Abstract
The epithelial to mesenchymal transition (EMT) is an important step for the developmental process. Recent evidences support that EMT allows the tumor cells to acquire invasive properties and to develop metastatic growth characteristics. Some of the transcription factors, which are actively involved in EMT process, have a significant role in the EMT–metastasis linkage. A number of studies have reported that EMT-inducing transcription factors (EMT-TFs), such as Twist, Snail, Slug, and Zeb, are directly or indirectly involved in cancer cell metastasis through a different signaling cascades, including the Akt, signal transducer and activator of transcription 3 (STAT3), mitogen-activated protein kinase (MAPK) and Wnt pathways, with the ultimate consequence of the downregulation of E-cadherin and upregulation of metastatic proteins, such as N-cadherin, vimentin, matrix metalloproteinase (MMP)-2, etc. This review summarizes the update information on the association of EMT-TFs with cancer metastasis and the possible cancer therapeutics via targeting the EMT-TFs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
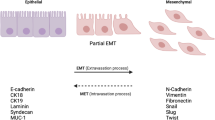
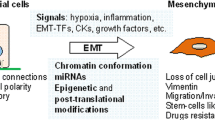
The epithelial to mesenchymal transition (EMT) is a biological process by which polarized epithelial cells loss the adherent and tight cell–cell junction, enhance the migratory capacity, elevate the resistance to apoptosis, greatly increase the production of extracellular matrix (ECM) components and gain the invasive properties to become mesenchymal cells. EMT and its reverse process, mesenchymal to epithelial transition (MET), are essential for embryonic development and several developmental processes, such as gastrulation, neural tube formation, mesoderm formation, wound healing, and organ fibrosis [1, 2]. The concept of EMT was first recognized in the field of embryology but has recently been reported to play a vital role in cancer progression and metastasis [3, 4].
Metastasis is a multistep process combining local invasion, intravasation, transport, extravasation, and colonization, by which cancer cells are spread from the site of primary tumors through the circulation to form secondary or metastatic tumors at a distant site or another nonadjacent organ [5]. As metastasis remains one of the most enigmatic aspects of the disease and causes most cancer deaths, it is one of the major threats in modern life [6]. EMT allows the tumor cells to acquire invasive properties and to develop metastatic growth characteristics. These events are facilitated by the reduction of cell–cell adhesion molecule E-cadherin; upregulation of more plastic mesenchymal proteins such as vimentin, N-cadherin, and smooth muscle actin; and deregulation of the Wnt pathway, and these break through the basement membrane [2]. Many EMT-inducing transcription factors (EMT-TFs), such as Twist1, Snail1, Snail2 (also named “Slug”), Zeb1, and Zeb2, can repress E-cadherin directly or indirectly [4, 7, 8]. These master EMT-TFs along with other factors facilitate EMT by repressing E-cadherin and other junctional proteins [2]. Association of EMT-TFs with cancer progression has become an interesting field of cancer research, especially in pathogenic and therapeutic aspects.
Role of EMT-TFs in metastatic cancers
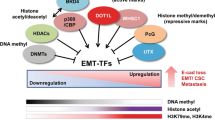
Several EMT-TFs factors act as master molecular switches that recognize the E-box DNA sequences in the promoter region of E-cadherin, recruit transcriptional cofactors and histone deacetylases, regulate the EMT process by responding to the known signaling pathways, and thereby repress its expression [9].
Twist
Twist is a member of the basic helix-loop-helix (bHLH) transcription factor family which is one of the important EMT-TFs [10]. Twist (also called Twist1) and Twist-related protein (Twist2) share extensive homology [11], while Twist1 is essential for the neural tube formation and thought to be involved in regulating the differentiation of osteogenic and chondrogenic cells from mesenchymal precursors during skeletal development and remodeling [12]. On the other hand, Twist2 maintains cells in a preosteoblast phenotype by inhibiting osteoblast maturation during osteoblast development through the similar mechanisms, but not identical to those utilized by Twist1 [13]. An aberrant Twist1 expression or its gene methylation is frequently found in metastatic carcinomas. In 2004, Yang et al. [14] first identified Twist1 as a pro-metastatic factor in murine isogenic breast cancer cell lines, and Sahlin et al. [15] indicated TWIST1 as a breast cancer susceptibility gene. The overexpression of Twist1 is also associated with the development and progression of prostate cancer, gynecological cancer like epithelial ovarian cancer, urological cancers like urothelial and bladder cancer, hepatocellular carcinoma (HCC), gastric cancer, colorectal carcinoma, and thyroid cancer [4].
In addition to overexpression, epigenetic changes (hypermethylation) in TWIST1 promoter have been known to be associated with different types of cancer [16–18]. Methylation of CpG islands in promoter regions of TWIST1 abolishes proper TATA-binding protein binding to the promoter, which permits cells to disassemble cell adherence, reduce DNA repairing capacity, and avoid apoptosis [19]. Hypermethylated TWIST1 provides a valuable tool for the diagnosis of cancer, but the exact mechanism remains still unknown as there is no direct correlation between Twist1 promoter methylation and TWIST1 protein or RNA expression [16].
Twist1 plays an essential role in cancer metastasis through different signaling pathways, including Akt (a serine/threonine-specific protein kinase), signal transducer and activator of transcription 3 (STAT3), mitogen-activated protein kinase (MAPK), Ras, transforming growth factor beta (TGFβ), and Wnt signaling. In addition to the classical oncogenic signaling pathways, some other signaling molecules like the increased activity of nuclear factor kappa B (NF-κB), neurotrophic receptor tyrosine kinase B (TrkB), and steroid receptor co-activator (SRC)-3 protein also induce cancer initiation and progression via Twist1 activation. Activated Twist1 has a significant role in tumor invasion and metastatic cancer development, as Twist1 downregulates E-cadherin and upregulates N-cadherin expression, which are the hallmarks of EMT [4, 9]. Moreover, Twist1 plays critical roles in some physiological process related to metastasis, such as angiogenesis, extravasation, invadopodia, vasculogenic mimicry, and chromosomal instability. Twist1 interacts with Mi2/nucleosome remodeling and deacetylase components and forms the protein complex Mi2/NuRD, which plays an essential role in cancer cell migration and invasion [20]. Experimental works showed that Twist1 is involved in tumor invasion and metastasis also by upregulating the expression of matrix metalloproteinases (MMPs) and downregulating the expression of TIMP, a naturally occurring specific inhibitor of MMPs [21, 22]. In addition, Twist1 is responsible for the loss of estrogen receptor (ER) activity by regulating ER function and thus is involved in the generation of hormone-resistant, ER-negative (ER−) breast cancer [23].
Recent evidences showed that the overexpression of Twist1 in cancer cells can promote the generation of a cancer stem cell (CSC) phenotype which possess self-renewal properties and are resistant to chemo/radiation therapy [4, 9, 24, 25]. In EMT-associated cancer stem cell traits, Twist1 directly activates the polycomb protein, Bmi1, which is frequently overexpressed in various types of human cancers and can confer drug resistance [24]. Twist1 also plays a critical role through the activation of β-catenin and Akt pathways which is thought to be associated with the generation of CSCs [25].
Snail
The Drosophila embryonic protein, Snail, is a zinc finger containing transcription factor which is required for proper development, such as mesoderm and neural crest formation and central nervous system (CNS) development of vertebrate and invertebrate embryos. The Snail superfamily includes Snail1 and Snail2 (also called Slug), and all the family members encode transcriptional repressors and share a similar organization with a highly conserved carboxy-terminal domain that binds to the E-box hexa-nucleotide DNA motif (5′-CACCTG-3′) in the human E-cadherin promoter [2, 26]. The expression of Snail/Slug is closely associated with cancer metastasis as it is a critical regulator of multiple signaling molecules such as epidermal growth factor (EGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), TGFβ, bone morphogenetic proteins (BMPs), Wnt, Notch, tumor necrosis factor alpha (TNF-α), and cytokines. These signaling molecules from tumor microenvironment have been shown to activate Snail/Slug in different cellular contexts. The activated Snail/Slug prominently induces EMT by downregulating E-cadherin and promoting cell migration, invasion, and tumor progression [26, 27]. It was reported that the overexpression of Snail is correlated with deacetylation of histones H3 and H4 at the E-cadherin promoter [28]. Snail can deacetylate histones H3 and H4 by the recruitment of a co-repressor complex containing histone deacetylase 1 (HDAC1) and HDAC2 (HDAC1/2) and Sin3A through the SNAG domain, leading to the repression of E-cadherin expression [28].
The overexpression of Snail1 has been found to be correlated with breast cancer, ovarian cancer, gastric cancer, hepatocellular carcinoma, colon cancer, and synovial sarcomas [27]. Snail and Slug are also critical for a cancer cell to acquire CSC-like properties toward resisting radio/chemotherapy-mediated cellular stress. They indirectly increase the activation of a self-renewal program through loss of binding to specific gene promoters including NANOG, HDAC1, TCF4, Krueppel-like factor 4 (KLF4), HDAC3, and GPC3 and further induce the expression of other stem cell markers including Oct4, Bmi1, and nestin as well as increase the number of cells with CD44+CD117+ (represent as ovarian CSC markers) [29].
Zeb
The Zeb family (Zeb1 and Zeb2) is a group of zinc finger/homeodomain transcription factors, which play a pivotal role in the formation of neural crest cells and the derivative structures during normal development of the vertebrate embryo [2, 30]. Both of the Zeb family members contain two widely separated zinc finger clusters (ZFCs), located toward the N-terminal (Nt-ZFC) and C-terminal (Ct-ZFC) ends of the protein and interacting with CACCT(G) E-box-like DNA sequences located in the target gene promoters [31]. Zeb proteins induce EMT by downregulating E-cadherin and upregulating a number of other mesenchymal markers, vimentin, fibronectin, N-cadherin, and MMPs, facilitating cell migration, invasion, and eventual metastasis to distant organs [2]. The expression of Zeb proteins is activated by several signaling molecules such as growth and steroid hormones, hypoxia-inducible factor-1 alpha (HIF-1α) in hypoxic conditions, FGF, insulin growth factor 1, platelet-derived growth factor (PDGF) receptor, Ras-ERK2-Fra1, NF-κB, and Janus kinase (JAK)/STAT3 and classical signaling pathways including TGFβ/Smad, Wnt, and Notch [9]. The overexpression of Zeb proteins is responsible for the increased aggressiveness and higher metastatic capacity in a wide range of primary human carcinomas including ovarian, breast, endometrium, lung, prostate, colon, gallbladder, pancreatic, and bladder cancer [32].
The epigenetic alterations, such as promoter hypermethylation and histone modifications of ZEB1, contribute to cancer metastasis. Although the functional mechanism of epigenetic alterations is not clear, some studies reported the prevalent hypermethylation and silencing of ZEB1, whose protein product suppresses E-cadherin expression, leading to the progression of different types of cancers [33–35]. It was also reported that Zeb maintains CSC-like properties and promotes tumorigenicity. Zeb represses the expression of stemness-inhibiting microRNAs (miRNAs), including miR-200, miR-183, and miR-203, which in turn increase stem cells markers (including Bmi1 and KLF4) and thereby act as a promoter of cancer stem cells [36].
A summary of EMT-TF expression patterns in different cancers has been presented in Table 1 [7, 17, 18, 29, 33–77].
EMT-TFs in cancer therapeutics
Metastatic cancers are among the major threats in human health. Despite the tremendous efforts in basic and clinical research, the pathogenic mechanism by which tumor cells escape the local environment and colonize distant organs is unclear [4, 78, 79]. The understanding of the mechanistic role of the EMT markers that have been associated with metastases and their regulation is thought to be essential to develop the potential treatment strategies of metastatic cancers.
Recently, Twist1 or Twist1-mediated signaling pathways have been indicated as the promising target in cancer therapeutics. Twist1 is thought to be inactivated by RNA interference (RNAi) by promoting cellular senescence and growth arrest which may control Twist1-mediated tumor metastasis. In a study, Zhuo et al. [80] utilized RNAi technology to knockdown Twist1 expression in A549 cell and reported that Twist1 depletion significantly sensitized A549 cell to cisplatin via MAPK/mitochondrial pathway. Their study suggested that Twist1 depletion might be a promising approach to treat cisplatin-resistant lung cancer [80]. Li et al. [81] reported that adriamycin treatment induces apoptosis in cancer cells, and their study indicated that Twist1 RNAi raises apoptosis rate and improves the efficacy in adriamycin-based chemotherapies for breast cancer. Knockdown of Twist1 by RNAi in anaplastic thyroid carcinoma cells reduces cell migration and local invasion and increases apoptosis [46]. Several chemotherapeutic agents are also able to downregulate Twist1 and Twist1-associated molecules to control tumor formation and cancer metastasis. The natural product curcumin was found to downregulate Twist1 and reduce malignant glioma growth by inhibiting the JAK1,2/STAT3 signaling pathway [82]. The cruciferous vegetable component sulforaphane (SFN) in combination with well-known polyphenol and flavonoid quercetin can eliminate cancer stem cell characteristics of pancreatic CSCs by downregulating Twist1 and other EMT proteins [83]. Quercetin reduces the migration ability of head and neck cancer-derived sphere cells partially by decreasing the productions of Twist1, N-cadherin, and vimentin [84]. The orchid component moscatilin inhibits migration and metastasis of human breast cancer MDA-MB-231 cells through inhibition of Akt and Twist signaling pathway [85]. Recently, Twist1 and its target protein N-cadherin were found to be downregulated by thymoquinone treatment in HeLa and MDA-MB-435 cells, while thymoquinone showed antimetastatic activity in these cancer cell lines [86].
Snail is another attractive target for the drug development of cancer therapeutics. Blocking the Snail activity has shown great potential to prevent cell migration, invasion, and cancer metastasis [26]. Shaoyao decoction (SYD), a traditional Chinese medicine prescription formulated by Liu Wan-Su is commonly used in treating ulcerative colitis [87]. SYD inhibits colorectal cancer (CRC) cell proliferation and induces CRC cell apoptosis by downregulating Snail and other EMT markers like N-cadherin, fibronectin, and vimentin. SYD might be an alternative therapy for colitis-associated CRC (caCRC) as it ameliorates caCRC by suppressing inflammation and inhibiting EMT [87]. Grape seed pro-anthocyanidins (GSPs), an efficient antioxidant and anticarcinogenic agent, ameliorates the radiation-induced lung injury through suppressing the TGFβ1/Smad3/Snail signaling pathway [88]. In a study, Lv et al. suggested that hydrogen sulfide (H2S) has a novel cancer therapeutic effects on breast cancer cells, as it reduces the expression of Snail and phospho-p38 (a signaling protein associated with apoptosis) through the activation of CSE/H2S pathway [89]. Forkhead box Q1 (FoxQ1), a member of the forkhead transcription factor family, is an important therapeutic target for pancreatic cancer treatment. Knocking down of FoxQ1 by small interfering RNA (siRNA) results in the inhibition of tumor formation and metastasis in pancreatic cancer stem-like cells via the reduction of Snail expression [90]. There is evidence that miRNA-148a suppresses the nuclear accumulation of Snail and metastasis of hepatoma cells by targeting Met/Snail signaling-like activated phosphorylation of Akt-Ser473 and inhibits the phosphorylation of GSK-3β-Ser9 [91]. Downregulating the expression of Snail was also found as a potential therapeutic approach for the treatment of metastasis and invasion of cervical carcinomas [92].
Snail2/Slug might be another target for cancer therapy. Slug expression was found to be significantly reduced in breast cancers after neoadjuvant chemotherapy [93]. Receptor tyrosine kinase (RTK) is one of the promising targets in molecularly targeted therapy for cancer. Axl is one of the most frequently activated RTK in liver cancer cell lines. Knocking down of Axl by RNAi significantly suppressed Slug expression and reduced the invasiveness of HCC cell lines [94]. Slug inhibition was also found as a target for the inhibition of metastatic capacity of prostate cancer by the combination of mTOR/Erk/HSP90 inhibitor (combination of rapamycin, CI-1040, and 17-AAG) treatment [95]. Among the miRNAs involved in breast cancer, miR-221 plays a crucial role, which actually downregulates the oncosuppressor p27Kip1 and upregulates Slug. By using antisense miRNA (antagomir) molecules, targeting miR-221 induces the downregulation of Slug and the upregulation of p27Kip1 [96]. Knockdown of Slug by using short hairpin RNA (shRNA) inhibits the proliferation and invasion of HCT116 colorectal cancer cells and lung cancer growth and metastasis [97, 98].
Both members of the Zeb family of transcription factors have the capacity to control cellular processes, and they could open up new possibilities for the treatment of advanced carcinomas [30]. Zeb1 was found to be highly expressed in lung adenocarcinoma A549 and H1299 cell lines. Knockdown of Zeb1 expression by lentivirus-delivered siRNA introduced a novel therapeutic target for the treatment of lung cancer, as it could decrease lung adenocarcinoma cell proliferation by delaying S-phase entry, induce cell apoptosis, and inhibit tumor formation in A549 and H1299 cell lines [99]. Inactivation of the retinoblastoma protein (RB) is the key determinant of breast cancer phenotype. Arima et al. [100] developed a screening program and thereby identified the cyclin-dependent kinase inhibitor (CDK4/6 inhibitor PD0332991) which reduces cell invasiveness and proliferation by downregulating Zeb1 expression and blocking RB phosphorylation. Shen et al. [101] transfected Zeb1 siRNA into the osteosarcoma MG-63 cells and found that the transfected MG-63 cells with Zeb1 siRNA result in reduced expression of Zeb1 and decrease the invasion ability of MG-63 cells, which might provide the information for the targeted therapy for osteosarcoma.
Thus, targeting EMT-TFs has a significant role in cancer therapeutics. Specified research in this area can aid more success in target-specific drug development for cancer treatments.
Conclusion
EMT is a key step in tumor progression and metastasis. Recently, EMT-TFs have been identified as molecular markers in some human metastatic cancers. Success in targeting EMT-TFs by small RNA technology or chemotherapeutic approach may show a new hope to control metastatic cancer. Study on the upstream or downstream molecules of these TFs in different signaling pathways might be useful in finding vital targets of cancer treatment. The better understanding of action of EMT-TFs on CSCs as well as metastasis will make target-specific cancer therapy easier. Since EMT-inducing signals are diverse and often context-dependent, the knowledge about the sequential changes in activity and expression pattern in EMT-TFs during EMT may provide the information on the mechanism of the progression of metastasis. More investigations are necessary for better understanding of the roles of individual EMT-TFs that may provide an insight into tumor formation and progression as well as provide a basis for innovative therapeutic approaches and diagnostic markers for metastatic cancers.
References
Wang Y, Zhou BP. Epithelial-mesenchymal transition in breast cancer progression and metastasis. Chin J Cancer. 2011;30:603–11.
Yang G, Yuan J, Li K. EMT transcription factors: implication in osteosarcoma. Med Oncol. 2013;30:697.
Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–9.
Khan MA, Chen HC, Zhang D, Fu J. Twist: a molecular target in cancer therapeutics. Tumor Biol. 2013;34:2497–506.
Khan MI, Adhami VM, Lall RK, Sechi M, Joshi DC, Haider OM, et al. YB-1 expression promotes epithelial-to-mesenchymal transition in prostate cancer that is inhibited by a small molecule fisetin. Oncotarget. 2014 Feb 19. (in press).
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64.
Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–96.
De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110.
Garg M. Epithelial-mesenchymal transition—activating transcription factors—multifunctional regulators in cancer. World J Stem Cells. 2013;5:188–95.
Yin K, Liao Q, He H, Zhong D. Prognostic value of Twist and E-cadherin in patients with osteosarcoma. Med Oncol. 2012;29:3449–55.
Šošić D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–80.
Horvai AE, Roy R, Borys D, O’Donnell RJ. Regulators of skeletal development: a cluster analysis of 206 bone tumors reveals diagnostically useful markers. Mod Pathol. 2012;25:1452–61.
Lee MS, Lowe G, Flanagan S, Kuchler K, Glackin CA. Human Dermo-1 has attributes similar to twist in early bone development. Bone. 2000;27:591–602.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39.
Sahlin P, Windh P, Lauritzen C, Emanuelsson M, Grönberg H, Stenman G. Women with Saethre-Chotzen syndrome are at increased risk of breast cancer. Gene Chromosome Cancer. 2007;46:656–60.
Gort EH, Suijkerbuijk KP, Roothaan SM, Raman V, Vooijs M, van der Wall E, et al. Methylation of the TWIST1 promoter, TWIST1 mRNA levels, and immunohistochemical expression of TWIST1 in breast cancer. Cancer Epidemiol Biomark Prev. 2008;17:3325–30.
Mehrotra J, Vali M, McVeigh M, Kominsky SL, Fackler MJ, Lahti-Domenici J, et al. Very high frequency of hypermethylated genes in breast cancer metastasis to the bone, brain, and lung. Clin Cancer Res. 2004;10:3104–9.
Okada T, Suehiro Y, Ueno K, Mitomori S, Kaneko S, Nishioka M, et al. TWIST1 hypermethylation is observed frequently in colorectal tumors and its overexpression is associated with unfavorable outcomes in patients with colorectal cancer. Gene Chromosome Cancer. 2010;49:452–62.
Locke I, Kote-Jarai Z, Fackler MJ, Bancroft E, Osin P, Nerurkar A, et al. Gene promoter hypermethylation in ductal lavage fluid from healthy BRCA gene mutation carriers and mutation-negative controls. Breast Cancer Res. 2007;9(1):R20.
Fu J, Zhang L, He T, Xiao X, Liu X, Wang L, et al. TWIST represses estrogen receptor-alpha expression by recruiting the NuRD protein complex in breast cancer cells. Int J Biol Sci. 2012;8:522–32.
Zhao XL, Sun T, Che N, Sun D, Zhao N, Dong XY. Promotion of hepatocellular carcinoma metastasis through matrix metalloproteinase activation by epithelial-mesenchymal transition regulator Twist1. J Cell Mol Med. 2011;15:691–700.
Okamura H, Yoshida K, Haneji T. Negative regulation of TIMP1 is mediated by transcription factor TWIST1. Int J Oncol. 2009;35:181–6.
Fu J, Qin L, He T, Qin J, Hong J, Wong J, et al. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Res. 2011;21:275–89.
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–92.
Li J, Zhou BP. Activation of β-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer. 2011;11:49.
Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of Snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13:963–72.
Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–61.
Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–19.
Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27(9):2059–68.
Gheldof A, Hulpiau P, van Roy F, De Craene B, Berx G. Evolutionary functional analysis and molecular regulation of the ZEB transcription factors. Cell Mol Life Sci. 2012;69:2527–41.
Browne G, Sayan AE, Tulchinsky E. ZEB proteins link cell motility with cell cycle control and cell survival in cancer. Cell Cycle. 2010;9:886–91.
Sánchez-Tilló E, Siles L, de Barrios O, Cuatrecasas M, Vaquero EC, Castells A, et al. Expanding roles of ZEB factors in tumorigenesis and tumor progression. Am J Cancer Res. 2011;1:897–912.
Acun T, Oztas E, Yagci T, Yakicier MC. SIP1 is downregulated in hepatocellular carcinoma by promoter hypermethylation. BMC Cancer. 2011;11:223.
Rodenhiser DI, Andrews J, Kennette W, Sadikovic B, Mendlowitz A, Tuck AB, et al. Epigenetic mapping and functional analysis in a breast cancer metastasis model using whole-genome promoter tiling microarrays. Breast Cancer Res. 2008;10:R62.
Li A, Omura N, Hong SM, Vincent A, Walter K, Griffith M, et al. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226–37.
Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–95.
Hosono S, Kajiyama H, Terauchi M, Shibata K, Ino K, Nawa A, et al. Expression of Twist increases the risk for recurrence and for poor survival in epithelial ovarian carcinoma patients. Br J Cancer. 2007;96:314–20.
Niu RF, Zhang L, Xi GM, Wei XY, Yang Y, Shi YR, et al. Upregulation of Twist induces angiogenesis and correlates with metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:385–94.
Luo GQ, Li JH, Wen JF, Zhou YH, Hu YB, Zhou JH. Effect and mechanism of the Twist gene on invasion and metastasis of gastric carcinoma cells. World J Gastroenterol. 2008;14:2487–93.
Yuen HF, Chan YP, Wong ML, Kwok WK, Chan KK, Lee PY, et al. Upregulation of Twist in oesophageal squamous cell carcinoma is associated with neoplastic transformation and distant metastasis. J Clin Pathol. 2007;60:510–4.
Song LB, Liao WT, Mai HQ, Zhang HZ, Zhang L, Li MZ, et al. The clinical significance of twist expression in nasopharyngeal carcinoma. Cancer Lett. 2006;242:258–65.
Yuen HF, Chua CW, Chan YP, Wong YC, Wang X, Chan KW. Significance of TWIST and E-cadherin expression in the metastatic progression of prostatic cancer. Histopathology. 2007;50:648–58.
McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–44.
Elias MC, Tozer KR, Silber JR, Mikheeva S, Deng M, Morrison RS, et al. TWIST is expressed in human gliomas and promotes invasion. Neoplasia. 2005;7:824–37.
Ou DL, Chien HF, Chen CL, Lin TC, Lin LI. Role of Twist in head and neck carcinoma with lymph node metastasis. Anticancer Res. 2008;28:1355–9.
Salerno P, Garcia-Rostan G, Piccinin S, Bencivenga TC, Di Maro G, Doglioni C, et al. TWIST1 plays a pleiotropic role in determining the anaplastic thyroid cancer phenotype. J Clin Endocrinol Metab. 2011;96:E772–81.
Singh S, Mak IW, Cowan RW, Turcotte R, Singh G, Ghert M. The role of TWIST as a regulator in giant cell tumor of bone. J Cell Biochem. 2011;112:2287–95.
Huang KT, Dobrovic A, Yan M, Karim RZ, Lee CS, Lakhani SR, et al. DNA methylation profiling of phyllodes and fibroadenoma tumours of the breast. Breast Cancer Res Treat. 2010;124:555–65.
Missaoui N, Hmissa S, Trabelsi A, Traoré C, Mokni M, Dante R, et al. Promoter hypermethylation of CDH13, DAPK1 and TWIST1 genes in precancerous and cancerous lesions of the uterine cervix. Pathol Res Pract. 2011;207:37–42.
Dhillon VS, Aslam M, Husain SA. The contribution of genetic and epigenetic changes in granulosa cell tumors of ovarian origin. Clin Cancer Res. 2004;10:5537–45.
Renard I, Joniau S, van Cleynenbreugel B, Collette C, Naômé C, Vlassenbroeck I, et al. Identification and validation of the methylated TWIST1 and NID2 genes through real-time methylation-specific polymerase chain reaction assays for the noninvasive detection of primary bladder cancer in urine samples. Eur Urol. 2010;58:96–104.
Schneider BG, Peng DF, Camargo MC, Piazuelo MB, Sicinschi LA, Mera R, et al. Promoter DNA hypermethylation in gastric biopsies from subjects at high and low risk for gastric cancer. Int J Cancer. 2010;127:2588–97.
Tsou JA, Galler JS, Siegmund KD, Laird PW, Turla S, Cozen W, et al. Identification of a panel of sensitive and specific DNA methylation markers for lung adenocarcinoma. Mol Cancer. 2007;6:70.
Kwon MJ, Kwon JH, Nam ES, Shin HS, Lee DJ, Kim JH, et al. TWIST1 promoter methylation is associated with prognosis in tonsillar squamous cell carcinoma. Hum Pathol. 2013;44:1722–9.
Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209.
Fan F, Samuel S, Evans KW, Lu J, Xia L, Zhou Y, et al. Overexpression of snail induces epithelial-mesenchymal transition and a cancer stem cell-like phenotype in human colorectal cancer cells. Cancer Med. 2012;1:5–16.
Zhu LF, Hu Y, Yang CC, Xu XH, Ning TY, Wang ZL, et al. Snail overexpression induces an epithelial to mesenchymal transition and cancer stem cell-like properties in SCC9 cells. Lab Invest. 2012;92:744–52.
Shin NR, Jeong EH, Choi CI, Moon HJ, Kwon CH, Chu IS, et al. Overexpression of Snail is associated with lymph node metastasis and poor prognosis in patients with gastric cancer. BMC Cancer. 2012;12:521.
Kim MK, Kim MA, Kim H, Kim YB, Song YS. Expression profiles of epithelial-mesenchymal transition-associated proteins in epithelial ovarian carcinoma. Biomed Res Int. 2014;2014:495754.
Roy HK, Smyrk TC, Koetsier J, Victor TA, Wali RK. The transcriptional repressor SNAIL is overexpressed in human colon cancer. Dig Dis Sci. 2005;50:42–6.
De Craene B, Denecker G, Vermassen P, Taminau J, Mauch C, Derore A, et al. Epidermal Snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential. Cell Death Differ. 2014;21:310–20.
Cai J. Roles of transcriptional factor Snail and adhesion factor E-cadherin in clear cell renal cell carcinoma. Exp Ther Med. 2013;6:1489–93.
Neal CL, Henderson V, Smith BN, McKeithen D, Graham T, Vo BT, et al. Snail transcription factor negatively regulates maspin tumor suppressor in human prostate cancer cells. BMC Cancer. 2012;12:336.
Castro Alves C, Rosivatz E, Schott C, Hollweck R, Becker I, Sarbia M, et al. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007;211:507–15.
Shih JY, Yang PC. The EMT regulator slug and lung carcinogenesis. Carcinogenesis. 2011;32:1299–304.
Shioiri M, Shida T, Koda K, Oda K, Seike K, Nishimura M, et al. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006;94:1816–22.
Yang HW, Menon LG, Black PM, Carroll RS, Johnson MD. SNAI2/Slug promotes growth and invasion in human gliomas. BMC Cancer. 2010;10:301.
Zhang K, Chen D, Jiao X, Zhang S, Liu X, Cao J, et al. Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab Invest. 2011;91:426–38.
Elloul S, Elstrand MB, Nesland JM, Tropé CG, Kvalheim G, Goldberg I, et al. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–43.
Uygur B, Wu WS. SLUG promotes prostate cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol Cancer. 2011;10:139.
Giannelli G, Bergamini C, Fransvea E, Sgarra C, Antonaci S. Laminin-5 with transforming growth factor-beta1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology. 2005;129:1375–83.
Spoelstra NS, Manning NG, Higashi Y, Darling D, Singh M, Shroyer KR, et al. The transcription factor ZEB1 is aberrantly expressed in aggressive uterine cancers. Cancer Res. 2006;66:3893–902.
Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, et al. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006;131:830–40.
Dohadwala M, Yang SC, Luo J, Sharma S, Batra RK, Huang M, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66:5338–45.
Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, et al. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68:2479–88.
Adachi Y, Takeuchi T, Nagayama T, Ohtsuki Y, Furihata M. Zeb1-mediated T-cadherin repression increases the invasive potential of gallbladder cancer. FEBS Lett. 2009;583:430–6.
Sayan AE, Griffiths TR, Pal R, Browne GJ, Ruddick A, Yagci T, et al. SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc Natl Acad Sci U S A. 2009;106:14884–9.
Celià-Terrassa T, Meca-Cortés O, Mateo F, de Paz AM, Rubio N, Arnal-Estapé A, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest. 2012;122:1849–68.
Scanlon CS, Van Tubergen EA, Inglehart RC, D’Silva NJ. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res. 2013;92:114–21.
Zhuo WL, Wang Y, Zhuo XL, Zhang YS, Chen ZT. Short interfering RNA directed against TWIST, a novel zinc finger transcription factor, increases A549 cell sensitivity to cisplatin via MAPK/mitochondrial pathway. Biochem Biophys Res Commun. 2008;369:1098–102.
Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q, Tang F, et al. Twist1-mediated adriamycin-induced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res. 2009;15:2657–65.
Weissenberger J, Priester M, Bernreuther C, Rakel S, Glatzel M, Seifert V, et al. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res. 2010;16:5781–95.
Srivastava RK, Tang SN, Zhu W, Meeker D, Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed). 2011;3:515–28.
Chang WW, Hu FW, Yu CC, Wang HH, Feng HP, Lan C, et al. Quercetin in elimination of tumor initiating stem-like and mesenchymal transformation property in head and neck cancer. Head Neck. 2013;35:413–9.
Pai HC, Chang LH, Peng CY, Chang YL, Chen CC, Shen CC, et al. Moscatilin inhibits migration and metastasis of human breast cancer MDA-MB-231 cells through inhibition of Akt and Twist signaling pathway. J Mol Med (Berl). 2013;91:347–56.
Khan MA, Yang M, Wei C, Gan L, Fu J. Thymoquinone downregulates n-cadherin, twist and snail expression and inhibits migration and invasion in cancer cells. Proceedings of Annual meeting of American Association of Cancer Research; April 05-09, 2014 at San Diego, USA (Abstract No. 5009).
Lin X, Yi Z, Diao J, Shao M, Zhao L, Cai H, et al. ShaoYao decoction ameliorates colitis-associated colorectal cancer by downregulating proinflammatory cytokines and promoting epithelial-mesenchymal transition. J Transl Med. 2014;12:105.
Huang Y, Liu W, Liu H, Yang Y, Cui J, Zhang P, et al. Grape seed pro-anthocyanidins ameliorates radiation-induced lung injury. J Cell Mol Med. 2014 Apr 24. (in press).
Lv M, Li Y, Ji MH, Zhuang M, Tang JH. Inhibition of invasion and epithelial-mesenchymal transition of human breast cancer cells by hydrogen sulfide through decreased phospho-p38 expression. Mol Med Rep. 2014 Apr 17. (in press).
Bao B, Azmi A, Aboukameel A, Ahmad A, Bolling-Fischer A, Sethi S, et al. Pancreatic cancer stem-like cells display aggressive behavior mediated via activation of FoxQ1. J Biol Chem. 2014 Apr 9. (in press).
Zhang JP, Zeng C, Xu L, Gong J, Fang JH, Zhuang SM. MicroRNA-148a suppresses the epithelial-mesenchymal transition and metastasis of hepatoma cells by targeting Met/Snail signaling. Oncogene. 2013 Sep 9. (in press).
Zhao W, Zhou Y, Xu H, Cheng Y, Kong B. Snail family proteins in cervical squamous carcinoma: expression and significance. Clin Invest Med. 2013;36:E223–33.
Riemenschnitter C, Teleki I, Tischler V, Guo W, Varga Z. Stability and prognostic value of Slug, Sox9 and Sox10 expression in breast cancers treated with neoadjuvant chemotherapy. Springerplus. 2013;2:695.
Lee HJ, Jeng YM, Chen YL, Chung L, Yuan RH. Gas6/Axl pathway promotes tumor invasion through the transcriptional activation of Slug in hepatocellular carcinoma. Carcinogenesis. 2013;35:769–75.
Ding G, Feng C, Jiang H, Ding Q, Zhang L, Na R, et al. Combination of rapamycin, CI-1040, and 17-AAG inhibits metastatic capacity of prostate cancer via Slug inhibition. PLoS One. 2013;8:e77400.
Piva R, Spandidos DA, Gambari R. From microRNA functions to microRNA therapeutics: novel targets and novel drugs in breast cancer research and treatment (Review). Int J Oncol. 2013;43:985–94.
Qian J, Liu H, Chen W, Wen K, Lu W, Huang C, et al. Knockdown of Slug by RNAi inhibits the proliferation and invasion of HCT116 colorectal cancer cells. Mol Med Rep. 2013;8:1055–9.
Wang YP, Wang MZ, Luo YR, Shen Y, Wei ZX. Lentivirus-mediated shRNA interference targeting SLUG inhibits lung cancer growth and metastasis. Asian Pac J Cancer Prev. 2012;13:4947–51.
Liu Y, Yan X, Liu N, Zhou J, Liu J, Pang H, et al. Lentivirus-delivered ZEB-1 small interfering RNA inhibits lung adenocarcinoma cell growth in vitro and in vivo. J Cancer Res Clin Oncol. 2012;138:1329–38.
Arima Y, Hayashi H, Sasaki M, Hosonaga M, Goto TM, Chiyoda T, et al. Induction of ZEB proteins by inactivation of RB protein is key determinant of mesenchymal phenotype of breast cancer. J Biol Chem. 2012;287:7896–906.
Shen A, Zhang Y, Yang H, Xu R, Huang G. Overexpression of ZEB1 relates to metastasis and invasion in osteosarcoma. J Surg Oncol. 2012;105:830–4.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (81172049), Science and Technology Innovation Team of Colleges and Universities of Sichuan Province (13TD0032), Health Department Foundation of Sichuan Province (130261), The Research Foundation of the Science and Technology Department of Sichuan Province (14JC0797), Luzhou City special foundation (2013LZLY-J10), and Luzhou Medical College grants for postdoctoral research (20130512, 20130513).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Mousumi Tania and Md. Asaduzzaman Khan have equal contribution.
Rights and permissions
About this article
Cite this article
Tania, M., Khan, M.A. & Fu, J. Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumor Biol. 35, 7335–7342 (2014). https://doi.org/10.1007/s13277-014-2163-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2163-y