Abstract
Increasing reports suggest that discovery of microRNAs (miRNAs) might provide a novel therapeutical target for human cancers, including osteosarcoma. Previous studies have shown that miR-32 was dysregulated in breast and endometrial cancer. However, its biological roles in osteosarcoma remain unclear. In the current study, we found that miR-32 was significantly down-regulated in osteosarcoma tissues, compared with the adjacent normal tissues. In vitro studies further demonstrated that miR-32 mimics were able to suppress, while its antisense oligos promoted cell proliferation in Saos-2 and U2OS cells. At the molecular level, our data further revealed that expression of Sox9 was negatively regulated by miR-32. Therefore, our results identify an important role for miR-32 in the osteosarcoma through regulating Sox9 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma (OS) has become the most common malignant bone tumor in children and young adults [1, 2]. Despite great efforts that have been made to discover its underlying mechanisms in recent years [1, 2], developing new treatment strategies is still urgent.
MicroRNAs (miRNAs), a highly conserved small noncoding RNA molecules of about 22 nucleotides, inhibit gene expression by binding to the 3′ untranslated region (3′-UTR) of mRNA sequence, leading to translational repression or degradation [3, 4]. Decades of studies have shown that abnormal expression of miRNAs in human cancers is tightly associated with cell proliferation, apoptosis, metastasis, and invasion, including osteosarcoma [5, 6]. Moreover, some miRNAs were shown to be associated with advanced tumor progression and may be an independent prognostic marker for osteosarcoma patients [7].
Previous studies have shown that miR-32 was up-regulated in prostate tumors [8]. Besides, miR-32 promoted growth, migration, and invasion in colorectal carcinoma cells, through suppression of phosphatase and tensin homologue (PTEN) [9]. Consistently, an inverse relationship between miR-32 and PTEN protein expression was identified in colorectal cancer tissues [10]. However, miR-32 was shown to inhibit the proliferation and invasion of gastric cancers [11], suggesting that miR-32 may act as a tumor suppressor or an oncogene in different cancers.
In the present study, we found that miR-32 was significantly down-regulated in osteosarcoma tissues, compared with the adjacent normal tissues. We showed that miR-32 negatively regulated Sox9 expression to suppress cell proliferation in Saos-2 and U2OS cells.
Materials and methods
Cell culture and transfection
Osteosarcoma cell lines (Saos-2 and U2OS cells) were obtained from American Type Culture Collection (Rockville, MD). Cells were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10 % fetal bovine serum (Gibco, USA). Cultures were maintained at 37 °C in a humidified atmosphere with 5 % CO2. miR-32 mimics, antisense oligos, and negative controls (NC) were obtained from Genepharm Company (Shanghai, China). For the transfection experiments, a complex of Lipofectamine 2000 (Invitrogen, CA, USA) and 20-nM microRNAs mentioned above was prepared following the manufacturer’s instructions.
Human tissue samples
Tumor tissues and adjacent nontumor normal tissues were collected from routine therapeutic surgery at our department. All samples were obtained with informed consent and approved by the hospital institutional review board. For the bisulfite DNA sequencing assays, modified DNA (50 ng) was amplified by PCR with Hotstart Taq (Qiagen) and primers specific to respective promoter regions of miR-32. PCR products were gel-extracted and subcloned into pMD19-T vectors (TaKaRa, Dalian, China) for DNA sequencing (BGI, Shenzhen, China).
Analysis of miRNA expression using TaqMan RT-PCR
Total RNA from tissue samples and cell lines was harvested using the miRNA Isolation Kit (Ambion, USA). Expression of mature miRNAs was assayed using Taqman MicroRNA Assay (Applied Biosystems) specific for hsa-miR-32. Briefly, 10 ng of total RNA was reverse transcribed to cDNA with specific stem-loop real-time (RT) primers. Quantitative real-time PCR was performed by using an Applied Biosystems 7900 Real-time PCR System and a TaqMan Universal PCR Master Mix. All the primers were obtained from the TaqMan miRNA Assays. Small nuclear U6 snRNA (Applied Biosystems) was used as an internal control.
Western blot
Cells or tissues were harvested and lysed with ice-cold lysis buffer (50 mM Tris-HCl, pH 6.8, 32 mM 2-ME, 2 % w/v SDS, 10 % glycerol). After centrifugation at 20,000 × g for 10 min at 4 °C, proteins in the supernatants were quantified and separated by 10 % SDS PAGE, transferred to NC membrane (Amersham Bioscience, Buckinghamshire, U.K.). After blocking with 10 % nonfat milk in PBS, membranes were immunoblotted with antibodies as indicated, followed by HRP-linked secondary antibodies (Santa Cruz, USA). The signals were detected by SuperSignal West Pico Chemiluminescent Substrate kit (Pierce, Rockford, IL) according to manufacturer’s instructions. Anti-Sox9, P53, PTEN, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Abcam company (USA). Protein levels of GAPDH were employed as loading controls.
BrdU assays
A cell proliferation enzyme-linked immunosorbent assay (bromodeoxyuridine (BrdU) kit; Beyotime) was used to analyze the incorporation of BrdU during DNA synthesis following the manufacturer’s protocols. All experiments were performed in triplicate. Absorbance was measured at 450 nm in the Spectra Max 190 ELISA reader (Molecular Devices, Sunnyvale, CA).
Cell invasion assays
Cell invasion abilities were analyzed using extracellular matrix-coated invasion chambers (Millipore, CA, USA) and quantitated with a colorimetric microplate reader at 570 nm, according to the manufacturer’s instructions.
Luciferase reporter assay
Total cDNA from Saos-2 cells was used to amplify the 3′UTR of Sox9 by PCR. The Sox9 3′UTR was cloned into pMir-Report (Ambion), yielding pMir-Report-Sox9. Mutations were introduced in potential miR-32 binding sites using the QuikChange site-directed mutagenesis Kit (Stratagene). The pRL-SV40 vector (Promega, USA) carrying the Renilla luciferase gene was used as an internal control to normalize the transfection efficiency. Luciferase values were determined using the Dual-Luciferase Reporter Assay System (Promega).
Statistical analysis
Data are expressed as the mean ± SEM from at least four separate experiments. Differences between groups were analyzed using Student’s t test or one-way ANOVA. A value of p < 0.05 was considered statistically significant.
Results
miR-32 was down-regulated in osteosarcoma tissues
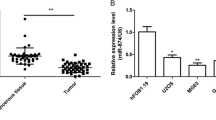
In order to determine the expression levels of miR-32 in osteosarcoma, TaqMan real-time PCR analysis was performed in 38 pairs of osteosarcoma tissues and pair-matched adjacent noncancerous tissues. As shown in Fig. 1a, our results demonstrated that miR-32 was significantly down-regulated in osteosarcoma tissues (Fig. 1a).
Expression levels of miR-32 in osteosarcoma tissues. a miR-32 expression was examined by TaqMan real-time PCR in human osteosarcoma tissues and adjacent noncancerous tissues (normal). n = 36. b DNA methylation levels of the CpG island near the transcription start site (TSS) of miR-32 in osteosarcoma and adjacent normal tissues. ***P < 0.001 between two groups
Previous studies have shown that higher DNA methylation of the CpG island could be associated with low expression of some miRNAs in human cancers [12, 13]. To test this hypothesis, we evaluated the DNA methylation status of the CpG island near the transcription start site (TSS) by bisulphite-treatment DNA sequencing. As expected, the DNA methylation levels of the CpG island were significantly elevated in osteosarcoma tissues (Fig. 1b), suggesting that reduced miR-32 expression in osteosarcoma may be associated with the DNA hypermethylation.
miR-32 overexpression inhibits cell proliferation and invasion
Next, to assess the function of miR-32 in tumorigenesis, miR-32 mimics and negative controls (NC) were transfected into Saos-2 and U2OS cells, respectively (Fig. 2a and b). As a result, miR-32 overexpression reduced cell number (Fig. 2c and d), inhibited cell proliferation and invasion in both cells (Fig. 2e–h).
miR-32 overexpression inhibits osteosarcoma cell proliferation and invasion. a, b Expression of miR-32 was determined in Saos-2 (a) and U2OS (b) cells after transfection of miR-32 mimics or negative controls (NC). c, d The growth curve of Saos-2 (c) and U2OS (d) cells after transfection of miR-32 mimics or negative controls (NC). e, f The cell proliferative potential (BrdU) was determined in Saos-2 (e) and U2OS (f). A450 absorption was assayed after transfection for 30 h. g, h Cell invasion assays were conducted in Saos-2 (g) and U2OS (h) cells after transfection for 30 h. *P < 0.05 **P < 0.01, ***P < 0.001 between two groups
miR-32 directly targets the Sox9 in osteosarcoma cells
Previous reports have shown that the tumor suppressor PTEN was a potential target of miR-32 in colorectal cancer cells [9, 10]. Therefore, we measured the protein levels of PTEN in Saos-2 and U2OS cells. Unexpectedly, PTEN protein contents were not affected in both cell transfection of miR-32 mimics (Supplementary Fig. 1A-B), suggesting distinct roles of miR-32 in osteosarcoma. Therefore, to screen the function target of miR-32 in osteosarcoma cells, bioinformatics software (miRWalk) was used. We found that the gene encoding sex-determining region Y-box 9 (Sox9) harbored a potential miR-32 binding site (Fig. 3a). Luciferase reporter assays using 3′-untranslated region (3′-UTR) of Sox9 gene further demonstrated that miR-32 mimics significantly reduced the activity of Sox9 3′-UTR, but not the binding motif mutant one (Fig. 3b). In agreement, miR-32 mimics significantly reduced the protein abundance of Sox9 in Saos-2 and U2OS cells (Fig. 3c and d). Moreover, protein levels of P53, which are negatively modulated by Sox9 [14, 15], were increased by miR-32 mimics (Fig. 3e, f), further indicating that Sox9 is a target of miR-32 in osteosarcoma cells.
miR-32 inhibits Sox9 protein expression in osteosarcoma cells. a Prediction of miR-32 binding sites in the 3′-UTR of human Sox9 gene. Potential binding site was highlighted in bold and the underlines represented the mutant bases. b Luciferase reporter assays in Saos-2 cells. Cells were transfected with 200 ng of wild-type 3′-UTR-reporter or mutant (Mut) constructs together with miR-32 mimics or negative controls (NC). c, d Protein levels of Sox9 were determined by western blot in Saos-2 (c) and U2OS (d) cells transfected with miR-32 mimics or negative controls (NC). e, f Protein levels of P53 were determined by Western blot in Saos-2 (e) and U2OS (f) cells transfected with miR-32 mimics or negative controls (NC). **P < 0.01, between two groups
Sox9 overexpression blocks the roles of miR-32
To verify the functional connection between miR-32 and Sox9, Saos-2 were transfected with lentiviruses containing Sox9 gene or empty vector (EV) after transfection of miR-32 mimics (Fig. 4a). As shown in Fig. 4b–d, Sox9 re-introduction reversed the anti-proliferation and anti-invasion roles of miR-32, underlining the specific importance of the Sox9 for miR-32 action. Similar results were also observed in U2OS cells (Supplementary Fig. 2A-D). Therefore, our results suggest that the roles of miR-32 in the development of osteosarcoma, at least in part, depend on its down-regulation of Sox9.
Sox9 re-introduction blocks the anti-proliferative roles of miR-32. a Sox9 protein expression was determined by Western blot in Saos-2 cells. Cells were pre-transfected with miR-32 mimics or negative control (NC) for 24 h and then transfected with lentiviruses containing Sox9 gene or empty vector (EV) for another 24 h. b–d The growth curve (b), cell proliferation (BrdU, c), and invasion abilities (d) were determined in Saos-2 cells. *P < 0.05 **P < 0.01, ***P < 0.001 between two groups
Inhibition of miR-32 promotes the proliferation and invasion of osteosarcoma cells
As described above, miR-32 plays a negative role in the proliferation and invasion of osteosarcoma cells. However, it remained unknown whether inhibition of miR-32 would promote tumor growth. Therefore, Saos-2 and U2OS cells were transfected with miR-32 antisense oligos to inhibit the roles of endogenous miR-32. As a result, we found that forced expression of the miR-32 antisense enhanced the number, proliferation, and invasion abilities of Saos-2 and MG63 cells, compared to negative control-transfected cells (Fig. 5a–f). Moreover, Sox9 protein contents were up-regulated, while P53 expression was inhibited by miR-32 antisense (Fig. 5g and h).
miR-32 antisense promotes the proliferation of osteosarcoma cells. a, b The growth curve of Saos-2 (a) and U2OS (b) cells after miR-32 antisense transfection compared to negative control (NC). c, d The cell proliferative potential (BrdU) was determined in Saos-2 (c) and U2OS (d) cells transfected with miR-32 antisense or negative control (NC). A450 absorption was assayed after transfection for 24 h. e, f Cell invasion assays were conducted in Saos-2 (e) and U2OS (f) cells after transfection for 30 h. g, h Protein levels of Sox9 and P53 were determined by Western blot in Saos-2 (g) and U2OS (h) cells miR-32 antisense or negative control (NC). *P < 0.05 **P < 0.01, ***P < 0.001 between two groups
Discussion
In this study, we demonstrated that miR-32 expression was down-regulated in osteosarcoma tissues. We proposed that the higher DNA methylation of the CpG island may contribute to the down-regulation of miR-32 in osteosarcoma. Furthermore, our in vitro studies revealed that overexpression of miR-32 inhibited, while its inhibition enhanced cell proliferation and invasion in Saos-2 and U2OS cells. Therefore, our study, for the first time, indicates that miR-32 might be a tumor suppressor in the progression of osteosarcoma. Together with previous studies [8–11], our results suggest that the functions of miR-32 in different tumors are diverse, which may rely on cellular context and its potential regulatory targets.
At the molecular level, our results revealed that Sox9 could be one of the direct targets of miR-32 in osteosarcoma cells. Sox9, a member of the Sox family of transcription factors, plays critical roles in the process of maintaining stem cell properties, lineage restriction, and terminal differentiation [16, 17]. Indeed, ablation of Sox9 in mice resulted in embryonic lethal due to severe heart defects [18]. Recent studies also demonstrated that Sox9 was usually overexpressed in cancers of the brain, lung, and prostate [19–21]. Mechanically, Sox9 promoted carcinogenesis, at least in part, through regulation of the Wnt pathway, P53 pathway, and epithelial-to-mesenchymal transition (EMT) [22, 23]. Besides, Sox9 was up-regulated in aggressive osteosarcoma tissues and associated with tumor progression and patients’ prognosis [24]. However, the molecular determinants of Sox9 expression in human cancers remain largely unexplored, although some studies have shown that certain miRNAs, such as miR-145 and miR-495, could down-regulate Sox9 in human glioma cells and mesenchymal stem cells, respectively [25, 26]. Therefore, our results regarding miR-32/Sox9 regulatory axis may contribute to the optimization of clinical treatments for osteosarcoma patients.
Taken together, we analyzed the expression and functions of miR-32 in osteosarcoma. Through gain- and loss-of-function studies, we found that miR-32 inhibited osteosarcoma cell proliferation and invasion. However, in vivo studies are still needed to investigate its precise roles in the tumor growth.
References
Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment—where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–32.
Botter SM, Neri D, Fuchs B. Recent advances in osteosarcoma. Curr Opin Pharmacol. 2014;16C:15–23.
Sun K, Lai EC. Adult-specific functions of animal microRNAs. Nat Rev Genet. 2013;14:535–48.
Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013;14:475–88.
Miao J, Wu S, Peng Z, et al. MicroRNAs in osteosarcoma: diagnostic and therapeutic aspects. Tumour Biol. 2013;34(4):2093–8.
Nugent M. MicroRNA function and dysregulation in bone tumors: the evidence to date. Cancer Manag Res. 2014;6:15–25.
Wang Z, Cai H, Lin L, et al. Upregulated expression of microRNA-214 is linked to tumor progression and adverse prognosis in pediatric osteosarcoma. Pediatr Blood Cancer. 2014;61(2):206–10.
Ambs S, Prueitt RL, Yi M, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68(15):6162–70.
Wu W, Yang J, Feng X, et al. MicroRNA-32 (miR-32) regulates phosphatase and tensin homologue (PTEN) expression and promotes growth, migration, and invasion in colorectal carcinoma cells. Mol Cancer. 2013;12:30.
Wu W, Yang P, Feng X, et al. The relationship between and clinical significance of MicroRNA-32 and phosphatase and tensin homologue expression in colorectal cancer. Gene Chromosome Cancer. 2013;52(12):1133–40.
Zhang J, Kuai X, Song M, et al. microRNA-32 inhibits the proliferation and invasion of the SGC-7901 gastric cancer cell line in vitro. Oncol Lett. 2014;7(1):270–4.
Weber B, Stresemann C, Brueckner B, Lyko F. Methylation of human microRNA genes in normal and neoplastic cells. Cell Cycle. 2007;6(9):1001–5.
Suzuki H, Maruyama R, Yamamoto E, Kai M. DNA methylation and microRNA dysregulation in cancer. Mol Oncol. 2012;6(6):567–78.
Panda DK, Miao D, Lefebvre V, et al. The transcription factor SOX9 regulates cell cycle and differentiation genes in chondrocytic CFK2 cells. J Biol Chem. 2001;276(44):41229–36.
Saegusa M, Hashimura M, Suzuki E, et al. Transcriptional up-regulation of Sox9 by NF-κB in endometrial carcinoma cells, modulating cell proliferation through alteration in the p14(ARF)/p53/p21(WAF1) pathway. Am J Pathol. 2012;181(2):684–92.
Wegner M. All purpose Sox: the many roles of Sox proteins in gene expression. Int J Biochem Cell Biol. 2009;42:381–90.
Matheu A, Collado M, Wise C, et al. Oncogenicity of the developmental transcription factor Sox9. Cancer Res. 2012;72(5):1301–15.
Akiyama H, Chaboissier MC, Martin JF, et al. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–28.
Kordes U, Hagel C. Expression of SOX9 and SOX10 in central neuroepithelial tumor. J Neurooncol. 2006;80:151–5.
Jiang SS, Fang WT, Hou YH, et al. Upregulation of SOX9 in lung adenocarcinoma and its involvement in the regulation of cell growth and tumorigenicity. Clin Cancer Res. 2010;16:4363–73.
Wang H, Leav I, Ibaragi S, et al. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res. 2008;68:1625–30.
Bastide P, Darido C, Pannequin J, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178(4):635–48.
Cheung M, Chaboissier MC, Mynett A, et al. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell. 2005;8:179–92.
Zhu H, Tang J, Tang M, Cai H. Upregulation of SOX9 in osteosarcoma and its association with tumor progression and patients’ prognosis. Diagn Pathol. 2013;8(1):183.
Rani SB, Rathod SS, Karthik S, et al. MiR-145 functions as a tumor-suppressive RNA by targeting Sox9 and adducin 3 in human glioma cells. Neuro Oncol. 2013;15(10):1302–16.
Lee S, Yoon DS, Paik S, et al. MicroRNA-495 inhibits chondrogenic differentiation in human mesenchymal stem cells by targeting Sox9. Stem Cells Dev. 2014 Mar 24.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
An erratum to this article is available at http://dx.doi.org/10.1007/s13277-017-5487-6.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Expression levels of PTEN in Saos-2 and U2OS cells. (A-B) Protein levels of PTEN were determined by western blot in Saos-2 (A) and U2OS (B) cells transfected with miR-32 mimics or negative controls (NC). (JPEG 81 kb)
Supplementary Fig. 2
Sox9 re-introduction blocks the anti-proliferative roles of miR-32 in U2OS cells. (A) Sox9 protein expression was determined by western blot in U2OS cells. Cells were pre-transfected with miR-32 mimics or negative control (NC) for 24 hr, and then transfected with lentiviruses containing Sox9 gene or empty vector (EV) for another 24 hr. (B-D) The growth curve (B), cell proliferation (BrdU, C) and invasion abilities (D) was determined in U2OS cells. *P < 0.05 **P < 0.01, ***P < 0.001 between two groups. (JPEG 107 kb)
About this article
Cite this article
Xu, JQ., Zhang, WB., Wan, R. et al. RETRACTED ARTICLE: MicroRNA-32 inhibits osteosarcoma cell proliferation and invasion by targeting Sox9. Tumor Biol. 35, 9847–9853 (2014). https://doi.org/10.1007/s13277-014-2229-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2229-x