Abstract
The 14-3-3 proteins are highly conserved molecules that are involved in many vital biologic processes and are associated with the progression of cancer. The role of 14-3-3ζ, a dimeric isoform of 14-3-3, in intrahepatic cholangiocarcinoma (ICC) was investigated in this study. The expression of 14-3-3ζ in tumour samples from patients with ICC was examined by Western blot and immunohistochemistry, and the correlation between its expression and various clinicopathological features was determined. Then, the capacity for invasion, migration and proliferation as well as the expression of epithelial–mesenchymal transition (EMT)-related markers in ICC cells were assessed after 14-3-3ζ depletion. Finally, the prognostic significance of 14-3-3ζ in patients with ICC was further evaluated by Kaplan–Meier and Cox regression analyses. The expression of 14-3-3ζ was significantly higher in ICC tissues compared to peritumoural tissues. High expression of 14-3-3ζ positively correlated with lymphatic metastasis and tumour–node–metastasis (TNM) stage. The inhibition of 14-3-3ζ expression was able to impair the invasion, migration and proliferation of ICC cells in vitro. The expression of 14-3-3ζ was significantly correlated with the expression of the EMT-related markers snail and E-cadherin in ICC samples. Moreover, the down-regulation of 14-3-3ζ also decreased the phosphorylation of extracellular signal-regulated kinase (ERK) in ICC cells. Clinically, patients with ICC with high 14-3-3ζ expression demonstrated a poor prognosis in terms of a short overall survival and a high recurrence rate of the disease. A multivariate analysis revealed that 14-3-3ζ overexpression was an independent prognostic indicator for patients with ICC. 14-3-3ζ may enhance the invasive and proliferative capacity of tumour cells and thus prompt the progression of ICC via the activation of ERK signalling and the induction of EMT. The overexpression of 14-3-3ζ may be used as a prognostic biomarker and therapeutic target in patients with ICC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common liver cancer and represents a diverse group of epithelial tumours with a late diagnosis and poor prognosis [1]. ICC is defined as a cholangiocarcinoma located in the second-degree bile ducts, and its incidence is increasing in Eastern Asia and in many Western countries [2, 3]. Although there are some treatment strategies such as surgical resection and palliative locoregional therapies, the overall survival (OS) of patients with ICC remains low, which is due to late diagnosis and early metastasis [4]. In this situation, a better understanding of the molecular mechanisms of this disease is needed in order to develop methods for early diagnosis and effective intervention.
The highly conserved 14-3-3 proteins are ubiquitous molecules that are involved in diverse biological processes such as metabolic regulation, cell cycle regulation, cell proliferation and apoptosis, as they integrate into different phospho-regulatory signalling pathways [5]. 14-3-3 often forms a complex with other molecules and regulates the function of a wide spectrum of proteins related to a variety of vital cellular processes such as growth and development. In addition, 14-3-3 is dysregulated in some diseases such as cancer [6]. As a dimeric isoform of the 14-3-3 protein, 14-3-3ζ exhibits an oncogenic potential through its interaction with target proteins involved in cancer initiation and progression [7]. Elevated expression of 14-3-3ζ has been observed in several tumours and is associated with drug resistance to certain anticancer therapies [8]. Studies have indicated that 14-3-3ζ overexpression may be a marker for recurrence and poor survival in patients with breast cancer, head and neck/oral squamous cell carcinomas and non-small cell lung carcinomas [9]. It has also been shown that depletion of 14-3-3ζ can increase the sensitivity of tumours to apoptosis-inducing agents. However, the up-regulation of 14-3-3ζ promotes resistance of tumour cells to apoptosis and increases their potential for invasion and metastasis by promoting epithelial–mesenchymal transition (EMT) [10–13]. Additionally, our previous study revealed that the 14-3-3ζ complex with αB-crystallin acts synergistically to promote the progression of hepatocellular carcinoma (HCC) by the constitutive activation of extracellular signal-regulated kinase (ERK) signalling [14].
In this study, we detected the expression of 14-3-3ζ protein and analysed the correlation between its expression and clinicopathological parameters in patients with ICC. Then, we evaluated the role of 14-3-3ζ in invasion, migration and proliferation of two human ICC cell lines. The role of 14-3-3ζ in the expression of markers related to EMT and the underlying mechanism were also explored. Finally, the prognostic implication of 14-3-3ζ in patients with ICC was determined.
Materials and methods
Patients and samples
The study samples included 30 frozen tissues for Western blot analysis and 120 formalin-fixed paraffin-embedded tissues that were randomly chosen from the tissue bank and pathology archives at Zhongshan Hospital after approval by the Zhongshan Hospital Research Ethics Committee. All specimens were from areas adjacent to the tumour margin and were obtained from patients with ICC who underwent a curative resection between 1999 and 2006 at the Liver Cancer Institute of Zhongshan Hospital, Fudan University (Shanghai, China). The histopathological diagnosis of ICC was based on World Health Organization criteria [15]. The seventh edition of the tumour–node–metastasis (TNM) classification system was also used [16]. Written informed consent was obtained from each patient. Follow-up data were collected until February 2009; the median duration of follow-up was 25 months (range, 4–120 months).
Cell lines
The human ICC cell line QBC939 was provided by the Shanghai Cancer Institute (Shanghai, China), and the cell line RBE was obtained from the Chinese Academy of Sciences Shanghai Branch Cell Bank (Shanghai, China). The cells were maintained in RPMI 1640 (Gibco, Carlsbad, CA) supplemented with 10 % foetal bovine serum (Gibco, Carlsbad, CA) and 1 % penicillin/streptomycin (Corning, Lowell, MA) at 37 °C in a humidified incubator with 5 % CO2.
Western blot and quantitative real-time polymerase chain reaction
Western blot and quantitative real-time polymerase chain reaction (qRT-PCR) were performed as described previously [14]. Briefly, 30 μg of protein was extracted from 30 cases of ICC samples and two ICC cell lines and was subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were then incubated with the corresponding antibodies, after which the bands were detected using an ECL substrate (Millipore, Bedford, MA) with a ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA). The antibodies used in the Western blot analysis are listed in Table S1.
Reverse transcription was performed with a kit (Takara, Dalian, China) to generate complementary DNA (cDNA) with 1 μg of RNA from each sample. Forward primers specific for 14-3-3ζ (5′-GATAAAAATGAGCTGGTTCA-3′) and a reverse primer (5′-TTAATTTTCCCCTCCTTCTCC-3′) were used. Gene amplification and detection were performed with the ABI PRISM 7900 Sequence Detection System (Applied Biosystems, Carlsbad, CA). PCR was performed with 1 μl cDNA and SYBR Green Real-time PCR Master Mix (Takara, Dalian, China), and the transcript levels were normalised to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The relative expression of 14-3-3ζ was analysed using the comparative cycle threshold (Ct) method according to the equation 2−ΔCt[ΔCt = Ct(14-3-3ζ) − Ct(GAPDH)]. All experiments were performed in triplicate.
Tissue microarrays and immunohistochemistry
Paraffin blocks used to construct the tissue microarrays (TMAs) were selected on the basis of suitable tissues with complete clinicopathologic and follow-up data. The construction of the TMAs and immunohistochemistry was performed as described in our earlier study [17, 18]. The antibodies used for immunohistochemistry are listed in Table S1. Images were captured by Leica QWin Plus v3 software (Leica Microsystems Imaging Solutions, Cambridge), and the average proportion in each field (three images) was used to represent a particular sample. Positive immunoreactivity for 14-3-3ζ was primarily expressed as brown staining in the cytoplasm. The criteria for the evaluation of 14-3-3ζ staining were established as follows: dark brown staining was scored as 2+, brown staining as + and weak brown or absent staining as − after an estimation of intensity. The mean area of positive staining (50 %) was used as a cut-off value to distinguish differential expression in proportion to measurement (score –, staining area ≤50 % of tumour section; score +, staining area >50 % of tumour section). After a comprehensive assessment of staining intensity and area presentation, which was performed by two independent researchers including a pathologist, patients with scores ≥2+ were considered to have high expression, and patients with scores <2+ were considered to have low expression. The intensity of E-cadherin and snail staining was evaluated as previously described [19].
Small interfering RNA
Transfections were performed with Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, Lipofectamine 2000 was incubated in Opti-MEM (Gibco, Carlsbad, CA) for 5 min at room temperature, then mixed with scrambled control or small interfering RNAs (siRNAs) diluted in an equal volume of Opti-MEM and incubated for another 20 min at room temperature. Finally, siRNA–Lipofectamine 2000 complexes (i.e. the interference or negative control group or only Lipofectamine 2000 as the mock group) were added to cells of 60 % confluence under Opti-MEM conditions in six-well plates. siRNA–Lipofectamine 2000 complexes in Opti-MEM were completely replaced with serum-containing regular medium after 6 h. Cells were lysed at 48 and 72 h after transfection for qRT-PCR and Western blot analysis, respectively. The sequences of the control and siRNAs are listed in Table S2.
Invasion, migration and proliferation assays and cell cycle analysis
Invasion and migration assays were performed as previously described [20]. Briefly, 48 h after siRNA interference, 1 × 105 cells were added to the top chambers of 24-well Transwell plates (Corning, Lowell, MA) coated with Matrigel (BD Biosciences, San Jose, CA). The bottom chambers were filled with complete media mixed with 10 % foetal bovine serum. Cells that migrated to the lower surface of the filter were considered to have invaded through the matrix and were counted after fixation and staining (incubation time: QBC939, 48 h; RBE, 36 h). The migration assay was performed in a similar manner except that the cells were seeded into the filter in plates that were not coated with Matrigel and were incubated for a shorter time (QBC939, 36 h; RBE, 24 h).
With regards to the proliferation assay, transfected cells were dispensed into a 96-well plate (QBC939, 3000 cells per 100 μl/well; RBE, 5000 cells per 100 μl/well). At the indicated time points, the Cell Counting Kit-8 solution (Dojindo, Kumamoto, Japan) was added to the cells followed by incubation for 2 h. The absorbance at 450 nm was measured to determine the number of viable cells in each well.
The cell cycle was analysed by flow cytometry. Briefly, the transfected cells were synchronised in G0 by serum starvation for 12 h and then released in complete medium containing 10 % foetal bovine serum for 24 h. After fixation in 70 % alcohol at −20 °C, the cells were washed and resuspended in cold PBS with propidium iodide and RNase A for 30 min on ice. Samples were analysed for DNA content using an EPICS ALTRA flow cytometer (Beckman Coulter, Fullerton, CA). All experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed with SPSS 16.0 software. The χ 2 test, Fisher’s exact probability and Student’s t test were used for comparisons between the groups. The probability of survival and the cumulative recurrence rate were evaluated using the Kaplan–Meier method, and the differences were assessed by log-rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. All tests were two tailed, and p < 0.05 was considered statistically significant.
Results
Expression of 14-3-3ζ was up-regulated in ICC tissues, and its expression correlated with tumour recurrence
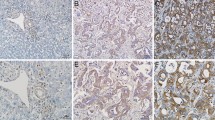
The expression of 14-3-3ζ in 30 ICC tumour and matched peritumoural tissues was analysed by Western blot. Compared with the peritumoural samples, semi-quantitative analysis showed that 14-3-3ζ protein expression was much higher in tumour tissues (0.66 ± 0.15 vs. 0.19 ± 0.05, p = 0.006, Fig. 1a). We then examined 14-3-3ζ expression in TMAs that consisted of 120 ICC and peritumoural samples by immunohistochemistry (Fig. 1b), and the results also revealed that 14-3-3ζ protein expression was much higher in tumour tissues than in peritumoural tissues (1.18 ± 0.11 vs. 0.77 ± 0.06, p = 0.001, Fig. 1c).
14-3-3ζ expression in tumour and peritumoural tissues from patients with intrahepatic cholangiocarcinoma. a The expression of 14-3-3ζ protein was detected in tumour and peritumoural tissues from patients with ICC by Western blot analysis. b Haematoxylin–eosin and 14-3-3ζ staining were illustrated in tumour and peritumoural tissues of intrahepatic cholangiocarcinoma patients with and without recurrence. Scale bar = 200 μm. c The expression of 14-3-3ζ in intrahepatic cholangiocarcinoma tumours is stronger than that of peritumoural tissues. d The expression of 14-3-3ζ in tumour tissues of patients with intrahepatic cholangiocarcinoma with recurrence is much higher than that in patients without recurrence. T tumour, P peritumour, HE haematoxylin–eosin
When these patients were classified into the 14-3-3ζ high and low expression groups, we investigated the relationship between 14-3-3ζ expression and clinicopathological features. The results showed that a high expression of 14-3-3ζ was significantly correlated with sex, lymphatic metastasis and TNM stage (Table 1). However, none of the other clinicopathological features such as age, hepatitis B surface antigen positivity, preoperative serum carbohydrate antigen 19-9, preoperative serum alpha-fetoprotein, microvascular invasion, tumour size, number or differentiation were correlated with 14-3-3ζ expression. Moreover, the results further revealed that patients with ICC who experienced a recurrence demonstrated a trend of high 14-3-3ζ expression compared with those who did not experience a recurrence (1.33 ± 0.13 vs. 0.70 ± 0.20, p = 0.013, Fig. 1d).
Silencing 14-3-3ζ expression in ICC cells resulted in a decreased ability for invasion, migration and proliferation
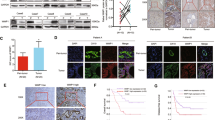
14-3-3ζ expression in two ICC cell lines, QBC939 and RBE, was knocked down by RNA interference. The second pair of siRNAs was found to have the highest inhibitory efficiency by qRT-PCR and Western blot (Fig. 2a, b). Thus, we chose this siRNA to assess its effect on the invasion, migration and growth of ICC cells. The results of Transwell assays with or without Matrigel showed that the average number of invading cells that were transfected with 14-3-3ζ-si-2 siRNA was significantly decreased in comparison with the 14-3-3ζ-si-nc-transfected cells (Fig. 2c, d). In the CCK-8 assay, the proliferation of ICC cells was restrained in 72 h after transfection of 14-3-3ζ-si-2 siRNA (Fig. 2e). Cell cycle analysis showed that 14-3-3ζ siRNA caused S phase arrest in ICC cells (Fig. 2f, g). All these data indicated that the migratory, invasive and proliferative ability of ICC cells was suppressed by the down-regulation of 14-3-3ζ.
Functional analysis after transfection with 14-3-3ζ small interfering RNA in ICC cells in vitro. a Quantitative real-time polymerase chain reaction and b Western blot analysis revealed the expression of 14-3-3ζ in QBC939 and RBE cells after RNA interference of 14-3-3ζ expression. c The migratory and invasive abilities of cancer cells were measured by Transwell plates and Matrigel invasion plates. Scale bar = 200 μm. d The numbers of invasive QBC939 and RBE cells that were transfected with small interfering RNA or a negative control were calculated. e Cell proliferation was detected by Cell Counting Kit-8 assay after knockdown of 14-3-3ζ. f, g Application of 14-3-3ζ small interfering RNA could cause S phase arrest of intrahepatic cholangiocarcinoma cells. si-nc small interfering RNA sequence negative control, si-1 small interfering RNA sequence 1, si-2 small interfering RNA sequence 2
High expression of 14-3-3ζ predicted a poor prognosis for patients with ICC
The 2- and 5-year survival rates for the 120 patients were 37.5 and 24.2 %, respectively, and the 2- and 5-year recurrence rates were 69.2 and 76.7 %, respectively. Expression of 14-3-3ζ exhibited considerable heterogeneity in the different tumour tissues. We classified all of the cohorts into high and low expression groups, and representative samples are shown in Fig. 3a. We found that patients with high expression of 14-3-3ζ had a significantly worse prognosis than those with low expression (Fig. 3b). The 2- and 5-year overall survival rates of patients with high expression of 14-3-3ζ were much lower than those with low expression (21.7 vs. 44.6 % and 7.8 vs. 28.4 %, respectively). The 2- and 5-year cumulative recurrence rates of patients with high expression of 14-3-3ζ were significantly higher than those with low expression (85.1 vs. 64.1 % and 91.7 vs. 71.7 %, respectively).
Overexpression of 14-3-3ζ was significantly correlated with poor prognosis and induced the activation of ERK signalling and epithelial–mesenchymal transition. a Representative images of haematoxylin–eosin and immunohistochemical staining for 14-3-3ζ. Scale bar = 200 μm. b Patients with intrahepatic cholangiocarcinoma with high 14-3-3ζ expression have a poorer prognosis in terms of overall survival and cumulative recurrence. c Two representative images of one patient with intrahepatic cholangiocarcinoma who exhibited 14-3-3ζlow, snaillow and E-cadherinhigh and one patient who exhibited 14-3-3ζhigh, snailhigh and E-cadherinlow. Scale bar = 200 μm. d A Western blot analysis indicated that snail was down-regulated and that E-cadherin was up-regulated by 14-3-3ζ interference in intrahepatic cholangiocarcinoma cells. e The down-regulation of 14-3-3ζ inhibited the phosphorylation of ERK1/2 in intrahepatic cholangiocarcinoma cells
14-3-3ζ expression, tumour number, differentiation, lymphatic metastasis and TNM stage were predictors for overall survival and cumulative recurrence based on a univariate analysis. In a multivariate Cox proportional hazards model, 14-3-3ζ staining was an independent prognostic factor for overall survival and cumulative recurrence (Table 2).
High expression of 14-3-3ζ induced EMT and activated ERK signalling
We performed immunohistochemistry to detect the expression of 14-3-3ζ and the EMT-related markers snail and E-cadherin in patients with ICC. The results showed that highly expressive levels of 14-3-3ζ positively correlated with snail (p < 0.001, r = 0.679) and negatively correlated with E-cadherin (p < 0.001, r = −0.356, Table S3). We also found a down-regulation of snail and an up-regulation of E-cadherin after the disruption of 14-3-3ζ by siRNA in ICC cells, which was consistent with the results from a clinical setting (Fig. 3c, d). Reduced magnitudes of ERK1/2 phosphorylation were observed in ICC cells that were transfected with 14-3-3ζ siRNA compared with the corresponding control cells (Fig. 3e). These results indicated that high levels of 14-3-3ζ may involve in EMT and activation of ERK signalling.
Discussion
14-3-3 proteins are able to interact with many different proteins based on their specific phosphor-serine/phosphor-threonine binding activity. Extensive evidence has supported the concept that dysregulation of 14-3-3 and its interaction with client proteins contributes to the development of a broad spectrum of human diseases. The specific isoform 14-3-3ζ was found to be overexpressed in cancer cell lines as well as in tumour samples and is associated with a poor prognosis of patients with breast or lung cancer [21, 22]. A recent study showed that there was an increased expression of 14-3-3ζ in intrahepatic cholangiocarcinoma compared with extrahepatic cholangiocarcinoma, and its overexpression was significantly correlated with tumour pathologic differentiation and stage, as determined by immunohistochemistry [23]. However, no study has addressed the role and prognostic significance of 14-3-3ζ in individuals with ICC. In this study, we showed that 14-3-3ζ expression was higher in tumours than in peritumoural tissues and that 14-3-3ζ expression exerted a significant effect on the invasive, migratory and proliferative capacity of ICC cells. Clinically, ICC patients with high expression of 14-3-3ζ had a poorer prognosis than those with low expression. The above data provide convincing evidence that 14-3-3ζ is implicated in the progression of ICC and may serve as a promising prognostic biomarker and a therapeutic target for patients with ICC.
Another interesting result from the present study was that 14-3-3ζ expression correlated with snail and E-cadherin expression in ICC samples. Moreover, the inhibition of 14-3-3ζ expression in ICC cells could inactivate the ERK signalling pathway. These data support the notion that 14-3-3ζ might foster the progression of ICC through the activation of the ERK pathway and by the induction of EMT. Cumulative evidence has indicated that 14-3-3 expression is controlled through genomic alterations including epigenetic silencing, gene amplification, post-transcriptional modulation of mRNA stability and proteasome-mediated degradation. 14-3-3 proteins function primarily as adaptor proteins by binding to various client proteins, which is influenced by modification of 14-3-3 proteins or client proteins within the 14-3-3 binding motifs [24]. Recent studies have reported that members of the 14-3-3 family, especially 14-3-3ζ, play an oncogenic role in multiple tumour types [11, 25]. The snail transcription factor functions as a main regulator of EMT events and directly regulates genes that affect cell adhesion, motility and polarity [26]. As a positive regulator of snail, 14-3-3ζ overexpression reduced cell–cell adhesion via the acceleration of EMT to promote the progression of cancer [13, 27]. In addition, 14-3-3 proteins are also involved in cell proliferation through the regulation of the cell cycle [28, 29]. Cell proliferation is often initiated by cell surface receptor tyrosine kinases (RTKs). Among RTK pathways, key molecules such as the Raf proteins could promote proliferation by signalling through the ERK cascade. 14-3-3ζ could form a complex with Raf to protect p-Raf from dephosphorylation [30]. Our research suggests that a modulation of 14-3-3ζ could activate the ERK signalling cascade and induce EMT.
Together, our results demonstrate that 14-3-3ζ plays an important role in tumour progression, and we provide evidence that 14-3-3ζ might enhance the invasive and proliferative abilities of ICC cells through the induction of EMT and activation of the ERK pathway. Although the precise mechanisms of 14-3-3ζ function on tumour development need to be further investigated, our study confirms the value of 14-3-3ζ in the prediction of prognosis and the development of new therapeutic interventions.
References
Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–54.
Everhart JE, Ruhl CE. Burden of digestive diseases in the United States. Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44.
Aitken A. 14-3-3 proteins: a historic overview. Semin Cancer Biol. 2006;16:162–72.
Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009;19:16–23.
Freeman AK, Morrison DK. 14-3-3 proteins: diverse functions in cell proliferation and cancer progression. Semin Cell Dev Biol. 2011;22:681–7.
Frasor J, Chang EC, Komm B, et al. Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res. 2006;66:7334–40.
Neal CL, Yu D. 14-3-3ζ as a prognostic marker and therapeutic target for cancer. Expert Opin Ther Targets. 2010;14:1343–54.
Niemantsverdriet M, Wagner K, Visser M, et al. Cellular functions of 14-3-3 zeta in apoptosis and cell adhesion emphasize its oncogenic character. Oncogene. 2008;27:1315–9.
Fan T, Li R, Todd NW, et al. Up-regulation of 14-3-3zeta in lung cancer and its implication as prognostic and therapeutic target. Cancer Res. 2007;67:7901–6.
Maxwell SA, Li Z, Jaye D, et al. 14-3-3zeta mediates resistance of diffuse large B cell lymphoma to an anthracycline-based chemotherapeutic regimen. J Biol Chem. 2009;284:22379–89.
Lu J, Guo H, Treekitkarnmongkol W, et al. 14-3-3zeta cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial-mesenchymal transition. Cancer Cell. 2009;16:195–207.
Huang XY, Ke AW, Shi GM, et al. AlphaB-crystallin complexes with 14-3-3zeta to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology. 2013;57:2235–47.
Wittekind C. Pitfalls in the classification of liver tumors. Pathologe. 2006;27:289–93.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Ke AW, Shi GM, Zhou J, et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology. 2009;49:491–503.
Zhang C, Bai DS, Huang XY, et al. Prognostic significance of Capn4 overexpression in intrahepatic cholangiocarcinoma. PLoS One. 2013;8:e54619.
Ke AW, Shi GM, Zhou J, et al. CD151 amplifies signaling by integrin alpha6beta1 to PI3K and induces the epithelial-mesenchymal transition in HCC cells. Gastroenterology. 2011;140:1629–41. e15.
Bai DS, Dai Z, Zhou J, et al. Capn4 overexpression underlies tumor invasion and metastasis after liver transplantation for hepatocellular carcinoma. Hepatology. 2009;49:460–70.
Neal CL, Yao J, Yang W, et al. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009;69:3425–32.
Zhao GY, Ding JY, Lu CL, et al. The overexpression of 14-3-3zeta and Hsp27 promotes non-small cell lung cancer progression. Cancer. 2014;120:652–63.
Wu Q, Liu CZ, Tao LY, et al. The clinicopathological and prognostic impact of 14-3-3 protein isoforms expression in human cholangiocarcinoma by immunohistochemistry. Asian Pac J Cancer Prev. 2012;13:1253–9.
Zhao J, Meyerkord CL, Du Y, et al. 14-3-3 proteins as potential therapeutic targets. Semin Cell Dev Biol. 2011;22:705–12.
Lin M, Morrison CD, Jones S, et al. Copy number gain and oncogenic activity of YWHAZ/14-3-3zeta in head and neck squamous cell carcinoma. Int J Cancer. 2009;125:603–11.
Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–61.
Hou Z, Peng H, White DE, et al. 14-3-3 binding sites in the snail protein are essential for snail-mediated transcriptional repression and epithelial-mesenchymal differentiation. Cancer Res. 2010;70:4385–93.
Bartkova J, Horejsi Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70.
Gardino AK, Yaffe MB. 14-3-3 proteins as signaling integration points for cell cycle control and apoptosis. Semin Cell Dev Biol. 2011;22:688–95.
Fischer A, Baljuls A, Reinders J, et al. Regulation of RAF activity by 14-3-3 proteins: RAF kinases associate functionally with both homo- and heterodimeric forms of 14-3-3 proteins. J Biol Chem. 2009;284:3183–94.
Acknowledgments
This study was supported by the National Key Sci-Tech Project (2012ZX10002011-002) the National Natural Science Foundation of China (81172023, 81071741 and 81030038), Shanghai Municipal Natural Science Foundation (14ZR1405800) and the Ph.D. Programs Foundation of the Ministry of Education of China (20110072120050).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Chi Zhang and Li-Xin Liu and Zhao-Ru Dong and Guo-Ming Shi contributed equally to this work. None of the material that appears in the article has been previously presented or published in any form.
Rights and permissions
About this article
Cite this article
Zhang, C., Liu, LX., Dong, ZR. et al. Up-regulation of 14-3-3ζ expression in intrahepatic cholangiocarcinoma and its clinical implications. Tumor Biol. 36, 1781–1789 (2015). https://doi.org/10.1007/s13277-014-2780-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2780-5