Abstract
Cancer-associated microRNAs have been stably detected in blood. The objective of this study was to identify a panel of circulating microRNAs with the potential to serve as biomarkers for estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2 (HER2)− breast cancer. We used microarray-based expression profiling to compare the levels of circulating microRNAs in blood samples from 11 ER+/HER2− advanced breast cancer patients plus 5 age-matched controls. MicroRNA levels were validated by reverse transcription quantitative polymerase chain reaction in 40 control subjects, 187 early breast cancer patients, and 45 metastatic breast cancer patients. Then, we assessed the association between the levels of microRNA and clinical outcomes of ER+/HER2− metastatic breast cancer. Initially, we found that miR-1280, miR-1260, and miR-720 were up-regulated in blood from breast cancer patients (P < 0.05). In validation, miR-1280 levels significantly increased in breast cancer patients and reflected tumor status (control<<early cancer<metastatic cancer). Among 37 metastatic breast cancer patients, miR-1280 levels significantly decreased after treatment in patients who responded to systemic treatment (P < 0.001). We confirmed that miR-1280 was not a classic microRNA, but a tRNALeu-derived fragment. These findings suggest that a circulating tRNA-derived microRNA, miR-1280, is differently expressed in breast cancer patients and may serve as a biomarker for ER-positive breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MicroRNAs are small molecules 22 nucleotides in length that regulate gene expression in a variety of biological processes, including cell cycle, differentiation, development, and metabolism, as well as human disease, such as diabetes and cancer. MicroRNA expression is frequently dysregulated in cancer and its microRNA expression patterns appear to be tissue-specific [1–4].
A microRNA can act as a tumor suppressor when its functional loss initiates or contributes to the malignant transformation of a normal cell [5, 6]. There are strong evidences that microRNAs function as oncogenes as well. The overexpression of miR-155, miR-21, or the miR-17-92 cluster has been frequently observed in various types of tumor cells; these microRNAs activate multiple genes, resulting in cellular proliferation, antiapoptotic effects, and metastasis [7–9]. In breast cancer, overall microRNA expression may clearly distinguish breast cancer tissue from normal breast tissue, and specific microRNA signatures may reflect the status of the estrogen receptor (ER), the progesterone receptor, and HER2/neu in cancer cells [3, 10, 11]. Other experiments have demonstrated that microRNAs are also involved in multiple steps in tumorigenesis and tumor growth, including angiogenesis and the renewal of cancer stem cells, which drew tremendous attention from cancer researchers [12–14].
Recently, cancer-associated microRNAs were detected in the blood of cancer patients, although the mechanism of export to the bloodstream from cancer cells remains unclear [15–18]. Interestingly, microRNAs in human plasma are present in a remarkably stable form that is protected from endogenous RNase activity [15]. Therefore, the unique patterns of circulating microRNAs may serve as noninvasive biomarkers of cancer development and prognosis [17, 19, 20].
Heneghan et al. evaluated seven known breast cancer-specific microRNAs using the blood of early breast cancer (EBC) patients; circulating miR-195 levels were elevated in operable breast cancer patients compared to age-matched healthy controls, and the levels decreased to control levels following curative tumor resection [16]. Zhao et al. performed expression profiling of circulating microRNAs in plasma samples from 20 patients with EBC and matched healthy controls [21]. They observed that 31 microRNAs were differentially expressed in Caucasian-American and 18 microRNAs in African-American patients, and in silico pathway analysis predicted contributions to critical cell functions, cancer development, and progression. However, Zhao et al. did not demonstrate the specificity of these circulating microRNAs for breast cancer, and many of the differentially expressed microRNAs were novel and had not been described in miRBase (www.mirbase.org). Additionally, their investigation failed to address the associations between specific microRNAs and breast cancer subtypes according to ER, progesterone receptor, and HER2 status.
Here, we report the identification of circulating microRNAs specific to ER- or progesterone receptor-positive and HER2-normal breast cancers. These microRNAs were differentially expressed in blood samples from breast cancer patients and matched healthy controls. We also investigated the clinical implications of these specific microRNAs in breast cancer patients.
Materials and methods
Patients and samples
This study consisted of two parts: discovery (microarray analysis) and validation (quantitative reverse transcription PCR (qRT-PCR) of selected microRNAs). Patients with ER- and /or progesterone receptor-positive and HER2-normal breast cancer were included in this study. Blood samples were collected prospectively from 272 women, including 187 consecutive EBC patients, 45 metastatic breast cancer (MBC) patients, and 40 healthy age-matched volunteers who served as controls for this study. We included patients who had normal ranged numbers of white blood cells (WBC) and red blood cells (RBC) in their peripheral blood.
Whole blood was collected in EDTA blood tubes (Monovette EDTA K; Sarstedt AG, Germany) and PAXgeneTM blood RNA tubes (Qiagen®, Valencia, CA, USA), which were frozen at −20 °C within 2 h and transferred to −80 °C within 1 day for long-term storage. None of the patients received anticancer treatment prior to blood collection. In addition, repeat blood samples were acquired from a subset of MBC patients after 2 or 3 months of systemic treatment (N = 37). Specific microRNAs identified in patient blood samples were evaluated in patient tumor tissues, tumor-associated normal tissues, a human mammary epithelial cell line, and breast cancer cell lines MCF-7 and T47D.
This study was approved by the Institutional Review Board of the National Cancer Center (NCCNCS-10-320), and written informed consent was obtained from every patient and control before blood was sampled.
Isolation of RNA
Total RNA was isolated from blood using the miRNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA was extracted from homogenized tissue using the RNeasy® Tissue Mini Kit (Qiagen) according to the manufacturer’s instructions. RNA concentration and quality were assessed via absorbance spectrometry on a NanoDropTM 2000 (Thermo®, Miami, FL, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA, USA).
MicroRNA microarray and data analysis
For control and test RNAs, the synthesis of target microRNA probes and hybridization were performed using the miRNA Labeling Reagent and Hybridization Kit (Agilent Technologies) according to the manufacturer’s instructions. All data normalization and selection of twofold changed genes were performed using GeneSpringGX 7.3 (Agilent Technologies). The averages of normalized ratios were calculated by dividing the average of the normalized signal channel intensity by the average of the normalized control channel intensity. The data have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE53179 [22].
Plasmids and transfection
Leu (AAG) chr14 TRNAL1 (chr14:21078291–21078372) was chemically synthesized (Bioneer Inc., Taejeon, South Korea). Expression vectors for transient transfection of the synthesized tRNA were generated by PCR amplification followed by insertion into the multiple cloning site of the pcDNA3.1 expression vector (Invitrogen Life Science). Host 293T cells were maintained in Dulbecco’s Modified Eagle Medium supplemented with 10 % FBS and 1 % penicillin/streptomycin. Transfection of 293T cells was achieved with Lipofectamine 2000 (Invitrogen Life Science) following the manufacturer’s instructions.
MicroRNA quantification by qRT-PCR
The identified microRNAs were validated using TaqMan Human MicroRNA assays for mature human microRNAs (Applied Biosystems, Cheshire, UK). The 7.5-μl reverse transcription (RT) reactions was performed using the Taqman microRNA reverse transcription kit (Applied Biosystems, Cheshire, UK) and incubated for 30 min at 16 °C, 30 min at 42 °C, 5 min at 85 °C, and then maintained at 4 °C. The qPCR was performed using the Taqman Universal Master Mix (Applied Biosystems, Cheshire, UK). RT products (2.5 μl) were used as templates in 10 μl reactions containing specific probes for each microRNA. The reactions were incubated in 384-well plates at 95 °C for 10 min, followed by 45 cycles at 95 °C for 15 s, and 60 °C for 1 min. The qPCR was done in Roche LightCycler 480 (Roche Applied Sciences, Penzberg, Germany) in duplicates. The cycle thresholds (C t) were calculated and normalized to miR-16 (miR-16; ABI), which was found the most-widely used endogenous control microRNA for RT-qPCR [15]. The relative expression levels of each miRNA were calculated using the comparative cycle thresholds (C T) method (2−ΔCT).
Northern blotting
Total RNA from 293T cells transfected with synthetic chr14 TRNAL1 was loaded onto a 15 % urea-polyacrylamide gel (Bio-Rad, USA), electrophoresed, and transferred to nylon membranes (Roche Diagnostics, Mannheim, Germany). RNA and membranes were cross-linked via exposure to UV light (120 mJ/cm2). For detecting miR-1280 and tRNALeu, 5′-AGGGTGGCGACGGT-3′ probes were labeled with non-radioactive digoxigenin (DIG) (Roche Diagnostics) according to the manufacturer’s protocol. Prehybridization was carried out using DIG Easy Hyb (Roche Diagnostics) at 37 °C, and hybridization was performed by adding digoxigenin-labeled probe to the prehybridization solution. After hybridization, the membranes were washed twice using the DIG Wash and Block Buffer Set (Roche Diagnostics). RNA was detected by exposure to anti-digoxigenin-alkaline phosphatase conjugate (Roche Diagnostics), and X-ray films were exposed to the membrane for chemiluminescence detection.
Statistical analysis
All values were expressed as means ± SDs. In analysis for microRNAs expression data, a nonparametric Wilcoxon rank sum test for comparison of differential expression between two groups was performed. To address the issue of multiple testing, adjusted P value was obtained by Benjamini and Hochberg’s adjustment. ANOVA was used to compare microRNA levels in EBC patients, MBC patients, and healthy controls with Bonferroni correction. The paired t test was used to detect changes in microRNA levels between baseline and post-treatment in patients with MBC. All statistical analyses were performed using SPSS software (version 21.0, IBM). Tumor response to systemic treatment was evaluated according to Response Evaluation Criteria In Solid Tumors (RECIST) version 1.0. Overall survival (OS) was defined as the length of time from the date of enrollment of this study to death from any cause or last follow-up date. OS was estimated using the Kaplan-Meier method and compared with logrank test. All tests were two-sided and considered statistically significant at P values <0.05.
Results
Differentially expressed microRNAs in blood samples from breast cancer patients
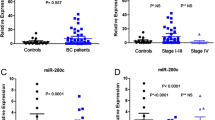
The blood samples were collected from 11 MBC patients and 5 controls for the discovery part and 187 EBC, 45 MBC patients, and 40 controls for the validation part. In the discovery arm of our investigation, global microRNA expression profiling was carried out using Agilent’s microRNA microarray platform. We identified 27 deregulated microRNAs in the whole blood of breast cancer patients compared to healthy controls (Fig. 1). Statistical analysis via t test with Benjamini-Hochberg adjustment revealed 16 significantly up-regulated microRNAs and 11 significantly down-regulated microRNAs (Table 1). We selected three microRNAs (miR-1260, miR-720, miR1280) that showed more than twofold up-regulated expression between the two groups for additional qRT-PCR validation. miR-21, miR-195, and let-7a were also included because the expression of these molecules in breast cancer tissue and in the blood of patients with breast cancer was reported in a previous study [16]. We then performed individual qRT-PCRs to quantify the expression of the microRNAs listed in Table 2. miR-1260, miR-720, as well as miR-1280 expression significantly differed between patients and controls (Fig. 2). Of those, miR-1280 was selected for further evaluation because others failed to discriminate tumors from normal samples and showed an irrelevance to tumor status.
Identification of miR-1280 in breast tumor tissue and breast cancer cell lines
Consistent with a previous report [23], we found that miR-1280 consists of tRNALeu-derived fragments (TdF) with an identical sequence at the 5′-terminal domain (Fig. 3a). miR-1280 expression was detected both in breast cancer tissue and in cancer cell lines, but not in tumor-associated normal tissue and a human mammary epithelial cell line (Fig. 3b). To clarify the relationship between miR-1280 and tRNALeu, we chemically synthesized a population of miR-1280 molecules matching tRNALeu, inserted these molecules into pcDNA3.1 expression vectors, and transiently transfected these vectors into 293T cells. Northern blotting revealed a clear increase in both the mature tRNA and a 22-nt band comigrating with the endogenous fragment observed in breast cancer cells compared to those with empty vectors, suggesting that miR-1280 was derived from tRNALeu (Fig. 3c).
miR-1280 is expressed in breast tumor cells. a Identical sequence of annotated human tRNAs with matched miR-1280 sequences. b miR-1280 levels in breast cancer cell lines and primary breast cancer tissue compared with normal breast tissue and a human mammary epithelial cell (HMEC) line. Each bar represents the mean of microRNA levels and vertical lines indicate plus or minus 1 standard deviation of the mean. c Northern blot of total RNA from 293T cells transiently transfected with empty vector or with vector encoding the Leu (AAG) chr14 TRNAL1. Bands correspond to the 22-nt tRNA fragment of miR-1280 and the 74-nt mature tRNA. EtBr, ethidium bromide
Association between miR-1280 (TdF-tRNALeu) levels in blood and breast cancer status
miR-1280 expression levels were confirmed with Taqman-based qRT-PCR in an independent cohort of breast cancer patients and controls using individual microRNA-specific primers (F: 5′ AT AAG CTT GGT AGC GTG GCC GAG; R: AT GAA TTC TGG TGG CAG CGG TGG 3′). This validation 1 cohort consisted of 50 EBC patients, 22 MBC patients, and 20 age-matched healthy controls. Levels of miR-1280 were significantly increased in the blood of breast cancer patients, especially MBC patients (Fig. 4a, P < 0.001). In a validation 2 cohort set comprised of 137 EBC patients, 23 MBC patients, and 20 age-matched healthy controls, miR-1280 expression levels in blood were higher in breast cancer patients than in controls; within this validation cohort, these levels were significantly higher in MBC patients (Fig. 4b).
Correlation between miR-1280 (TdF-tRNALeu) levels in blood and treatment response
A subset of our MBC patients had undergone post-systemic treatment (such as cytotoxic chemotherapy or endocrine therapy) before blood samples were collected (N = 37), allowing us to assess the effect of treatment on circulating miR-1280 levels. miR-1280 expression in blood decreased significantly after 2–3 months of systemic treatment (Fig. 5a). These changes differed according to treatment response. In patients who responded to treatment (complete response, partial response, or stable disease), miR-1280 levels significantly decreased after treatment (P < 0.001, Fig. 5b). However, the expression of miR-1280 increased after treatment in the blood of patients with non-responding tumors (P = 0.035, Fig. 5c). In addition, there was a tendency of better survival in patients with decreasing miR-1280 levels than those with increasing or stable ones (P < 0.058) (Fig. 5d).
Changes of the levels of miR-1280 following systemic treatment in metastatic breast cancer. a Decreased blood levels of miR-1280 in MBC patients after at least 2–3 months after systemic treatment. Direction of blood level changes of miR-1280 reflected treatment response both in patients with response (N = 30) (b) and those without response (N = 7) (c). d Overall survival according to the direction of change of miR-1280 levels (decreasing (N = 21) vs. no change/increasing (N = 16))
Discussion
The list of microRNA candidates encoded by the human genome continues to grow due to the application of high-throughput sequencing technology. However, many microRNAs have been removed from miRBase and reclassified as other small RNAs generated via non-canonical pathways dependent or independent of the RNAse III enzymes Drosha and Dicer [24–26]. Next to microRNAs, the most abundant group of small RNAs consists of fragments derived from tRNA (tRNA-derived RNA fragments, TdF) [26]. Previous reports demonstrated that TdF are not random by-products of tRNA biogenesis but are short RNAs with specific sequences and structures that have specific biological roles in living cells [26, 27]. However, the functional role of these small RNAs, which may act as microRNAs, has not yet been fully defined. Recently, Maute et al. showed that the tRNA-derived microRNA CU1276 was generated in a DICER1-dependent manner from tRNAGly in mature B cells [27]. CU1276 was down-regulated in lymphoma cells, suppressing the proliferation of the cells and modulating the molecular response to DNA damage via the repression of replication protein A1 (RPA1).
Previously, microRNAs were aberrantly expressed in the breast cancer tissue and in the serum of patients categorized into four subtypes (ER+/HER2−, ER+/HER2+, ER−/HER2+, and ER−/HER2−) [10, 28]. Given these data, this study focused on the ER-positive/HER2-normal subtype. Of the 27 microRNAs that were differentially expressed in this study (Table 1), we found that miR-1280 expression levels significantly differed between the blood of breast cancer patients and healthy controls. miR-1280 expression was detected exclusively in breast cancer cells represented by primary tissues and breast cancer cell lines. These levels were higher in MBC patients than in EBC patients, suggesting that miR-1280 may reflect the tumor burden, similar to traditional tumor markers.
In addition, we observed that the changes in miR-1280 expression levels reflected the effects of systemic treatment. Previous investigations demonstrated a relationship between circulating microRNAs and treatment response, especially in EBC [16, 29]. From our data, the levels of miR-1280 decreased in patients who responded to systemic therapies; the direction was opposite in patients who did not respond to treatment. It is still too early to reach conclusions about the clinical utility of this marker, since the current investigation included a small number of patients.
Currently, there was no data about the role of miR-1280 especially associated with cancer. Schopman et al. suggested that miR-1280 should be one of tRNA-derived microRNAs based on the fact that its nucleic acid sequence was consistent with that of tRNAleu, which we demonstrated in this study [23].
Some tRNA-derived microRNAs regulate the expression of various genes, as do the microRNAs [26, 27]. According to our data, tRNA-derived microRNAs may be useful markers for breast cancer. However, the biogenesis and function of miR-1280 should be subject to further investigation. Additional studies will be valuable for defining the clinical utility of miR-1280 levels in terms of treatment response.
Conclusion
Blood-based microRNA signatures associated with breast cancer may be identifiable. Expression of miR-1280, a tRNA-derived microRNA, was detected in blood samples from ER-positive breast cancer patients; this expression may serve as a biomarker that reflects treatment response.
Abbreviations
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- EBC:
-
Early breast cancer
- MBC:
-
Metastatic breast cancer
- PCR:
-
Polymerase chain reaction
- qRT-PCR:
-
Quantitative reverse transcription PCR
- DIG:
-
Digoxigenin
- SD:
-
Standard deviation
- RECIST:
-
Response Evaluation Criteria In Solid Tumors
- OS:
-
Overall survival
- RPA1:
-
Replication protein A1
References
Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–69.
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–66.
Iorio M, Ferracin M, Liu C-G, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–70.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8.
Garzon R, Calin G, Croce C. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–79.
Garzon R, Marcucci G, Croce C. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9(10):775–89.
Kluiver J, Poppema S, de Jong D, Blokzijl T, Harms G, Jacobs S, et al. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J Pathol. 2005;207(2):243–9.
Chan J, Krichevsky A, Kosik K. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65(14):6029–33.
Ventura A, Young A, Winslow M, Lintault L, Meissner A, Erkeland S, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132(5):875–86.
Lowery A, Miller N, Devaney A, McNeill RE, Davoren PA, Lemetre C, et al. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res. 2009;11(3):R27.
Blenkiron C, Goldstein L, Thorne N, Spiteri I, Chin S-F, Dunning M, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8(10):R214.
Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104(51):20350–5.
Shimono Y, Zabala M, Cho R, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603.
Shenouda S, Alahari S. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28(3–4):369–78.
Mitchell P, Parkin R, Kroh E, Fritz B, Wyman S, Pogosova-Agadjanyan E, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–8.
Heneghan H, Miller N, Lowery A. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251(3):499–505.
Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102(7):1174–9.
Wong T-S, Liu X-B, Wong B. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res. 2008;14(9):2588–92.
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010;28(10):1721–6.
Sun Y, Wang M, Lin G, Sun S, Li X, Qi J, et al. Serum microRNA-155 as a potential biomarker to track disease in breast cancer. PLoS One. 2012;7(10):e47003.
Zhao H, Shen J, Medico L, Wang D, Ambrosone C. A pilot study of circulating miRNAs as potential biomarkers of early stage breast cancer. PLoS One. 2010;5(10):e13735.
Comparison of microRNA expression with whole blood between ER positive breast cancer patients and normal controls. http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE53179.
Schopman NC, Heynen S, Haasnoot J, Berkhout B. A miRNA-tRNA mix-up: tRNA origin of proposed miRNA. RNA Biol. 2010;7(5):573–6.
Cheloufi S, Dos Santos C, Chong M, Hannon G. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465(7298):584–9.
Yang J, Lai E. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell. 2011;43(6):892–903.
Lee Y, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev. 2009;23(22):2639–49.
Maute RL, Schneider C, Sumazin P, Holmes A, Califano A, Basso K, et al. tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc Natl Acad Sci U S A. 2013;110(4):1404–9.
Chan M, Liaw C, Ji S, Tan H, Wong C, Thike A, et al. Identification of circulating microRNA signatures for breast cancer detection. Clin Cancer Res. 2013;19(16):4477–87.
Jung E-J, Santarpia L, Kim J, Esteva F, Moretti E, Buzdar A, et al. Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer. 2012;118(10):2603–14.
Acknowledgments
This work was supported by NCC Grant 0910220 and NCC Grant 1110260.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, I.H., Kang, J.H., Lee, K.S. et al. Identification and clinical implications of circulating microRNAs for estrogen receptor-positive breast cancer. Tumor Biol. 35, 12173–12180 (2014). https://doi.org/10.1007/s13277-014-2525-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2525-5