growth arrest and cell apoptosis. Our results also indicated that overexpression of HOXA5 could significantly stimulate p21 expression in NSCLC cells, which suggested that HOXA5 involved in NSCLC cell proliferation partly via regulating p21 expression in NSCLC cells. p21 is an inhibitor of cyclin-dependent kinases and could be activated through p53-dependent or p53-independent pathways, and lots of evidence indicated that p21 plays a key role in regulation of the cell cycle, especially in G1 arrest [27, 28]. Our results indicated that p21 maybe involved in HOXA5 overexpression-mediated cell growth arrest in p53-independent pathway.
In summary, the expression of HOXA5 was lost in NSCLC tissues and cells, which is mediated partly by DNA methylation. Decreased HOXA5 expression may be a negative prognostic factor and higher risk for NSCLC patients. This study showed that HOXA5 regulates the proliferation ability of NSCLC cells partly through regulation of p21. These findings further the understanding of NSCLC pathogenesis and progression and facilitate the development of HOXA5-directed diagnostics and therapeutics against this deadly disease. However, the potential underlying pathways involved in HOXA5-mediated NSCLC cell migration suppression are needed to be further investigated in future work.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi:10.3322/caac.21166.
Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, et al. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8(9):784–96. doi:10.1016/S1470-2045(07)70246-2.
Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2(10):777–85. doi:10.1038/nrc907.
Bhatlekar S, Fields JZ, Boman BM. HOX genes and their role in the development of human cancers. J Mol Med (Berl). 2014;92(8):811–23. doi:10.1007/s00109-014-1181-y.
McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68(2):283–302.
Garcia-Fernandez J. The genesis and evolution of homeobox gene clusters. Nat Rev Genet. 2005;6(12):881–92. doi:10.1038/nrg1723.
Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6(12):893–904. doi:10.1038/nrg1726.
Kelly ZL, Michael A, Butler-Manuel S, Pandha HS, Morgan RG. HOX genes in ovarian cancer. J Ovarian Res. 2011;4:16. doi:10.1186/1757-2215-4-16.
Eklund EA. The role of HOX genes in myeloid leukemogenesis. Curr Opin Hematol. 2006;13(2):67–73. doi:10.1097/01.moh.0000208467.63861.d6.
Calvo R, West J, Franklin W, Erickson P, Bemis L, Li E, et al. Altered HOX and WNT7A expression in human lung cancer. Proc Natl Acad Sci U S A. 2000;97(23):12776–81. doi:10.1073/pnas.97.23.12776.
Ramachandran S, Liu P, Young AN, Yin-Goen Q, Lim SD, Laycock N, et al. Loss of HOXC6 expression induces apoptosis in prostate cancer cells. Oncogene. 2005;24(1):188–98. doi:10.1038/sj.onc.1207906.
Liao WT, Jiang D, Yuan J, Cui YM, Shi XW, Chen CM, et al. HOXB7 as a prognostic factor and mediator of colorectal cancer progression. Clin Cancer Res. 2011;17(11):3569–78. doi:10.1158/1078-0432.CCR-10-2533.
Abe M, Hamada J, Takahashi O, Takahashi Y, Tada M, Miyamoto M, et al. Disordered expression of HOX genes in human non-small cell lung cancer. Oncol Rep. 2006;15(4):797–802.
Plowright L, Harrington KJ, Pandha HS, Morgan R. HOX transcription factors are potential therapeutic targets in non-small-cell lung cancer (targeting HOX genes in lung cancer). Br J Cancer. 2009;100(3):470–5. doi:10.1038/sj.bjc.6604857.
Mandeville I, Aubin J, LeBlanc M, Lalancette-Hebert M, Janelle MF, Tremblay GM, et al. Impact of the loss of Hoxa5 function on lung alveogenesis. Am J Pathol. 2006;169(4):1312–27. doi:10.2353/ajpath.2006.051333.
Packer AI, Mailutha KG, Ambrozewicz LA, Wolgemuth DJ. Regulation of the Hoxa4 and Hoxa5 genes in the embryonic mouse lung by retinoic acid and TGFbeta1: implications for lung development and patterning. Dev Dyn. 2000;217(1):62–74.
Raman V, Martensen SA, Reisman D, Evron E, Odenwald WF, Jaffee E, et al. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature. 2000;405(6789):974–8. doi:10.1038/35016125.
Stasinopoulos IA, Mironchik Y, Raman A, Wildes F, Winnard Jr P, Raman V. HOXA5-twist interaction alters p53 homeostasis in breast cancer cells. J Biol Chem. 2005;280(3):2294–9. doi:10.1074/jbc.M411018200.
Duriseti S, Winnard Jr PT, Mironchik Y, Vesuna F, Raman A, Raman V. HOXA5 regulates hMLH1 expression in breast cancer cells. Neoplasia. 2006;8(4):250–8. doi:10.1593/neo.05766.
Yoo KH, Park YK, Kim HS, Jung WW, Chang SG. Epigenetic inactivation of HOXA5 and MSH2 gene in clear cell renal cell carcinoma. Pathol Int. 2010;60(10):661–6. doi:10.1111/j.1440-1827.2010.02578.x.
Kim DS, Kim MJ, Lee JY, Lee SM, Choi JY, Yoon GS, et al. Epigenetic inactivation of Homeobox A5 gene in nonsmall cell lung cancer and its relationship with clinicopathological features. Mol Carcinog. 2009;48(12):1109–15. doi:10.1002/mc.20561.
Gyorffy B, Lanczky A, Szallasi Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr Relat Cancer. 2012;19(2):197–208. doi:10.1530/ERC-11-0329.
Storti P, Donofrio G, Colla S, Airoldi I, Bolzoni M, Agnelli L, et al. HOXB7 expression by myeloma cells regulates their pro-angiogenic properties in multiple myeloma patients. Leukemia. 2011;25(3):527–37. doi:10.1038/leu.2010.270.
Chile T, Fortes MA, Correa-Giannella ML, Brentani HP, Maria DA, Puga RD, et al. HOXB7 mRNA is overexpressed in pancreatic ductal adenocarcinomas and its knockdown induces cell cycle arrest and apoptosis. BMC Cancer. 2013;13:451. doi:10.1186/1471-2407-13-451.
Kim SY, Hwang SH, Song EJ, Shin HJ, Jung JS, Lee EY. Level of HOXA5 hypermethylation in acute myeloid leukemia is associated with short-term outcome. Korean J Lab Med. 2010;30(5):469–73. doi:10.3343/kjlm.2010.30.5.469.
Chen H, Chung S, Sukumar S. HOXA5-induced apoptosis in breast cancer cells is mediated by caspases 2 and 8. Mol Cell Biol. 2004;24(2):924–35.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75(4):805–16.
Hengst L, Gopfert U, Lashuel HA, Reed SI. Complete inhibition of Cdk/cyclin by one molecule of p21(Cip1). Genes Dev. 1998;12(24):3882–8.
Acknowledgments
This work was supported by the National Natural Scientific Foundation of China (No. 81372397 to Kai-hua Lu, No. 81302012 to Wei Li) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Mei-ling Zhang and Feng-qi Nie contributed equally to this work and should be regarded as joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(XLS 10 kb)
Rights and permissions
About this article
Cite this article
Zhang, Ml., Nie, Fq., Sun, M. et al. HOXA5 indicates poor prognosis and suppresses cell proliferation by regulating p21 expression in non small cell lung cancer. Tumor Biol. 36, 3521–3531 (2015). https://doi.org/10.1007/s13277-014-2988-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2988-4