Abstract
Estrogen receptor 1 (ESR1) and estrogen receptor 2 (ESR2) may play a role in the development of prostate cancer. Many studies focused on ESR1 rs9340799 and ESR2 rs1256049 polymorphisms to explore associations with prostate cancer risk. These studies showed inconsistent and conflicting results. The aim of this meta-analysis was to investigate the pooled association of ESR1 rs9340799 and ESR2 rs1256049 polymorphisms with prostate cancer risk. A systematic literature search was conducted to identify related studies (up to February 2014) in several online databases including PubMed, Google Scholar, CNKI and Wanfang online libraries. A total of 16 eligible articles were enrolled in this updated meta-analysis. The result suggested that ESR1 rs9340799 polymorphism was significantly associated with prostate cancer in overall populations (GG+GA vs. AA: P = 0.002; G vs. A: P = 0.004), Caucasians (GG+GA vs. AA: P = 0.008; G vs. A: P = 0.016) and Africans (GG+GA vs. AA: P = 0.005; G vs. A: P = 0.006), but not in Asians (GG+GA vs. AA: P = 0.462; G vs. A: P = 0.665). The result also showed that there was a significant association between ESR2 rs1256049 polymorphism and prostate cancer in Caucasians (AA+AG vs. GG: P = 0.016; A vs. G: P = 0.005), but no association in overall populations (AA+AG vs. GG: P = 0.826; A vs. G: P = 0.478), Asians (AA+AG vs. GG: P = 0.177; A vs. G: P = 0.703) and Africans (AA+AG vs. GG: P = 0.847; A vs. G: P = 0.707). The cumulative meta-analysis and sensitivity analysis showed the results were robust. In conclusion, this meta-analysis indicated that ESR1 rs9340799 polymorphism was associated with prostate cancer risk in overall populations, Caucasians and Africans, while ESR2 rs1256049 polymorphism was associated with prostate cancer risk in Caucasians. However, the biological mechanisms need to be further investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer, one of the most common medical problems in males, accounted for 10 % of cancer-related male deaths [1]. Like other complex diseases, prostate cancer is caused by many factors [2]. Hormonal factors are also considered to play a fundamental role in the pathogenesis of prostate cancer [3]. Estrogen as a kind of sex steroid hormone has been implicated in the stimulation of aberrant prostate growth, development and progression of prostate cancer [4, 5]. The direct effects of estrogens are mediated by estrogen receptor (ESR), which interacts with other cell-signaling pathways to influence cell behavior. There are two major ESR subtypes: ESR1 and ESR2, and their encoded genes are respectively located on chromosome 6q25.1 and chromosome 14q23.1 [6, 7]. There have been evidences to suggest that the genetic polymorphisms in ESR1 and ESR2 genes can cause transcription change or affect the stability of the transcript [8, 9], which may have an influence on the risk of prostate cancer. Several single nucleotide polymorphisms (SNPs) have been identified in ESR1 and ESR2 genes, but two common SNPs have showed significant effects on the expression and function of the receptor: one is ESR1 rs9340799 at intron 1 which is also known as –351A>G variant or XbaI A/G [8]; the other is ESR2 rs1256049 at exon 5 which is also known as 1082G>A variant or RsaI G/A [9].
Lots of studies have been conducted in the last few years to evaluate the rs9340799 polymorphism on ESR1 and rs1256049 polymorphism on ESR2 and their association with prostate cancer. However, the results were inconsistent. A few studies initially discovered a significant association of ESR1 rs9340799 and ESR2 rs1256049 polymorphism with prostate cancer risk [10–17], but these results have not been replicated [18–25]. The meta-analysis on ESR1 rs9340799 polymorphism was first reported by Ding et al. [26] and then updated by Wang et al. [27]. However, it has been noticed that results of these two reports were inconsistent, due to different eligible studies they included. In addition, Gu et al. [28] recently performed a meta-analysis and concluded that ESR1 rs9340799 polymorphism increased the risk of prostate cancer only in Africans, but the appropriate genetic model was not chosen in this study. On the other hand, there was a lack of meta-analysis concerning the association between ESR2 rs1256049 polymorphism and prostate cancer risk.
In order to assess the influence of ESR1 rs9340799 and ESR2 rs1256049 polymorphisms on prostate cancer risk, a meta-analysis including 16 eligible articles was performed under the most appropriate genetic model and cumulative meta-analysis and sensitivity analysis were conducted to ensure the pooled results were robust.
Materials and methods
Search strategy and inclusion criteria
To identify all the published studies based on the association between ESR1 rs9340799 and ESR2 rs1256049 polymorphisms and the risk of prostate cancer, we performed a systematic literature search in PubMed, Google Scholar, CNKI and Wanfang online libraries (up to February 2014) using the terms “single nucleotide polymorphism or SNP or variants” and “Prostate cancer or carcinoma” and “ESR or ESR1 or ESR2 or estrogen receptor alpha or estrogen receptor beta.” We also conducted a manual search of references identified in the retrieved articles to find other relevant studies.
We defined strict criteria for inclusion of studies: (a) studies evaluated ESR1 rs9340799 and ESR2 rs1256049 gene polymorphisms and prostate cancer risk; (b) the design of studies must be clinical case-control; (c) the numbers of the genotype or allele were reported in the article or could be obtained from authors or other source; (d) the study with the largest sample size or with the latest data was selected if overlapped publications existed. Accordingly, studies were excluded if any of the following conditions applied: (a) abstracts, reviews, conference reports; (b) insufficient data referring to genotype.
Data extraction
Two authors independently extracted all the data based on the inclusion criteria listed above. Any disagreement was subsequently resolved by discussing with a third author. The following data were extracted from each article: name of first author, year of publication, ethnicity of the population, sample size, genotype and allele distributions in prostate cancer cases and healthy controls.
Assessment of study quality
We assessed methodological quality according to the approach by Hu et al. [29] and the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (www.cochrane.org). Three independent authors (C. Fu, W.Q. Dong and A. Wang) assessed the methodological quality of included studies, and disagreement was resolved by consensus and discussion.
Statistical analysis
Data management and analysis were performed using Stata (version 10.1, Stata Corp., College Station, TX, USA). Since included studies were retrospective and case-control studies, the association between ESR1 rs9340799, ESR2 rs1256049 polymorphism and risk of prostate cancer was measured by the odds ratio (OR) with 95 % confidence intervals (CI), which was according to methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions. The statistical significance of the summary OR was determined with the Z-test, a P value less than 0.05 was considered statistically significant. OR1, OR2 and OR3 were calculated for the genotype: (1) GG vs. AA (OR1), GA vs. AA (OR2), and GG vs. GA (OR3) for the ESR1 rs9340799 polymorphism; (2) AA vs.GG (OR1), AG vs. GG (OR2), and AA vs. AG (OR3) for the ESR2 rs1256049 polymorphism. These pairwise differences were used to determine the most appropriate genetic model [30]: (a) If OR1 = OR3 ≠ 1 (P 1 < 0.05 and P 3 < 0.05), and OR2 = 1 (P 2 > 0.05), a recessive model is suggested. (b) If OR1 = OR2 ≠ 1 (P 1 < 0.05 and P 2 < 0.05), and OR3 = 1 (P 3 > 0.05), a dominant model is suggested. (c) If OR2 = 1/OR3 ≠ 1 and OR1 = 1, an over dominant model is suggested. (d) If OR1 > OR2 > 1 and OR1 > OR3 > 1 (or OR1 < OR2 < 1 and OR1 < OR3 < 1), a codominant model is suggested. The Cochran’s Q test and I 2 test were used to evaluate potential heterogeneity between studies [31, 32]. A P value less than 0.05 and values of I2 more than 50 % were considered statistically significant. If there was a significant heterogeneity across the included studies, the random effect model was used [33]. To explore the source of the heterogeneity and evaluate the ethnic-specific effects, subgroup analyses were performed by ethnicity. To access the stability of pooled results, sensitivity analyses were performed by removing one study each time. Cumulative meta-analysis was initially performed by date of publications to evaluate the trend of pooled results as studies accumulated over time [34]. Begg’s test [35] was applied to evaluate the evidence for publication bias, a P value less than 0.05 was considered statistically significant. If publication bias existed, the Duval and Tweedie nonparametric “trim and fill” method was used to adjust for it [36]. Hardy–Weinberg equilibrium (HWE) was evaluated by using chi-square test in case and control groups for each study [37]. P values less than 0.05 was considered significant departure from HWE.
Results
Characteristics of included studies
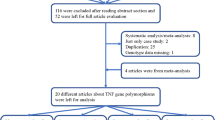
The flow chart in Fig. 1 summarized the literature review process. A total of 16 eligible articles met the inclusion criteria and were included in this meta-analysis [10–25]. Specifically, 16 studies were selected from 12 articles [10–16, 18–22] on ESR1 rs9340799 polymorphism and eight studies were selected from six articles [14, 17, 20, 23–25] on ESR2 rs1256049 polymorphism. The criteria that covered the representativeness of cases and controls, the ascertainment of cases and controls, genotyping examination, and association assessment, indicated the methodological quality of included studies was generally good. Main characteristics of the eligible studies were showed in Table 1. Studies on ESR1 rs9340799 enrolled 3,716 cases and 4,626 controls, including 11 Caucasian studies, three Asian studies and two African studies. Comparatively, studies on ESR2 rs1256049 enrolled 7,796 cases and 8,927 controls, including three Caucasian studies, four Asian studies and one African study. All identified studies showed no deviation from HWE (P > 0.05) except for one study [14]. The results of the meta-analysis are summarized in Table 2.
Association between ESR1 rs9340799 polymorphism and risk of prostate cancer
The estimated OR1, OR2 and OR3 of ESR1 rs9340799 polymorphism for overall populations were 1.31 (95 % CI 1.03–1.68, P = 0.029), 1.35 (95 % CI 1.11–1.63, P = 0.002), 1.00 (95 % CI 0.83–1.19, P = 0.963), respectively. These estimates suggested a dominant model (GG+GA vs. AA) was the best fit. The pooled meta-analysis result showed an increased risk of prostate cancer among G carriers (GG and GA) as compared with AA (OR = 1.34, 95 % CI: 1.11–1.60, P = 0.002) (Fig. 2). However, publication bias might exist from Begg’s test (P = 0.032; Table 2). This might be caused by overall populations with high OR estimates. Subgroup analysis was further performed by Ethnicity (Fig. 2). Unexpectedly, the risk of prostate cancer was significantly higher among G carriers in Caucasians (OR = 1.36, 95 % CI 1.09–1.71, P = 0.008) and Africans (OR = 1.78, 95 % CI 1.19–2.66, P = 0.005) but not in Asians (OR = 1.11, 95 % CI 0.84–1.47, P = 0.462). With regard to the subgroup of Caucasians, Begg’s test suggested the possibility of publication bias (P = 0.020; Table 2) and visual inspection of the funnel plot revealed asymmetry (Fig. 3a). We took an adjustment for the likely effect of bias using “trim and fill” method, which imputes four hypothetical negative unpublished studies to mirror the positive studies that cause funnel plot asymmetry. The imputed studies gave a symmetrical funnel plot (Fig. 3b) and showed a pooled OR of 1.29 (95 % CI 1.04–2.48, P = 0.015), which was only a slight change from our estimate of 1.36.
Summary odds ratio (OR) for the association between ESR1 rs9340799 polymorphism and prostate cancer risk (upper panel, based on GG+GA vs. AA; lower panel, based on G vs. A). A random-effects model was used for all analyses. Horizontal lines represent 95 % confidence intervals (CIs). Boxes represent the OR in each study, with the size of each box is proportional to the weight of each study. Diamonds represent summary effects with corresponding 95 % CIs
The association between ESR1 rs9340799 polymorphism and risk of prostate cancer was also reported under allele model (G vs. A). It showed the same pattern of results as that under dominant model (Fig. 2), which suggested a significant association between allele G and prostate cancer risk in overall populations (OR = 1.20, 95 % CI 1.06–1.35, P = 0.004), Caucasians (OR = 1.20, 95 % CI 1.03–1.38, P = 0.016), Africans (OR = 1.53, 95 % CI 1.13–2.07, P = 0.006), and a null association in Asians (OR = 1.06, 95 % CI 0.83–1.35, P = 0.665). No publication bias was indicated here (Table 2).
In the cumulative meta-analysis for Caucasians, the evidence was observed to support a significant association of ESR1 rs9340799 polymorphism with prostate cancer risk under the dominant and allele model (Fig. 4). As shown in Fig. 5, sensitivity analysis did not influence the results, which were stable and statistically robust.
Association between ESR2 rs1256049 polymorphism and risk of prostate cancer
The estimated OR1, OR2 and OR3 of ESR2 rs1256049 polymorphism for overall populations were 1.41 (95 % CI 1.02–1.91, P = 0.035), 1.29 (95 % CI 1.13–1.54, P = 0.018), 1.37 (95 % CI 0.91–2.02, P = 0.414), respectively. These estimates also suggested a dominant model (AA+AG vs. GG) was the best fit. As shown in Fig. 6, no significant association was detected between A carriers (AA and AG) and the risk of prostate cancer in overall populations (OR = 0.98, 95 % CI 0.82–1.18, P = 0.826). However, in the stratified analysis by ethnicity, a statistically significant association was found in Caucasians (OR = 1.17, 95 % CI 1.03–1.33, P = 0.016) but was not observed in Asians (OR = 0.85, 95 % CI 0.66–1.08, P = 0.177) and Africans (OR = 1.03, 95 % CI 0.79–1.33, P = 0.847).
Summary odds ratio (OR) for the association between ESR2 rs1256049 polymorphism and prostate cancer risk (upper panel, based on AA+AG vs. GG; lower panel, based on A vs. G). A random-effects model was used for all analyses. Horizontal lines represent 95 % confidence intervals (CIs). Boxes represent the OR in each study, with the size of each box is proportional to the weight of each study. Diamonds represent summary effects with corresponding 95 % CIs
Similar results were observed under allele model (A vs. G) (Fig. 6), which revealed that allele A was a risk factor for prostate cancer in Caucasians (OR = 1.19, 95 % CI 1.06–1.35, P = 0.005), and there was no association between allele A and prostate cancer risk in overall populations (OR = 1.05, 95 % CI 0.91–1.22, P = 0.478), Asians (OR = 0.96, 95 % CI 0.78–1.19, P = 0.703) and Africans (OR = 1.05, 95 % CI 0.82–1.34, P = 0.707).
There was no evidence of publication bias in dominant and allele model according to the results of Begg’s test (Table 2).
Discussion
Estrogens have significant direct and indirect effects on aberrant prostate growth, development and progression of prostate cancer, in which ESR1 and ESR2 are two key factors [4, 5]. ESR1 as an oncogenic factor promotes cell proliferation and survival, whereas ESR2 is a protective factor that is anti-carcinogenic and proapoptotic [38, 39]. It is known that the genetic polymorphisms in ESR1 and ESR2 genes can cause transcription change or affect the stability of the transcript [8, 9]. Thus, polymorphisms of ESR1 gene located on chromosome 6 [6] and ESR2 gene located on chromosome 14 [7] may be a risk factor for prostate cancer.
In recent years, there were several ESR1 and ESR2 gene polymorphisms that had been identified as candidates for prostate cancer research. Moreover, ESR1 rs9340799 and ESR2 rs1256049 polymorphisms had been extensively studied. Since the first study [18] reported the association between ESR1 rs9340799 polymorphism and prostate cancer in 2001, many subsequent studies have showed inconsistent results [10–16, 19–21]. There were three meta-analysis studies attempting to get conclusive results on the association of ESR1 rs9340799 polymorphism with prostate cancer risk [26–28], but they failed. Obviously, the combined results remained conflicting, which might be caused by that different number of eligible studies were enrolled and the most appropriate genetic model was not used to evaluate the association between ESR1 rs9340799 polymorphism and prostate cancer. In fact, the results can be misleading when an inappropriate model was assumed [40]. For the ESR2 rs1256049 polymorphism, there were also many studies reporting inconsistent results [14, 17, 20, 23–25], but no meta-analysis had been performed to analyze the combined results.
Therefore, it was essential for us to perform a refined meta-analysis choosing the most appropriate genetic model and including all relevant studies. In this study, a total of 16 studies pertained to ESR1 rs9340799 polymorphism, which enrolled 3,716 cases and 4,626 controls, and eight studies involved ESR2 rs1256049 polymorphism, which enrolled 7,796 cases and 8,927 controls. We used a “model free” approach to show the most appropriate genetic model. For ESR1 rs9340799 polymorphism, a dominant model (GG+GA vs. AA) was suggested. The results showed that G carriers (GG and GA) had increased prostate cancer risk compared with AA. In the subgroup analysis by ethnicity, the significant association was observed in Caucasians and Africans, but not in Asians. It is possible that different genetic backgrounds may account for these differences. However, there was still significant heterogeneity in Caucasians that might influence the results. We then conducted the cumulative meta-analysis and sensitivity analysis which suggested high stability and reliability of our results. When the association between ESR1 rs9340799 polymorphism and risk of prostate cancer was reported under allele model (G vs. A), it showed the same pattern of results as that under dominant model. For ESR2 rs1256049 polymorphism, a dominant model (AA+AG vs. GG) was also suggested. There was no significant association between A carriers (AA and AG) and prostate cancer risk. When stratified analysis was performed by ethnicity, significant association was found in Caucasians, but not in Asians and Africans. Similar results were observed under an allele model (A vs. G). The implications of ESR1 rs9340799 and ESR2 rs1256049 polymorphisms have not been elucidated. The ESR1 rs9340799 polymorphism is located at intron 1, which is an enhancer region. This intronic polymorphism may modify the splicing of messenger RNA (mRNA) transcripts, resulting in significant changes in gene function [8, 41]. The ESR2 rs1256049 polymorphism is a silent mutation in codon 328 and the G to A mutation does not change amino acid in the protein. However, this polymorphism may have a direct effect on modifying the secondary structure of the mRNA and leading to changes in mRNA stability and translation [9, 42].
Some potential limitations of this meta-analysis should be considered. First, selection bias might have played a role because the genotype distribution among cases and controls of one study deviated from HWE. Second, the number of case and control in Asians and Africans was not sufficiently large. Third, we searched articles from the database in English and Chinese, so articles with potentially high-quality data that were published in other languages were not included because of potential medical translation inaccuracies. Nonetheless, our meta-analysis had some advantages. First, it showed pooled results under the most appropriate genetic model, and cumulative meta-analysis and sensitivity analysis were conducted to validate the robustness of pooled results. Second, although possible publication bias was suggested between ESR1 rs9340799 polymorphism and prostate cancer risk, adjustment for the likely effect of bias using “trim and fill” method only showed a slight change, indicating that the conclusion should be unbiased.
Conclusions
In conclusion, the present study demonstrates that ESR1 rs9340799 polymorphism was significantly associated with prostate cancer in overall populations, Caucasians and Africans, while ESR2 rs1256049 polymorphism was significantly associated with prostate cancer only in Caucasians. Given that Prostate cancer is a multifactorial disease, future studies should be expected to explore the possible functional role of ESR1 rs9340799 and ESR2 rs1256049 polymorphisms in prostate cancer.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Becker N. Epidemiology of prostate cancer. Radiologe. 2011;51:922–9.
McDougall JA, Li CI. Trends in distant-stage breast, colorectal, and prostate cancer incidence rates from 1992 to 2004: potential influences of screening and hormonal factors. Horm Cancer. 2010;1:55–62.
Harkonen PL, Makela SI. Role of estrogens in development of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:297–305.
Ellem SJ, Risbridger GP. The dual, opposing roles of estrogen in the prostate. Ann N Y Acad Sci. 2009;1155:174–86.
Menasce LP, White GR, Harrison CJ, Boyle JM. Localization of the estrogen receptor locus (ESR) to chromosome 6q25.1 by FISH and a simple post-FISH banding technique. Genomics. 1993;17:263–5.
Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–65.
Herrington DM, Howard TD, Brosnihan KB, McDonnell DP, Li X, Hawkins GA, et al. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E-selectin but not C-reactive protein. Circulation. 2002;105:1879–82.
Maguire P, Margolin S, Skoglund J, Sun XF, Gustafsson JA, Borresen-Dale AL, et al. Estrogen receptor beta (ESR2) polymorphisms in familial and sporadic breast cancer. Breast Cancer Res Treat. 2005;94:145–52.
Beuten J, Gelfond JA, Franke JL, Weldon KS, Crandall AC, Johnson-Pais TL, et al. Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1869–80.
Sissung TM, Danesi R, Kirkland CT, Baum CE, Ockers SB, Stein EV, et al. Estrogen receptor α and aromatase polymorphisms affect risk, prognosis, and therapeutic outcome in men with castration-resistant prostate cancer treated with docetaxel-based therapy. J Clin Endocrinol Metab. 2011;96:E368–72.
Balistreri CR, Caruso C, Carruba G, Miceli V, Candore G. Genotyping of sex hormone-related pathways in benign and malignant human prostate tissues: data of a preliminary study. OMICS. 2011;15:369–74.
Szendroi A, Speer G, Tabak A, Kosa JP, Nyirady P, Majoros A, et al. The role of vitamin D, estrogen, calcium sensing receptor genotypes and serum calcium in the pathogenesis of prostate cancer. Can J Urol. 2011;18:5710–6.
Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Estrogen Receptors Alpha (rs2234693 and rs9340799), and Beta (rs4986938 and rs1256049) Genes polymorphism in prostate cancer: evidence for association with risk and histopathological tumor characteristics in Iranian men. Mol Carcinog. 2012;51 Suppl 1:E104–17.
Jurecekova J, Sivonova MK, Evinova A, Kliment J, Dobrota D. The association between estrogen receptor alpha polymorphisms and the risk of prostate cancer in Slovak population. Mol Cell Biochem. 2013;381:201–7.
Hernandez J, Balic I, Johnson-Pais TL, Higgins BA, Torkko KC, Thompson IM, et al. Association between an estrogen receptor alpha gene polymorphism and the risk of prostate cancer in black men. J Urol. 2006;175:523–7.
Chen YC, Kraft P, Bretsky P, Ketkar S, Hunter DJ, et al. Sequence variants of estrogen receptor beta and risk of prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2007;16:1973–81.
Modugno F, Weissfeld JL, Trump DL, Zmuda JM, Shea P, Cauley JA, et al. Allelic variants of aromatase and the androgen and estrogen receptors: toward a multigenic model of prostate cancer risk. Clin Cancer Res. 2001;7:3092–6.
Suzuki K, Nakazato H, Matsui H, Koike H, Okugi H, Kashiwagi B, et al. Genetic polymorphisms of estrogen receptor alpha, CYP19, catechol-O-methyltransferase are associated with familial prostate carcinoma risk in a Japanese population. Cancer. 2003;98:1411–6.
Fukatsu T, Hirokawa Y, Araki T, Hioki T, Murata T, Suzuki H, et al. Genetic polymorphisms of hormone-related genes and prostate cancer risk in the Japanese population. Anticancer Res. 2004;24:2431–7.
Cunningham JM, Hebbring SJ, McDonnell SK, Cicek MS, Christensen GB, Wang L, et al. Evaluation of genetic variations in the androgen and estrogen metabolic pathways as risk factors for sporadic and familial prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:969–78.
Gupta L, Thakur H, Sobti RC, Seth A, Singh SK. Role of genetic polymorphism of estrogen receptor-alpha gene and risk of prostate cancer in north Indian population. Mol Cell Biochem. 2010;335:255–61.
Sonoda T, Suzuki H, Mori M, Tsukamoto T, Yokomizo A, Naito S, et al. Polymorphisms in estrogen related genes may modify the protective effect of isoflavones against prostate cancer risk in Japanese men. Eur J Cancer Prev. 2010;19:131–7.
Nicolaiew N, Cancel-Tassin G, Azzouzi AR, Grand BL, Mangin P, Cormier L, et al. Association between estrogen and androgen receptor genes and prostate cancer risk. Eur J Endocrinol. 2009;160:101–6.
Sun YH, Yang B, Wang XH, Xu CL, Gao XF, Gao X, et al. Association between single-nucleotide polymorphisms in estrogen receptor beta gene and risk of prostate cancer. Chin J Surg. 2005;43:948–51.
Ding X, Cui FM, Xu ST, Pu JX, Huang YH, Zhang JL. Variants on ESR1 and their association with prostate cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2012;13:3931–6.
Wang YM, Liu ZW, Guo JB, Wang XF, Zhao XX, Zheng X. ESR1 Gene polymorphisms and prostate cancer risk: a HuGE review and meta-analysis. PLoS One. 2013;21:e66999.
Gu Z, Wang G, Chen W. Estrogen receptor alpha gene polymorphisms and risk of prostate cancer: a meta-analysis involving 18 studies. Tumor Biol. 2014. doi:10.1007/s13277-014-1785-4.
Hu ZY, Yu Q, Pei Q, Guo C. Dose-dependent association between UGT1A1*28 genotype and irinotecan-induced neutropenia: low doses also increase risk. Clin Cancer Res. 2010;16:3832–42.
Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J. A method for meta-analysis of molecular association studies. Stat Med. 2005;24:1291–306.
Wang T, Wang B. Association between glutathione S-transferase M1/glutathione S-transferase T1 polymorphisms and Parkinson's disease: a meta-analysis. J Neurol Sci. 2014;338:65–70.
Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672–3.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Mullen B, Muellerleile P, Bryant B. Cumulative meta-analysis: a consideration of indicators of sufficiency and stability. Pers Soc Psychol Bull. 2001;27:1450–62.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Dual S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98.
Salanti G, Amountza G, Ntzani EE, Ioannidis JP. Hardy–Weinberg equilibrium in genetic association studies: an empirical evaluation of reporting, deviations, and power. Eur J Hum Genet. 2005;13:840–8.
Bonkhoff H, Berges R. The evolving role of oestrogens and their receptors in the development and progression of prostate cancer. Eur Urol. 2009;55:533–42.
Attia DM, Ederveen AG. Opposing roles of ER-alpha and ER-beta in the genesis and progression of adenocarcinoma in the rat ventral prostate. Prostate. 2012;72:1013–22.
Minelli C, Thompson JR, Abrams KR, Thakkinstian A, Attia J. The choice of a genetic model in the meta-analysis of molecular association studies. Int J Epidemiol. 2005;34:1319–28.
Ushiyama T, Ueyama H, Inoue K, Nishioka J, Ohkubo I, Hukuda S. Estrogen receptor gene polymorphism and generalized osteoarthritis. J Rheumatol. 1998;25:134–7.
Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–16.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, C., Dong, WQ., Wang, A. et al. The influence of ESR1 rs9340799 and ESR2 rs1256049 polymorphisms on prostate cancer risk. Tumor Biol. 35, 8319–8328 (2014). https://doi.org/10.1007/s13277-014-2086-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2086-7