Abstract
The aim of our study was to investigate the application of contrast-enhanced ultrasound (CEUS) and its quantification analysis for the prediction of early treatment response of sorafenib on rabbit VX2 liver tumor model. Rabbits were implanted VX2 tumor mass to establish a liver tumor model. Fourteen days after tumor implantation, rabbits presented with single liver tumor were randomly divided into two groups. Rabbits in treatment group were given by gavage once a day for 14 days with sorafenib suspension at a dose of 30 mg/kg, whereas rabbits in control group were given saline by gavage of the same volume. CEUS was performed before treatment and 3, 7, 14 days after treatment for the analysis of tumor size, enhancement pattern, and necrosis range. The time intensity curve (TIC) was used to obtain quantitative parameters of enhancement patterns. Before sorafenib administration, tumor volumes ranged from 0.24 to 0.75 cm3 (mean 0.49 ± 0.18 cm3) in treatment group and 0.24 to 0.44 cm3 (mean 0.30 ± 0.12 cm3) in control group. The dynamic enhancement patterns of tumors were homogeneous hyper-enhancement (n = 8), heterogeneous hyper-enhancement (n = 4), and peripheral rim-like enhancement (n = 2). All tumors of the treatment group presented with peripheral rim-like enhancement with large necrotic area after sorafenib administration, whereas tumors of the control group showed heterogeneous hyper-enhancement (n = 5) and peripheral rim-like enhancement (n = 2). There was a significant difference in area under the curve (AUC) before and after sorafenib treatment (P = 0.045). CEUS may be of value in the evaluation of early therapeutic response after sorafenib administration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Angiogenesis plays an important role in the growth and development of hepatocellular carcinoma (HCC) characterized by an increase of histological microvessel density (MVD) or vascular endothelium growth factor (VEGF) expression on tissue biopsies, and hypervascular enhancement pattern on imaging [1]. Sorafenib is a multikinase inhibitor targeting the Raf serine/threonine kinases and the VEGFR1-3, PDGFR-β, c-Kit, Flt3, and p38 tyrosine kinases, which blocks the VEGF and PDGF dependent angiogenesis pathway [2]. Several studies have shown that these therapeutics can help improve patients’ overall or progression-free survival [3, 4]. Effective monitoring of the new therapeutic approach requires imaging techniques that can detect early microvascular changes within tumors. The anti-angiogenic therapy can lead to internal tumor necrosis prior to or in the absence of volumetric regression [5]. Assessment of tumor response based on changes in tumor size such as the response evaluation criteria in solid tumors (RECIST) has been shown to be insufficient for targeted therapies. Therefore, early and accurate assessment of tumor response after targeted therapy is of utmost important. Recently, functional imaging modalities, including dynamic contrast-enhanced MRI (CEMRI), contrast-enhanced computed tomography (CECT), and positron emission tomography (PET) have been applied to monitor therapeutic response rather than morphological criteria [6–8].
Ultrasound imaging is an inexpensive and widely available technique. Color Doppler Ultrasound provides accurate analysis for visualization of the vasculature. However, it can not show the microvessels smaller than approximately 200 um in diameter [9]. Nowadays, the use of ultrasound contrast agent (UCA) and specific imaging software has provided a technique to display the vascularization of organ and lesion in real time. It has the potential advantage in the imaging of angiogenesis and monitoring of response to anti-angiogenic treatment. Contrast-enhanced ultrasound (CEUS) is a useful technique for visualization of the microvessels. Unlike CT or MRI contrast agent, ultrasonic microbubbles remain strictly in vessels, which is more suitable for the evaluation of blood flow [10].
The aim of our study was to investigate the dynamic changes of CEUS perfusion parameters and histopathology of the liver bearing VX2 tumors in rabbits after sorafenib therapy.
Materials and methods
Rabbit VX2 liver tumor models
Twenty male New Zealand white rabbits weighing 2–3 kg were used in this study. The rabbit VX2 liver model was established according to the literatures [11, 12]. First, VX2 tumor tissue in the thigh of donor rabbit was dissected and cut into cubes of 1–2 mm3 in size under sterile conditions. Then, 18 recipient rabbits were anesthetized by auricular vein injection of ketamine and diazepam (1:1). Through a median abdominal incision below the xiphoid, the left lobe of liver was exposed, where a 1-cm deep tunnel was made using ophthalmologic forceps. The VX2 tumor tissue cube was inserted into the tunnel which was then blocked by gelatin sponge to prevent the dropping out of tumor tissue and bleeding. The abdominal wall was sutured, and penicillin was intramuscular injected for 3 days after operation to prevent infection. Gray-scale ultrasound was performed to monitor the tumor growth consecutively and measure the size of tumor.
Experiment design
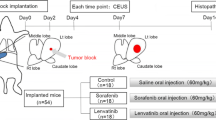
The protocol was approved by the Animal Care and Use Committee of Zhongshan Hospital. Fourteen days after tumor implantation, rabbits presented with single liver tumor were randomly divided into two groups. Rabbits in treatment group were given by gavage once a day for 14 days with sorafenib suspension at a dose of 30 mg/kg [13], whereas those in control group were given saline of the same volume. Conventional gray-scale ultrasound and CEUS was scheduled to be performed before treatment and 3, 7, 14 days after treatment. The rabbits were euthanized with diazepam 14 days after treatment. The specimens of liver were evaluated histologically.
Contrast-enhanced ultrasound
CEUS was performed using IU 22 (Philips, Bothell, WA, USA) equipped with a 3–9 MHz linear array transducer. A dose of 0.1 ml/kg of sulfur hexafluoride microbubbles (Sonovue, Bracco, Milan, Italy) was injected into auricular vein in bolus via a scalp needle followed by a flush of 1 ml of 0.9 % normal saline. Tumor microvessels were assessed by low mechanical index CEUS associated with quantification analysis software (QLAB, Philips, WA, USA). All of the ultrasonic parameters including mechanical index, gain, depth, dynamic range, and focal zone were constant throughout the whole study. The lesion was observed continuously for 2 min after injection of contrast, and all of the images were recorded on hard disk for further analysis.
Imaging analysis
All the imaging analysis was performed by one doctor who had no access to any information of the cases. First, gray-scale ultrasound was used for the evaluation of tumor location, echogenicity, and volume. The maximum length, width, and thickness of the tumors were measured in the largest slices. Tumor volume was calculated using the following formula [14]: length × width × thickness (mm)/2. Then, on the images in the arterial phase, analysis of enhancement characteristics was as follows: (1) enhancement level compared with the surrounding liver parenchyma (hyperechoic, hypoechoic, or isoechoic); (2) enhancement pattern (homogeneous, heterogeneous, peripheral); (3) presence of necrosis. The tumor necrosis rate was calculated as the no enhancement area divided by the entire area of the tumor (Fig. 1). Third, region of interest (ROI) was drawn according to lesion margins so as to cover the entire tumor. The time intensity curve (TIC) was automatically depicted by the software according to consecutive images (Fig. 2), with adjustments for motion compensation when necessary. Six quantitative perfusion parameters were extracted from the TIC: peak intensity (PI), time to peak (TTP), time from peak to one half, slope coefficient during wash-in, mean transit time (MTT), and area under the curve (AUC).
Histological examination
Angiogenesis was assessed by calculating the microvessel density (MVD). The pathological sections were first screened at low power (×100) to identify three areas with the greatest numbers of microvessels or vascularity. Then, microvessels of each area were counted at high magnification (×400). The MVD was calculated as the mean value of microvessels in the three most vascularized areas. The mean counts for each specimen were recorded as the microvessel density (MVD).
Statistical analysis
Statistical analysis was performed using SPSS software v 17.0 (SPSS Inc, Chicago, IL, USA) for Windows. Quantitative data were expressed as mean ± SD. The independent samples t test and one-way ANOVA test was used to analyze the quantitative data. Spearman correlation test was used to analyze the correlation between time and perfusion parameter. A p value <0.05 was considered indicative of statistical significance.
Results
The implanted VX2 orthotopic liver tumors grew successfully in 18 rabbits. However, 4 rabbits were ruled out from this study due to the following reasons: one rabbit had multiple lesions within the liver after VX2 tumor implantation; one rabbit died of anesthetic accident before CEUS; one rabbit died of pulmonary edema as a result of drugs misled into trachea during gavage; and one rabbit died of severe infection. Consequently, 14 rabbits divided into treatment group (n = 7) and control group (n = 7) were included in our study.
Before sorafenib administration, tumor volumes ranged from 0.24 to 0.75 cm3 (mean 0.49 ± 0.18 cm3) in treatment group and 0.24 to 0.44 cm3 (mean 0.30 ± 0.12 cm3) in control group. There was no statistical significant difference in volume between the two groups (P = 0.110). Before treatment, the liver tumor lesions appeared hypoechoic (n = 11) or hyperechoic (n = 3) on gray-scale ultrasound. Three types of enhancement patterns of the lesions were shown on CEUS: homogeneous hyper-enhancement (n = 8), heterogeneous hyper-enhancement (n = 4), and peripheral rim-like enhancement (n = 2) (Fig. 3). All tumor lesions showed quick wash-out after hyper-enhancement.
Although the tumor in treatment group seem to grow more slowly than that in control group, no statistical significant differences were detected in the tumor volume on the 3rd, 7th, and 14th day between the two groups (P > 0.05) (Table 1). As time goes on, tumors began to show peripheral or heterogeneous enhancement with various thickness and irregular inner margin of the enhanced areas. All tumors in treatment group presented with peripheral rim-like enhancement with large necrotic area after sorafenib administration (Fig. 4), whereas tumors in control group showed heterogeneous hyper-enhancement (n = 5) and peripheral rim-like enhancement (n = 2). The mean necrotic ratios in treatment group were larger than those in control group. But we can only find statistical significant difference in necrotic ratio on the 7th day after sorafenib administration between the two groups (Table 2).
a–d Rabbit with VX2 tumor before and after sorafenib treatment. A tumor in the left liver before treatment showed heterogeneous hyper-enhancement (a) followed by quick wash-out on CEUS imaging (b). Seven days after sorafenib administration, the tumor appeared as peripheral rim-like enhancement with a little UCA entry in the surrounding of tumor (c). Gross pathological imaging (d) showed residual tumor (arrow head) around necrosis area (arrow)
The AUC of treatment group decreased from 971.4 before sorafenib administration to 879.0, 720.5, and 535.5 on 3rd, 7th, and 14th day after treatment, respectively, with statistical significant difference. Spearman correlation test showed that the change of AUC was correlated to time (r s = −0.615, t = 0.004). However, no statistical significant differences were found in the change of other quantitative parameters including PI, TTP, time from peak to one half, MTT, and slope coefficient during wash-in in both treatment and control group. Table 3 showed the comparison of quantitative parameters in ROI of treatment group and control group.
In pathology, necrosis occurred within the tumor in both treatment and control group. Abdominal wall metastases were found in two rabbits in treatment group and one in control group. Two rabbits in control group developed ascites and liver metastases. The mean MVD was 6.9 ± 2.7 in treatment group and 13.9 ± 2.3 in control group (Fig. 5). There was a statistical significant difference in microvessels between the two groups (P = 0.001).
Discussion
To evaluate the early treatment response during the anti-angiogenic therapy is of great importance, for it might help physicians to select the best treatment options. In our study, rabbit VX2 orthotopic liver tumor model was used for the evaluation of antiangiogenic therapy. And in our study, we found that CEUS allowed us to detect a reduction in tumor vascularity in the early stage after the initiation of sorafenib therapy.
Rabbit VX2 orthotopic liver tumor model is the most commonly used big animal model to imitate primary or secondary liver cancers in humans. The VX2 tumor is a highly malignant anaplastic squamous cell carcinoma with biological behaviors including highly invasive, early hematogenous spread and high lethality [15]. Luo et al. [11] reported that most VX2 tumors were hypervascular within 14 days after tumor implantation. However, they began to develop peripheral vessels and central hypovascular areas after 14 days, which were shown as necrotic areas by pathologic examination. Conventional gray-scale US, color Doppler US, and CEUS were useful in sequential evaluation of VX2 tumor growth and characteristic vascularity [11]. Rabbit hepatic VX2 carcinoma is mainly fed by hepatic artery, and there is frequent occurrence of arteriovenous fistula [16]. This characteristic can be showed on CEUS as a quick wash-in followed by wash-out enhancement pattern.
Our study has demonstrated that continuous changes in rabbit VX2 liver tumors after the treatment of sorafenib can be monitored using dynamic CEUS. Apart from measurement of tumor volume, we can also observe the early change of enhancement pattern, calculate the necrosis ratio, and use QLAB software to analyze the TIC. The RECIST criteria, which are typically used to assess the treatment response to sorafenib for HCC, are quite insufficient in the early stage after sorafenib administration. It is shown in our study that no statistical significant differences were detected in the mean tumor volume at 3, 7, and 14 days between treatment and control group, while the mean necrotic ratios in treatment group were larger than those in control group on day 7. This suggested that tumor tend to necrosis with fewer blood volumes after sorafenib treatment.
Quantification analysis of time intensity curves has been reported to correlate with some critical clinical outcomes. Sugimoto et al. [17] found that a decrease in AUC during wash-in on day 14 was strongly correlated with tumor response in HCC, and AUC during wash-in on day 7 was the most relevant parameter for both overall and progression-free survival. Another study of Frampas et al. [18] showed a decrease in the AUC of more than 40 % at month 1 may be a potential surrogate marker of tumor response during targeted therapy. Our findings were consistent with these studies. The quantitative parameter most reliably predicting therapeutic response to sorafenib was AUC, which was related to the total blood volume in the investigated area. In pathological examination, we found a reduction of tumor MVD in sorafenib treatment group. The change of AUC was consistent with pathology. This result tends to suggest that some perfusion quantitative parameter is able to identify a decrease in tumor blood volume after treatment.
Some documents have reported that other quantitative parameters such as PI and TTP were useful in the predicting efficacy of anti-angiogenic therapy [19]. These two parameters were related to the flux per unit volume of vessels in tumor. Yoshida et al. [13] used CEUS for the evaluation of anti-angiogenesis treatment in rabbit model. They found that TTP of tumors was prolonged in the sorafenib group, which did not change or was shortened in the control group. However, in our study, no significant difference was found in PI and TTP of the two groups. There are several explanations for this difference. During its early stages, the VX2 tumor presents solid nature with little necrosis in the center. However, tumor growth could be faster after 2 weeks of tumor inoculation, resulting to a large necrotic area in the control group. This appearance can be shown on CEUS. In our study, the entire tumor including the necrosis area was included in ROI when we used TIC analysis, whereas some present studies only analyzed part of the region of tumors which excluded necrosis. As mentioned above, tumors in control group may show necrosis for a lack of blood supply when growing rapidly. This biological characteristic of VX2 tumor can result in the blood flow change similar to anti-angiogenesis treatment. Therefore, the appropriate time point to start anti-angiogenic therapy was of great importance. Based on previous literatures, we chose 14 days after implantation for the optimal time to start treatment in our study [20]. However, we may start pharmacological intervention even earlier to avoid affection by the spontaneous necrosis of tumor itself.
The main limitation of our study was the flaw of the chosen animal tumor model. Although we implanted VX2 tumor into rabbit liver to simulate blood supply of human HCC in vivo, VX2 as a squamous carcinoma still has some different biological characteristics from liver malignancy of human. As a fast-growing xenograft model, rabbit VX2 liver tumor lacks of a cirrhosis background. Therefore, it does not precisely reflect the behavior of HCC in human beings. Second, CEUS is an operator-dependent examination and the results can be affected by many factors. Although the function of motion compensation was used to reduce the impact of breath and heartbeat movements, we still found that breath may lead to the instability of images in ROI and thus affect the analysis results. Moreover, the sample size of our study was relatively small. And the results of CEUS were not compared with that of contrast CT or MRI according to mRECIST criteria.
In conclusion, the early changes in tumor perfusion observed may be predictive of tumor response. Quantitative parameter such as AUC is useful for the evaluation of total tumor blood flow, thus, predicting treatment efficacy. However, because of the limitation of animal models and quantitative analysis software, further study is still needed in this field.
References
International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49(2):658–64.
Huynh H. Molecularly targeted therapy in hepatocellular carcinoma. Biochem Pharmacol. 2010;80(5):550–60.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57(4):821–9.
Sun S, Schiller JH. Angiogenesis inhibitors in the treatment of lung cancer. Crit Rev Oncol Hematol. 2007;62(2):93–104.
Cyran CC, Schwarz B, Paprottka PM, Sourbron S, von Einem JC, Dietrich O, et al. In vivo monitoring of sorafenib therapy effects on experimental prostate carcinomas using dynamic contrast-enhanced MRI and macromolecular contrast media. Cancer Imaging. 2013;13(4):557–66.
Goggi JL, Bejot R, Moonshi SS, Bhakoo KK. Stratification of 18 F-labeled PET imaging agents for the assessment of antiangiogenic therapy responses in tumors. J Nucl Med. 2013;54(9):1630–6.
Anderson H, Price P, Blomley M, Leach MO, Workman P. Measuring changes in human tumour vasculature in response to therapy using functional imaging techniques. Br J Cancer. 2001;85(8):1085–93.
Rouffiac V, Bouquet C, Lassau N, Opolon P, Koscielny S, Peronneau P, et al. Validation of a new method for quantifying in vivo murine tumor necrosis by sonography. Investig Radiol. 2004;39(6):350–6.
Lamuraglia M, Bridal SL, Santin M, Izzi G, Rixe O, Paradiso A, et al. Clinical relevance of contrast-enhanced ultrasound in monitoring anti-angiogenic therapy of cancer: current status and perspectives. Crit Rev Oncol Hematol. 2010;73(3):202–12.
Luo W, Zhou X, Zheng X, He G, Yu M, Li Q, et al. Role of sonography for implantation and sequential evaluation of a VX2 rabbit liver tumor model. J Ultrasound Med. 2010;29(1):51–60.
Zhou P, Zhou P, He W, Wang LH, Li XH, Tian SM, et al. The influence of blood supply on high intensity focused ultrasound: a preliminary study on rabbit hepatic VX2 tumors of different ages. Acad Radiol. 2012;19(1):40–7.
Yoshida K, Hirokawa T, Moriyasu F, Liu L, Liu GJ, Yamada M, et al. Arterial-phase contrast-enhanced ultrasonography for evaluating anti-angiogenesis treatment: a pilot study. World J Gastroenterol. 2011;17(8):1045–50.
Zhang YJ, Chen MS, Li JQ, Zhang YQ, Zhong C, Liang HH. Radiofrequency ablation combined with ethanol injection for liver cancer treatment—an experimental study. Ai Zheng. 2006;25(9):1092–6.
Lin WY, Chen J, Lin Y, Han K. Implantation of VX2 carcinoma into the liver of rabbits: a comparison of three direct-injection methods. J Vet Med Sci. 2002;64(7):649–52.
He W, Wang W, Zhou P, Wang YX, Zhou P, Li RZ, et al. Enhanced ablation of high intensity focused ultrasound cardiovasc with microbbles: an experimental study on rabbit hepatic VX2 tumors. Cardiovasc Intervent Radiol. 2011;34(5):1050–7.
Sugimoto K, Moriyasu F, Saito K, Rognin N, Kamiyama N, Furuichi Y, et al. Hepatocellular carcinoma treated with sorafenib: early detection of treatment response and major adverse events by contrast-enhanced US. Liver Int. 2013;33(4):605–15.
Frampas E, Lassau N, Zappa M, Vullierme MP, Koscielny S, Vilgrain V. Advanced hepatocellular carcinoma: early evaluation of response to targeted therapy and prognostic value of perfusion CT and dynamic contrast enhanced-ultrasound. Preliminary results. Eur J Radiol. 2013;82(5):e205–11.
Shiozawa K, Watanabe M, Kikuchi Y, Kudo T, Maruyama K, Sumino Y. Evaluation of sorafenib for hepatocellular carcinoma by contrast-enhanced ultrasonography: a pilot study. World J Gastroenterol. 2012;18(40):5753–8.
Wang Z, Yang G, Nie P, Fu J, Wang X, Liu D. Dynamical observation on biological progression of VX2 liver tumors to identify the optimal time for intervention in animal models. PLoS One. 2013;8(8):e74327.
Acknowledgments
This research is supported by the national Nature Science Foundation of China (81371577)
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Hai-Xia Yuan contributed equally to this work and should be considered co-first author.
Rights and permissions
About this article
Cite this article
Kong, WT., Yuan, HX., Cai, H. et al. Early treatment response to sorafenib for rabbit VX2 orthotic liver tumors: evaluation by quantitative contrast-enhanced ultrasound. Tumor Biol. 36, 2593–2599 (2015). https://doi.org/10.1007/s13277-014-2877-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2877-x