Abstract
Dystroglycan (DG), a multifunctional protein dimer of non-covalently linked α and β subunits, is best known as an adhesion and transduction molecule linking the cytoskeleton and intracellular signaling pathways to extracellular matrix proteins. Loss of DG binding, possibly by degradation or disturbed glycosylation, has been reported in a variety of cancers. DG is abundant at astroglial endfeet forming the blood–brain barrier (BBB) and glia limitans; so, we examined if loss of expression is associated with glioma. Expression levels of α-DG and β-DG were assessed by immunohistochemistry in a series of 78 glioma specimens to determine the relationship with tumor grade and possible prognostic significance. α-DG immunostaining was undetectable in 44 of 49 high-grade specimens (89.8 %) compared to 15 of 29 low-grade specimens (51.72 %) (P < 0.05). Moreover, loss of α-DG expression was an independent predictor of shorter disease-free survival (DFS) (hazards ratio (HR) = 0.142, 95 % confidence interval (CI) 0.033 − 0.611, P = 0.0088). Reduced expression of both α-DG and β-DG was also a powerful negative prognostic factor for DFS (HR = 2.556, 95 % CI 1.403–4.654, P = 0.0022) and overall survival (OS) (HR = 2.193, 95 % CI 1.031–4.666, P = 0.0414). Lack of α-DG immunoreactivity is more frequent in high-grade glioma and is an independent predictor of poor clinical outcome. Similarly, lack of both α-DG and β-DG immunoreactivity is a strong independent predictor of clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dystroglycan (DG), originally isolated from skeletal muscle as part of the dystrophin–glycoprotein complex (DGC), is a transmembrane glycoprotein expressed in numerous tissues at the interface between the basement membrane and cell membrane [1–3]. It is composed of two subunits encoded by a single gene [4], the highly glycosylated extracellular α-DG with varying molecular mass (120 − 180 kDa) in different tissues and the 43-kDa transmembrane β-DG that acts as a bridge between α-DG and the cytoskeleton [2, 4, 5]. Within the central nervous system (CNS), DG is abundant at two basal lamina interfaces formed by astrocytes, foot processes abutting cerebral blood vessels, an important component of the blood–brain barrier (BBB), and foot processes that constitute the glia limitans at the pial surface of the brain and spinal cord. Brain DG has also been localized to neuronal elements in several locations, including the retina, hippocampus, and cerebellar cortex, where it may form a structural element of GABAergic synapses [6]. In many tissues, including the CNS, DG is a receptor for several extracellular proteins, including laminin, agrin, perlecan, and neurexin, that allows these extracellular proteins to alter intracellular structure and function [2, 7, 8].
Changes in DG interactions with extracellular matrix (ECM) proteins may contribute to the development, progression, and metastasis of tumors [2, 4, 5]. By linking the ECM to the cytoskeleton, DG maintains tissue integrity [7], and loss of this association is a seminal event in tumorigenesis. In fact, previous studies of human epithelial tumors have suggested that DG is a tumor suppressor [5, 8–13]. Furthermore, Satz et al. [14] suggested that DG also stabilizes the glial limitans basement membrane during cerebral cortex development and Myshrall et al. [15] concluded that DG on radial glia endfeet was required to maintain pial basement membrane integrity. The BBB is disturbed in glioma, which may enhance the invasive capacity of the tumor [16, 17].
In light of the multifarious roles of DG in controlling cell morphology, motility, proliferation, cell–cell adhesion, and cell–ECM interaction [4, 5, 8–13], we speculated that changes in DG may be associated with the development of glioma, a highly invasive and malignant primary tumor in adults with no effective therapy and low survival rate. The aim of this study was to measure expression of DG in glioma of both low and high grades to establish possible relationships among DG function, tumor aggression, and clinical outcome.
Methods
Study participants and tissue samples
This study is a retrospective review of 78 Chinese glioma patients who underwent surgery at the First Affiliated Hospital of Harbin Medical University between May 2002 and July 2010. No patient received chemotherapy or radiotherapy before operation. All cases were classified according to WHO criteria [18]. The period of follow-up ranged from 24 to 129 months. The formalin-fixed, paraffin-embedded specimens were retrieved from the archives of the Department of Pathology in our institute, and two experienced pathologists confirmed the histological diagnosis of each lesion. Tissue specimens were biopsy and cut from the middle of tumor obtained for immunohistochemistry analysis. The Institutional Review Committee approved the study design, and informed consent was obtained from all patients.
Immunohistochemical detection of DG
Immunohistochemical analyses were performed on routinely processed, formalin-fixed, paraffin-embedded glioma specimens using an avidin–biotin complex immunoperoxidase kit (Vectastain ABC, Vector Laboratories, Burlingame, CA). Briefly, successive 2-μm thick tissue sections were cut from tissue blocks selected for the presence of representative lesions and mounted on charged and precleaned slides. Sections were dewaxed, rehydrated, heated in a microwave (10 min at 750 W in 1 mM EDTA buffer, pH 8.0) for antigen retrieval, and incubated in 0.3 % hydrogen peroxide methanol solution for 30 min to block endogenous peroxidase activity. After blocking the tissue with goat serum for 1 h at room temperature, the primary antibody was applied overnight at 4 °C in a high-humidity chamber. Immunolabeling was visualized using the Vectastain diaminobenzidine kit (Vector Laboratories), and sections were counterstained with 1 % modified Harris hematoxylin. The mouse monoclonal anti-α-DG (clone VIA4-1, Upstate Biotechnology, Lake Placid, NY) and anti-β-DG (clone 43DAG1/8D5, Abcam, UK) were used at a dilution of 1:100 in phosphate-buffered serum (PBS) with 1 % horse serum. The mouse monoclonal matrix metalloproteinase 9 antibody anti-MMP-9 (2C3, Santa Cruz, CA) was used at a dilution of 1:50 in PBS with 1 % horse serum. Slides were rated for antigen expression by two investigators with no knowledge of the corresponding clinicopathological data. Skeletal muscle samples (known to express DG and MMP-9) served as a positive control for each antibody. Both positive and negative control slides were included in each batch of slides. For anti-α-DG and anti-β-DG immunoreactivity, only clear staining of the cell membrane was regarded as positive. For anti-MMP-9 studies, the degree of immunoreactivity in individual tissue sections was considered high only in cases of unequivocal staining of the cytoplasm in more than 25 % of cells in each high-power field (×400). At least five fields within regions of interest were examined, and the percentage of cells expressing α-DG and β-DG was calculated semiautomatically by a computer-assisted cellular image analyzer (Image-Pro Plus; Media Cybernetics, Silver Spring, MD). The results obtained were standardized to controls and stratified into three categories in term of staining intensity: 1 = weak, 2 = moderate, and 3 = strong. Samples were also stratified in term of percentage of positive cells as follows: 0 = 0 %, 1 = 1–10 %, 2 = 11–50 %, and 3 = >50 % positive cells. A staining score was defined taking into account both intensity of staining and percentage of positive cells and was calculated as % positive cells staining intensity, and was stratified as follows: negative = 0, low = 1, moderate = 2–3, and strong >3. High is >3, and low is <3.
Statistical analysis
The association between DG (or MMP-9) and clinicopathological parameters was tested using Pearson’s chi-squared test. Disease-free survival (DFS) was defined as the interval between surgery and the first documented evidence of recurrence at either the original or distant sites. Overall survival was defined as the interval between surgery and death from the disease. Disease-free and overall survival curves were constructed using the Kaplan–Meier method, and the log-rank test was used for comparison. Univariate risk ratio was calculated, and multivariate relative risks were calculated using Cox proportional hazards regression. All calculations were performed using the SAS statistical software package (SAS Institute Inc.), and P < 0.05 was considered statistically significant.
Results
Localization of α-DG and β-DG in human glioma specimens
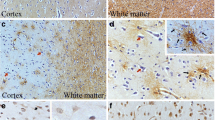
The expression levels of α-DG, β-DG, and MMP-9 were evaluated by immunostaining in a series of 78 primary human glioma samples obtained from patients diagnosed with astrocytoma grade I/II (n = 29) or grade III/IV (n = 49). For α-DG and β-DG (Tables 1 and 2), only clear membrane staining was regarded as high, and we found that high staining was mainly located along the vascular basal lamina and perivascular region of the tumor core (Fig. 1, ②A, ②B, ③B, ④B; Fig. 2, ①A, ③A, ③B, ④B). For MMP-9, high staining was mainly found in the cytoplasm of high-grade specimens (data not shown).
Examples of low-grade human glioma stained with anti-α-DG (VIA-4) (①A ~ ④A) and anti-β-DG (8D5) (①B ~ ④B). ① ~ ④ represent four different cases. Low expression of α-DG (①A, ③A, ④A). Low expression of β-DG (①B). High expression of α-DG (②A). High expression of β-DG (②B, ③B, ④B). Original magnifications × 400
Examples of high-grade human glioma stained with anti-α-DG (VIA-4) (①A ~ ④A) and anti-β-DG (8D5) (①B ~ ④B). ① ~ ④ represent four different cases. Low expression of α-DG (②A, ④A). Low expression of β-DG (①B, ②B). High expression of α-DG (①A, ③A). High expression of β-DG (③B, ④B). Original magnifications × 400×
Differential expression of α-DG in high- and low-grade human glioma and correlation with clinical outcome
In all the 78 cases of glioma, α-DG immunostaining was detected. A significantly greater proportion was in the high-grade (III/IV) group (low-grade 15 of 29 or 51.72 %, high-grade 44 of 49 or 89.8 %; P < 0.05). MMP-9 immunostaining was detected in 37 of 78 specimens, with a significantly greater fraction of MMP-9 samples rated as high expression showing no detectable expression of α-DG (low expression of MMP-9 and low expression of α-DG 24 of 41 specimens or 58.54 %; high expression of MMP-9 and low expression of α-DG 35 of 37 or 94.59 %; P < 0.05). Thus, α-DG expression was inversely correlated with both tumor grade and MMP-9 expression. In contrast, there was no significant association between α-DG expression and either gender, age, or tumor size (Table 1).
When α-DG staining was analyzed in relation to clinical outcome, disease recurrence and disease-related death were more frequent in patients with α-DG-low tumors. When tumors were grouped according to α-DG expression status, 45 of 59 cases with low α-DG expression (76.3 %) but only 2 of the 19 cases with high α-DG expression (10.5 %) recurred during follow-up (P < 0.0001) (Table 1). Enhanced DFS was also confirmed by Kaplan–Meier curves, which displayed a significant separation between the α-DG (high) and α-DG (low) patient groups (P < 0.05 by log-rank test) (Fig. 3a). Similarly, 28 of 59 patients with low α-DG expression (47.5 %) but only 1 of the 19 patients with high expression of α-DG (5.3 %) died of the disease during follow-up (P < 0.001) (Table 1). Thus, patients whose tumors expressed less α-DG were more likely to die due to the disease compared to those with α-DG expressing tumors, a result confirmed by Kaplan–Meier curves (P < 0.05 by log-rank test) (Fig. 3c).
In a univariate analysis, we found that age, tumor size, tumor grade, MMP-9 expression, and α-DG expression were associated with shorter DFS (Table 3) and overall survival (Table 4). To further explore the prognostic significance of α-DG, we built a Cox regression model including the parameters gender, age, tumor size, tumor grade, MMP-9 expression, and α-DG expression. High-grade glioma (grade III/IV) (hazards ratio (HR) = 3.972, 95 % CI 1.556 − 10.142, P = 0.0039), MMP-9 expression (HR = 2.081, 95 % CI 1.058 − 4.092, P = 0.0336), and low expression of α-DG (HR = 0.142, 95 % CI 0.033 − 0.611, P = 0.0088) were independent predictors of shorter DFS according to multivariate analysis (Table 5). A similar Cox regression model indicated that tumor grade (HR = 7.522, 95 % CI 2.268 − 24.951, P = 0.0010) was an independent predictor of shorter overall survival according to multivariate analysis (Table 6).
β-DG immunostaining was not strongly correlated to clinicopathological parameters except MMP-9 immunostaining
Expression of β-DG was also assessed by immunostaining in the same series of 78 glioma samples using the specific monoclonal antibody 8D5 (Fig. 1, ①B, ②B, ③B, ④B; Fig. 2, ①B, ②B, ③B, ④B). No immunostaining was observed in 39 of 78 cases, and β-DG expression did not correlate with gender, age, tumor size, tumor grade, or survival rate (Table 5). A significantly greater fraction of specimens with low expression of β-DG were also rated as high expression of MMP-9 (low expression of MMP-9 and low expression of β-DG 11 of 41 or 26.83 %; high expression of MMP-9 and low expression of β-DG 28 of 37 or 75.68 %; P < 0.05). Thus, like α-DG, there was a negative association between MMP-9 and β-DG expression. Moreover, a greater fraction of cases with low expression of β-DG recurred (28/39 or 71.8 % vs 19/39 or 48.7 %; P = 0.0373) (Table 2). However, a Cox regression model and Kaplan–Meier curves did not confirm a significant relationship between low β-DG expression and recurrence (data not shown).
Prognostic significance of combined α-DG and β-DG expression
We constructed a second Cox regression model including both α-DG and β-DG expressions in addition to gender, age, tumor size, tumor grade, and MMP-9 expression. Lack of α-DG and β-DG expression was confirmed to be an independent predictor of shorter DFS and overall survival (OS) (Tables 7 and 8). Moreover, when considered together as one parameter, low expression of both α-DG and β-DG was a powerful negative prognostic factor for both DFS (HR = 2.556, 95 % CI 1.403–4.654, P = 0.0022) and OS (HR = 2.193, 95 % CI 1.031–4.666, P = 0.0414) (Tables 7 and 8). Similarly, Kaplan–Meier curves of patients whose tumors were rated as low expression for α-DG and β-DG versus all remaining patients showed significant separation for both DFS and OS (P < 0.05 by log-rank test) (Fig. 3b, d).
Discussion
Loss of stable cell attachment to the basement membrane and disrupted ECM intracellular signaling due to DG degradation or dysfunction may be critical for tumor growth and metastasis [2, 4]. In this study, the potential prognostic significance of dystroglycan α-DG and β-DG expression levels was investigated in a series of human glioma specimens. We observed a clear reduction in α-DG expression in high-grade tumors (III/IV), which correlated with poor clinical outcome (Tables 1 and 4 and Fig. 3a, c). These findings support the tumor-suppressor function of DG in astrocytes, in accord with previous observations in several human epithelial tumors [5, 8–13].
In contrast to α-DG expression, transmembrane β-DG subunit expression was not reduced in high-grade glioma and did not correlate with prognosis in Cox proportional hazards regression (Tables 4 and 6). However, since β-DG was correlated with recurrence by Pearson’s chi-squared test (Table 2), we built a second Cox regression model including both α-DG and β-DG expression along with gender, age, tumor size, tumor grade, and MMP-9 expression and did find that loss of α-DG was an independent predictor of shorter DFS (Table 4). Moreover, dual loss of α-DG and β-DG was a powerful prognostic indicator (Tables 7 and 8).
This study is among the first to analyze the association between the expression of DG and DFS of patients with glioma. As such, it provides crucial information that may improve patient assessment prior to treatment. Tumor grade also has prognostic significance for survival, with our group and others [19–21] determining that an advanced WHO grade is a significant predictor of shorter OS. Taken together, these data and our findings that expression of α-DG is significantly associated with tumor grade support the hypothesis that decreased expression of DG contributes to tumor progression and invasion, and consequently, survival. Moreover, the data suggest that determining DG expression and tumor grade concurrently may increase the accuracy of prognosis and lead to better-informed treatment decisions. Profiling of MMP-9 expression may further increase prognostic accuracy, given our finding that increased expression of MMP-9 was a third independent prognostic indicator for shorter DFS. This result confirms those of other studies by Sun et al. and Zhao et al. [22, 23], who found that expression of MMP-9 in tumor samples was an independent predictor of survival.
Staining of both antigens was mainly located along the vascular basal lamina and perivascular region in the tumor core (Fig. 1, ②A, ②B, ③B, ④B; Fig. 2, ①A, ③A, ③B, ④B), regardless of tumor grade, in agreement with previous observations [24, 25]. In healthy tissue, α-DG functions as receptor for extracellular proteins, including laminin, perlecan, and agrin [26], while β-DG is the transmembrane subunit connecting α-DG to the intracellular cytoskeleton and downstream signaling pathways [27–32]. In the CNS, DG is abundant at the glial limitans, an important component of the BBB [6]. DG has been implicated in early mouse brain development, in myelination and regulation of the nodal architecture of peripheral nerves, and synaptogenesis [2, 14, 15, 33]. Thus, it is highly likely that interfering with these roles, particularly control of proliferation, basement membrane adhesion, and signaling through kinases such as MAPK, could lead to glioma progression and invasion.
In this study, the α subunit of the DG complex was almost always absent in high-grade glioma, while the β subunit did not display significant variations between different grades. Since DG subunits are encoded by a single gene and produced by posttranslational cleavage of a single precursor molecule [2], detection of the β-DG subunit in most of the high-grade gliomas lacking α-DG immunoreactivity (Tables 1 and 2) indicates that gene expression is not lost during tumor progression; rather, specific posttranslational mechanism affecting α-DG processing are altered in glioma as reported in epithelial tumors [12, 26]. α-DG is highly glycosylated, and the anti-α-DG antibody used in our study (clone VIA4-1, Upstate) only recognizes the glycosylated domain of α-DG. Thus, reduced immunoreactivity represented a reduction in α-DG glycosylation. Loss of α-DG glycosylation has been suggested to promote tumor invasion and metastasis by allowing detachment of carcinoma cells from the BM. Indeed, Shimojo et al. [12] demonstrated that reduction of α-DG glycosylation rather than of core protein expression in prostate carcinoma cells is associated with highly infiltrative histological patterns as assessed by the Gleason grading system. In a future study, we will investigate the extent α-DG glycosylation and α-DG core protein expression in high- and low-grade glioma.
Many studies suggested that matrix metalloproteinases such as MMP-9 and MMP-2 may degrade DG by disrupting the interface between the C-terminal domain of α-DG and the N-terminal extracellular domain of β-DG [34, 35], a phenomenon observed under both physiological conditions [36] and pathological conditions [37]. In epithelial tumors, β-DG was degraded into a 31-kDa fragment without the N-terminal [7, 8, 38]. However, in contrast to epithelial tumors, Calogero et al. [19] found that almost primary glioma cells, subcultured glioma cells, and cells within human glioma tissues expressed integral β-DG (43 kDa). In the study, the anti-β-DG antibody (clone 43DAG1/8D5, Abcam) used recognizes 16 amino acids at the extreme C terminus of human β-DG. Thus, reduction of β-DG was not due entirely to proteolysis by MMP-9. However, further study for the relationship between β-DG and MMP-9 is demanded.
Conclusions
The results demonstrate that (i) reduction of high α-DG expression is more frequent in high-grade glioma and (ii) low α-DG expression and low expression of both α-DG and β-DG are independent predictors of poor clinical outcome. Larger scale clinical studies and basic investigations into the molecular mechanisms for DG reduction in glioma are warranted.
References
Winder SJ. The complexities of dystroglycan. Trends Biochem Sci. 2001;26:118–24.
Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207.
Waite A, Brown SC, Blake DJ. The dystrophin-glycoprotein complex in brain development and disease. Trends Neurosci. 2012;35:487–96.
Sgambato A, Brancaccio A. The dystroglycan complex: from biology to cancer. J Cell Physiol. 2005;205:163–9.
Brennan PA, Jing J, Ethunandan M, Gorecki D. Dystroglycan complex in cancer. Eur J Surg Oncol. 2004;30:589–92.
Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–5.
Losasso C, Di Tommaso F, Sgambato A, Ardito R, Cittadini A, Giardina B, et al. Anomalous dystroglycan in carcinoma cell lines. FEBS Lett. 2000;484:194–8.
Sgambato A, Migaldi M, Montanari M, Camerini A, Brancaccio A, Rossi G, et al. Dystroglycan expression is frequently reduced in human breast and colon cancers and is associated with tumor progression. Am J Pathol. 2003;162:849–60.
Sgambato A, Camerini A, Amoroso D, Genovese G, De Luca F, Cecchi M, et al. Expression of dystroglycan correlates with tumor grade and predicts survival in renal cell carcinoma. Cancer Biol Ther. 2007;6:1840–6.
Moon YW, Rha SY, Zhang X, Jeung HC, Yang WI, Kwon O, et al. Increments of alpha-dystroglycan expression in liver metastasis correlate with poor survival in gastric cancer. J Surg Oncol. 2009;100:459–65.
Sgambato A, Camerini A, Genovese G, De Luca F, Viacava P, Migaldi M, et al. Loss of nuclear p27(kip1) and alpha-dystroglycan is a frequent event and is a strong predictor of poor outcome in renal cell carcinoma. Cancer Sci. 2010;101:2080–6.
Shimojo H, Kobayashi M, Kamigaito T, Shimojo Y, Fukuda M, Nakayama J. Reduced glycosylation of alpha-dystroglycans on carcinoma cells contributes to formation of highly infiltrative histological patterns in prostate cancer. Prostate. 2011;71:1151–7.
Bao X, Fukuda M. A tumor suppressor function of laminin-binding alpha-dystroglycan. Methods Enzymol. 2010;479:387–96.
Satz JS, Ostendorf AP, Hou S, Turner A, Kusano H, Lee JC, et al. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J Neurosci. 2010;30:14560–72.
Myshrall TD, Moore SA, Ostendorf AP, Satz JS, Kowalczyk T, Nguyen H, et al. Dystroglycan on radial glia end feet is required for pial basement membrane integrity and columnar organization of the developing cerebral cortex. J Neuropathol Exp Neurol. 2012;71:1047–63.
Wolburg H, Noell S, Fallier-Becker P, Mack AF, Wolburg-Buchholz K. The disturbed blood-brain barrier in human glioblastoma. Mol Aspects Med. 2012;33:579–89.
Gritsenko PG, Ilina O, Friedl P. Interstitial guidance of cancer invasion. J Pathol. 2012;226:185–99.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 who classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109.
Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–73.
Yang P, Wang Y, Peng X, You G, Zhang W, Yan W, et al. Management and survival rates in patients with glioma in China (2004-2010): a retrospective study from a single-institution. J Neurooncol. 2013;113:259–66.
Siangprasertkij C, Navalitloha Y. A multivariate analysis of patients with glioma: a treatment outcome and prognostic factor for survival. J Med Assoc Thai. 2008;91:491–6.
Sun ZF, Wang L, Gu F, Fu L, Li WL, Ma YJ. Expression of Notch1, MMP-2 and MMP-9 and their significance in glioma patients. Zhonghua Zhong Liu Za Zhi. 2012;34:26–30.
Zhao J, Li G, Zhao Z, Wang J, Gao G, He S. Matrix metalloproteinase-9 expression is increased in astrocytic glioma and associated with prognosis of patients. Jpn J Clin Oncol. 2012;42:1060–5.
Calogero A, Pavoni E, Gramaglia T, D'Amati G, Ragona G, Brancaccio A, et al. Altered expression of alpha-dystroglycan subunit in human gliomas. Cancer Biol Ther. 2006;5:441–8.
Noell S, Wolburg-Buchholz K, Mack AF, Ritz R, Tatagiba M, Beschorner R, et al. Dynamics of expression patterns of aqp4, dystroglycan, agrin and matrix metalloproteinases in human glioblastoma. Cell Tissue Res. 2012;347:429–41.
de Bernabe DB, Inamori K, Yoshida-Moriguchi T, Weydert CJ, Harper HA, Willer T, et al. Loss of alpha-dystroglycan laminin binding in epithelium-derived cancers is caused by silencing of large. J Biol Chem. 2009;284:11279–84.
Constantin B. Dystrophin complex functions as a scaffold for signalling proteins. Biochim Biophys Acta. 1838;2014:635–42.
Chand D, Song L, deLannoy L, Barsyte-Lovejoy D, Ackloo S, Boutros PC, et al. C-terminal region of teneurin-1 co-localizes with dystroglycan and modulates cytoskeletal organization through an extracellular signal-regulated kinase-dependent stathmin- and filamin a-mediated mechanism in hippocampal cells. Neuroscience. 2012;219:255–70.
Zhou YW, Munoz J, Jiang D, Jarrett HW. Laminin-alpha1 LG4-5 domain binding to dystroglycan mediates muscle cell survival, growth, and the AP-1 and NF-kappaB transcription factors but also has adverse effects. Am J Physiol Cell Physiol. 2012;302:C902–14.
Budinger GR, Urich D, DeBiase PJ, Chiarella SE, Burgess ZO, Baker CM, et al. Stretch-induced activation of AMP kinase in the lung requires dystroglycan. Am J Respir Cell Mol Biol. 2008;39:666–72.
Zhou Y, Jiang D, Thomason DB, Jarrett HW. Laminin-induced activation of Rac1 and JNKp46 is initiated by Src family kinases and mimics the effects of skeletal muscle contraction. Biochemistry. 2007;46:14907–16.
Spence HJ, Dhillon AS, James M, Winder SJ. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 2004;5:484–9.
Masaki T, Matsumura K, Saito F, Yamada H, Higuchi S, Kamakura K, et al. Association of dystroglycan and laminin-2 coexpression with myelinogenesis in peripheral nerves. Med Electron Microsc. 2003;36:221–39.
Sbardella D, Inzitari R, Iavarone F, Gioia M, Marini S, Sciandra F, et al. Enzymatic processing by MMP-2 and MMP-9 of wild-type and mutated mouse beta-dystroglycan. IUBMB Life. 2012;64:988–94.
Zhong D, Saito F, Saito Y, Nakamura A, Shimizu T, Matsumura K. Characterization of the protease activity that cleaves the extracellular domain of beta-dystroglycan. Biochem Biophys Res Commun. 2006;345:867–71.
Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J, Jaworski J, et al. Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J Biol Chem. 2007;282:16036–41.
Court FA, Zambroni D, Pavoni E, Colombelli C, Baragli C, Figlia G, et al. MMP2-9 cleavage of dystroglycan alters the size and molecular composition of Schwann cell domains. J Neurosci. 2011;31:12208–17.
Shang ZJ, Ethunandan M, Gorecki DC, Brennan PA. Aberrant expression of beta-dystroglycan may be due to processing by matrix metalloproteinases-2 and -9 in oral squamous cell carcinoma. Oral Oncol. 2008;44:1139–46.
Acknowledgments
This work was supported by the Heilongjiang Postdoctoral Financial Assistance (LBH-Z12177), Education Department of Heilongjiang Province in China (12521290), and Research Foundation of Health and Family Planning Commission of Heilongjiang Province in China (2012-563)
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Dong, XH., Ma, Y. et al. Reduction of α-dystroglycan expression is correlated with poor prognosis in glioma. Tumor Biol. 35, 11621–11629 (2014). https://doi.org/10.1007/s13277-014-2418-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2418-7