Abstract
The current treatment for advanced nonsmall cell lung cancer (NSCLC) remains unsatisfactory due to resistance to chemotherapy and ionizing radiation. The ubiquitin-proteasome system (UPS) regulates multiple cellular processes that are crucial for the proliferation and survival of all kinds of cells. Carbobenzoxyl-leucinyl-leucinyl-leucinal-H (MG132), a specific and selective reversible inhibitor of the 26S proteasome, represents a novel approach for cancer therapy. However, whether MG132 can potentiate the effect of radiation against the growth and metastasis of NSCLC is not clear. We found that MG132 inhibited the proliferation of human NSCLC cell lines (A549 and H1299) in a dose- and time-dependent manner by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Then MG132 at a nontoxic dose (100 nM) was selected for following studies. Pretreatment of A549 and H1299 cells with 100 nM MG132 before ionizing radiation (IR) potentiated the anticancer effect of IR. Moreover, pretreatment with 100 nM MG132 before IR-enhanced radiation induced cell cycle arrest by decreased CyclinD1 but increased Wee1 expression in A549 and H1299 cells. In addition, pretreatment of MG132 combined with IR significantly suppressed cell migration and invasion abilities in NSCLC cell lines, which was accompanied by decreased expression of matrix metalloproteinase (MMP)-2 and MMP-9 in NSCLC cell lines. Taken together, our results demonstrate that MG132 enhances the antigrowth and antimetastatic effects of irradiation in NSCLC cells by modulating expression of cell cycle and invasion- related genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonsmall cell lung cancer (NSCLC) accounts for approximately 80–85 % of all cases of lung cancer and is the leading cause of cancer-related death in sexual-independent populations [1]. The overall cure rate and survival rate of patients with advanced NSCLC remain low, with a median survival time of 8–11 months and a 1-year survival rate of 30 % [2–4]. Radiation therapy plays a significant role in the management of human lung cancer [5]. Unfortunately, few patients experience complete responses to radiotherapy, mainly because of the tolerance of the surrounding normal tissues. Meanwhile, solid tumors tend to lessen the efficacy of radiation therapy owing to a hypoxic region [6]. Thus, novel approaches to enhance the effects of ionizing radiation (IR) and reduce the radioresistance are warranted. Particularly, for the treatment of NSCLC, the combination of anticancer molecules with IR has been reported to improve the survival rate and been able to reduce the administration dose and the toxicity [7]. Many efforts for the screening and development of radiosensitizer have been reported to enhance the radiotherapeutic efficacy [8, 9]. Finally, the goal of the combination of chemotherapy and radiotherapy is the control of tumors and improvement of patient survival.
The ubiquitin-proteasome system (UPS) is critical for the proliferation and survival of all cells, particularly cancerous cells. Proteasome inhibition offers us a new way to inhibit the function of ubiquitin, which has become a very attractive anticancer target [10]. The first element of this pathway being investigated as a target is the proteasome, as proteasome degrades about 80 % of all intracellular proteins related to the regulation of cell cycle, proliferation, apoptosis, angiogenesis, metastasis, and resistance to chemotherapy and radiotherapy [11–13]. Meanwhile, IR has been reported to activate nuclear factor-κB (NF-κB) in both in vitro and in vivo models [14–16]. Activated NF-κB binds to specific DNA sequences in target genes, designated as κB elements and regulates transcription of over 400 genes involved in multiple biological processes, such as immunoregulation, growth regulation, proliferation, inflammation, carcinogenesis, apoptosis, and metastasis [17, 18]. Proteasome inhibitors including bortezomib (boronic acid dipeptide derivative) and carbobenzoxyl-leucinyl-leucinyl-leucinal-H (MG132) have shown anticancer activity and are known as a sensitizer to DNA-damaging agents, IR, and DNA cross linker for cancer therapy [19–21]. It is well known that bortezomib is an already approved agent for the treatment of multiple myeloma and has been under evaluation in phases I and II clinical trials against various malignancies including NSCLC [22]. Moreover, the combination of bortezomib with IR induced NF-κB inhibition and apoptosis, which is successful in the control of head and neck [23], colorectal [24], and androgen-independent prostate cancer cells [12]. MG132 has also been reported to sensitize PC-3 prostate cancer cells to IR via NF-κB inhibition [25, 26] and improve the radiotherapeutic effect in NSCLC [27]. However, proteasome inhibitors including MG132 have been studied at high concentration (μM) inducing apoptosis, which could not be applicable to clinical treatment for human cancer [12, 20, 27]. Moreover, whether low-dose MG132 can potentiate the effect of radiation against NSCLC remains unknown.
In this report, we investigated the effect of MG132 at a nontoxic dose (100 nM) on growth, metastasis, and its accurate mechanism in NSCLC cell lines. We found that MG132 significantly enhanced the antigrowth and antimetastatic effects of X-ray irradiation by regulating the expression of the proliferation and invasion-related genes including cyclin D1, P53, Wee1, and matrix metalloproteinases (MMPs).
Materials and methods
Cell culture and IR treatment
The NSCLC cell lines, A549 and H1299, were maintained in Dulbecco's modified Eagle's medium (DMEM; HyClone, Logan, UT, USA) containing 10 % fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100-U/mL penicillin, 100-μg/mL streptomycin at 37 °C in a humidified 5 % CO2 incubator. MG132 (Sigma-Aldrich, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) and diluted by DMEM (without FBS) to different concentrations. Cells were treated with indicated concentration of MG132 and irradiated 6 h later. Cells were irradiated using X-ray from linear accelerators (Varian, Palo Alto, CA, USA) with a dose rate of 200 cGy/min; 1.5-cm bolus was used as compensators.
Cell viability assay
Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded in 96-well plates at a density of 2.5 × 103/well 1 day prior to treatment. Then, cells were treated with MG132 or/and irradiation. After treatment, 20 μL of 5 mg/mL MTT solution was added into each well and incubated for 4 h. After the supernatant was removed, 100 μL of DMSO was added, and then placed in a microplate reader to measure OD value. Cell viability rate (VR) was calculated according to the following formula: VR = (OD in observed group / OD in 0 Gy group) × 100 %. All assays were repeated three times in quintuplicate.
Focus formation assay
For focus formation assay, cells were seeded into 6-well plate at 200 cells/well 24 h prior to MG132 treatment and then incubated with or without nontoxic dose (100 nM) of MG132 for 6 h at room temperature followed by removal of the supernatant and addition of MG132-free fresh culture solution. The cells were incubated for 12 days to allow colony formation and stained with 0.5 % crystal violet solution in 10 % methanol. The colonies composing more than 50 cells were counted using a microscope. The experiment was repeated three times.
Cell cycle distribution analysis
To determine whether MG132 affected the cell cycle distribution induced by IR in NSCLC cells, flow cytometry was used. Cells were seeded into 35-mm culture dishes and cultured at 70 % confluence before MG132 treatment. After incubated with DMSO or 100 nM of MG132 for 6 hs, cells were irradiated at 0 or 6 Gy. Cells were allowed to grow for another 48 h. Cells were digested by 0.25 % trypsin, fixed with 70 % ethanol, and stored overnight at 4 °C. Then, the cells were centrifuged, washed with phosphate-buffered saline (PBS) twice, labeled with propidium iodide (PI; 50 μg/mL), and then shielded from light for 30PBSmin before analyses by flow cytometry (Beckman Coulter, Inc., CA, USA).
In vitro wound healing-scratch assay
Cells were seeded into 35-mm culture dish and were cultured at 80–90 % confluence prior to MG132 treatment. After incubated with or without nontoxic dose (100 nM) of MG132 for 6 h, cells were irradiated at 0 or 4 Gy at room temperature. Confluent cell monolayers were then scratched using a 10-μL pipette tip, followed by removal of the supernatant and addition of fresh culture solution. The wound healing was observed using a microscope. The analysis of the wound healing was performed using ImageJ software (MD, USA). Assays were performed independently for three times [28].
Cell migration assay
Cells were pretreated with 100 nM MG132 or DMSO for 6 h prior to 4 Gy X-ray irradiation. After digested by 0.25 % trypsin, 1 × 104 cells (in 100 μL DMEM without FBS) were seeded into the upper part of a Transwell® chamber (24-well, 8.0 μm pore membranes; Corning, NY, USA). In the lower part of the chamber, 500 μL DMEM medium with 10 % FBS was added. Cells were allowed to grow for another 24 h, and then, the upper part of the chamber was removed and 50 μL of 5 mg/mL MTT solution was added into each lower part of the chamber. After incubation for 4 h at 37 °C, the supernatant was removed and 500 μL DMSO was added. The cell migration rate (MR) was calculated according to the following formula: MR = (OD in observed group / OD in control group) × 100 %. All assays were repeated three times in triplicates.
Boyden chamber cell invasion assay
After incubated with or without nontoxic dose (100 nM) of MG132 for 6 h, cells were irradiated at 0 or 4 Gy at room temperature; 1 × 104 cells (in 100 μL DMEM without FBS) were seeded into the upper part of a Transwell® chamber (transwell filter inserts in 6.5-mm diameter with a pore size of 5 μm; Corning Inc., Corning, NY, USA), which was 30 min precoated with 50 μL Matrigel. In the lower part of the chamber, 600-μL DMEM medium with 10 % FBS was added. The cells were incubated for 24 h to allow cells to migrate through the Matrigel. Then, the cells remaining on the upper surface were removed five times with a cotton swab moistened in PBS. Migrated cells on the lower surface were fixed in methanol for 10 min at room temperature followed by staining with 2 % crystal violet in methanol for 20 min. All assays were repeated three times in triplicates [29].
Western blot analysis
Cells were harvested at 48 h after treatment with MG132 or 8 Gy irradiation. Cells were then incubated with ice-cold RIPA buffer (50 mM Tris, pH 7.2, 150 mM NaCl, 1 % NP-40, 1 % sodium deoxycholate, 0.05 % SDS, and 1 mM PMSF) on ice. The supernatants obtained after centrifugation were quantified with an enhanced BCA protein assay kit (Beyotime, Nantong, China). Proteins were separated by sodium dodecyl sulfate poly-acrylamide gel electrophoresis (SDS-PAGE) through 10 % gel and transferred to polyvinylidene fluoride (PVDF) membrane. Membrane was blocked with 5 % skim milk in PBS containing 0.1 % Tween-20 (PBST) for 1 h at room temperature. Membrane was probed with antibodies against CyclinD1, P53, Wee1, MMP-2, and MMP-9 overnight at 4 °C. Antibodies against CyclinD1 and P53 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Wee1 antibody was obtained from Abcam company (Abcam, Cambridge, UK). MMP-2 and MMP-9 antibodies were obtained from Epitomics company (Epitomics, CA, USA). Membrane was washed three times in PBST and incubated with peroxidase-conjugated mouse or rabbit secondary antibody for 2 h at room temperature. Membrane was washed in PBST, and the detection was done using enhanced chemiluminescence. The analysis of relative protein expression level was performed using ImageJ software (MD, USA).
Statistical analysis
Statistical analysis was performed with the use of SAS 8.0 statistical software. Data are presented as mean ± SEM. The Student’s t test was used to measure the difference between the two groups. The differences between more than two groups were tested for significance using one-way analysis of variance. P < 0.05 was considered statistically significant.
Results
Enhancement of antiproliferative effect of MG132 with or without radiation in NSCLC cells
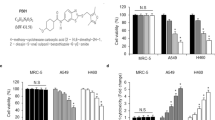
We first determined whether MG132 alone at various concentrations inhibits the proliferation of human NSCLC cells. The results clearly indicated that MG132 alone inhibited cell proliferation in both A549 and H1299 cells in a dose- and time-dependent manner (Fig. 1a, b). The 50 % inhibition concentration (IC50) of MG132 against A549 and H1299 cells was 1.43 and 1.04 μM, respectively. To evaluate the ability of MG132 to enhance the antitumor and antimetastatic effects of IR in NSCLC cells, 100 nM MG132, which resulted in ~90 % cell viability, was used for the following experiments as a nontoxic dose.
Proteasome inhibitor MG132 inhibits cell proliferation in A549 and H1299 cells in vitro. a A549 and b H1299 cells were incubated with various concentrations of MG132 for 24, 36, or 48 h. Cell viability was measured as described in “Materials and methods.” The viability of the untreated cells was normalized as 100 %. Data are presented as the means ± SEM of three independent experiments. c A549 and d H1299 cells were incubated with nontoxic dose of MG132 for 0, 2, 4, or 6 h before 0 or 4 Gy irradiation. Then, the cells were incubated for another 48 h, and the cell viability was measured. The viability of control cells was normalized as 100 %. Data are presented as the mean ± SEM of three independent experiments
We next evaluated whether pretreatment of MG132 enhanced the antiproliferative effect of IR in NSCLC cells. The results showed that 100 nM of MG132 inhibited cell proliferation of both cell lines in a pretreatment time-dependent manner (Fig. 1c, d). Given that 6 h of MG132 pretreatment before irradiation sensitized NSCLC cells to IR-induced cell death, this pretreatment time was used for further studies.
MG132 at nontoxic dose results in decreased focus formation and sufficiently enhances antigrowth effect of radiation in NSCLC cells
To investigate whether MG132 at a low dose could affect focus formation and enhance antigrowth effect of radiation, we first examined clonogenicity after treatment of 100 nM MG132 or equivalent DMSO for 6 h without IR. As shown in Fig. 2a, b, compared with DMSO-treated cells, the relative colony numbers of both cell lines after treatment of 100 nM MG132 were significantly decreased (P < 0.05) in both A549 and H1299 cells. However, cell viability of both cell lines was not attenuated by the pretreatment of 100 nM MG132 alone, whereas when combined with IR, 100 nM MG132 significantly enhanced cytotoxicity in an irradiation dose-dependent manner (Fig. 2c, d). These results demonstrated that MG132 at nontoxic dose (100 nM) resulted in decreased focus formation and sufficiently enhanced antigrowth effect of radiation in NSCLC cells.
Proteasome inhibitor MG132 decreases focus formation and enhances the antigrowth effect of IR in A549 and H1299 cells. a A549 and b H1299 cells were treated with 100 nM MG132 or equivalent DMSO for 6 h, and then, the medium was changed. Cells were incubated for another 12 days to allow colony formation. The colonies comprising over 50 cells were counted. The colony number of untreated cells was normalized as 100 %, and relative colony number was plotted. Data are presented as the means ± SEM (*P < 0.05, compared with the DMSO-treated control cells). c A549 and d H1299 cells were treated with 100 nM MG132 for 6 h prior to IR. After incubation for 72 h, cell viability was measured as described in “Materials and methods.” The viability of untreated cells was normalized to 100 %, and cell viability was plotted. Data are presented as the mean ± SEM (*P < 0.05, compared with the IR plus DMSO-treated cells)
Enhancement of cell cycle arrest of G1 in A549 cells and G2/M in H1299 cells due to the treatment with nontoxic dose MG132 before IR
Whether MG132 modulated cell cycle progression after IR remains elusive. A549 and H1299 cells were untreated or pretreated with 100 nM MG132 for 6 h. Then, cells were exposed to IR, and 48 h later, cell cycle was measured. As shown in Fig. 3a, b, compared with the DMSO-pretreated control cells, the pretreatment of 100 nM MG132 alone did not change the distribution of cell cycle in the two cell lines; 6 Gy X-ray irradiation alone induced a slight increase of G1 phase A549 cells and G2/M phase H1299 cells, whereas pretreatment with 100 nM MG132 for 6 h in combination with IR significantly increased radiation-induced G1 arrest in A549 cells and G2/M arrest in H1299 cells (P < 0.05).
Effect of MG132 and IR on cell cycle distribution in lung cancer cell. a A549 and b H1299 cells were pretreated with 100 nM MG132 or equivalent DMSO for 6 h prior to IR. After 24 h, both attached and floating cells were harvested for cell cycle analysis. Data are presented as the mean ± SEM of three independent experiments. (*P < 0.05, compared with the IR plus DMSO-treated cells)
MG132 modulates migration of human NSCLC cells at a nontoxic dose with or without radiation
To investigate whether MG132 at a nontoxic dose could significantly enhance the radiation-induced antimigration in NSCLC cells, we first performed scratch assays. As shown in Fig. 4a, b, compared with the control cells, the migration ability of both cell lines was modestly suppressed when cells were treated with IR alone in 24 h later. Whereas, cells treated with 100 nM MG132 for 6 h before exposing to IR significantly decreased the ability to migrate in the both cell lines and in vitro wound healing was remarkably inhibited compared with the control cells that closed the gap fastest at the same time point. We next examined the migration ability of NSCLC cells by the Boyden chamber cell migration assay and results showed that the cell MR of both cell lines treated with 100 nM MG132 plus IR were markedly decreased (Fig. 4c, d). These results indicated that the migration ability of NSCLC cells was significantly reduced by MG132 at a range of nontoxic dose (nM) in combination with IR.
Proteasome inhibitor MG132 at a nontoxic dose enhances the radiation-induced antimigration in A549 and H1299 cells. In vitro scratch-wound-healing assay was performed in a A549 and b H1299 cells. Cells were pretreated with 100 nM MG132 or equivalent DMSO for 6 h prior to IR. Wound healing was observed 24 h after IR. The migration of c A549 and d H1299 cells were determined by Boyden chamber cell migration assay. Cells were pretreated with 100 nM MG132 or equivalent DMSO for 6 h prior to IR. Cells were incubated for additional 24 h after irradiation to allow cells to migrate through membranes. Cells that migrated in the lower part of the chamber were stained. Data are presented as the mean ± SEM of triplicate experiments (*P < 0.05, compared with the plus DMSO-treated cells).
MG132 reduces invasion of human NSCLC cells at a nontoxic dose with or without IR
To investigate whether MG132 at a nontoxic dose could significantly enhance the anti-invasion effect of radiation in NSCLC cells, we performed the Boyden chamber-based cell invasion assay to examine the invasiveness of the two cell lines. We found that 100-nM MG132-pretreated cells for 6 h prior to IR significantly decreased the number of cells that penetrated the Matrigel-coated membrane (Fig. 5a, b). These results indicated that the invasiveness of the both cell lines was significantly reduced by MG132 pretreatment before IR at a range of nontoxic dose (nM).
Proteasome inhibitor MG132 at a nontoxic dose enhances the radiation-induced anti-invasion in A549 and H1299 cells. a A549 and b H1299 cells with indicated treatments were incubated for 24 h after radiation to allow cells to migrate through the Matrigel and the cells that through the Matrigel were stained by crystal violet (×400). Cell invasion rate of each group was calculated and plotted. Data are presented as the mean ± SEM (*P < 0.05, compared with the DMSO-treated control cells)
Enhancement of antigrowth and antimetastasis effects of radiation by nontoxic dose MG132 through regulating the expression of genes involved in proliferation and invasion
Whether MG132 can regulate the expression of genes associated with proliferation and invasion was examined by Western blot. The results revealed that pretreatment of 100 nM MG132 for 6 h before 8 Gy irradiation significantly inhibited the expression of cyclin D1, MMP-2, and MMP-9 while it upregulated the expression of P53 in A549 cells (Fig. 6a). Comparatively, in P53-null cell line H1299, pretreatment with MG132 prior to IR increased expression of Wee1, accompanying a reduction of MMP-2 and MMP-9 (Fig. 6b). These results suggested the different mechanisms of MG132 pretreatment against IR-induced antiproliferative and antimetastasis in A549 and H1299 cells.
Proteasome inhibitor MG132 regulates the expression of genes associated with proliferation and invasion in A549 and H1299 cells. The protein expression modulated by MG132 or/and IR was examined by Western blot analysis. Cells were harvested at 48 h after treatments with MG132 or equivalent DMSO for 6 h before irradiated with or without 8 Gy irradiation. The analysis of relative protein expression level was performed using ImageJ software (MD, USA)
Discussion
Radiation therapy is an established treatment modality and plays a significant role in the management of human lung cancer. However, radiotherapy is limited by the total dose that can be given without damaging normal tissues and the development of cancer radioresistance [30]. In this study, we investigated the efficacy and mechanism of MG132 for enhancing the antitumor and antimetastatic effects of radiation in human NSCLC cells. We found that MG132 inhibited cell proliferation in both cell lines in a dose- and time-dependent manner. Then, we determined that MG132 at nontoxic dose (nM) resulted in a decreased focus formation in both cell lines. This molecule also enhanced cytotoxicity, cell cycle arrest, antimigration, and anti-invasion induced by IR. In P53 wild-type A549 cells, MG132 plus IR inhibited the expression of cyclin D1, MMP-2, and MMP-9, followed by upregulation of P53. Comparatively, in P53-null H1299 cells, pretreatment with MG132 prior to IR increased expression of Wee1, accompanying a reduction of MMP-2 and MMP-9.
The mechanism of MG132 for the enhancement of antigrowth and antimetastasis effects of radiation has not been clearly illustrated so far. It has been reported that activation of the transcription factor NF-κB is frequently appeared in tumors and inhibition of the NF-κB signaling cascade may augment the efficacy of radiotherapy [31]. Our results showed that pretreatment of MG132 downregulated cyclin D1, MMP-2, and MMP-9, all of which are regulated by NF-κB signal pathway [32]. These molecules are associated with the improved antitumor and antimetastatic effects of radiation by MG132 in NSCLC cells [33]. Thus, the NF-κB activation which is downregulated by treatment of proteasome inhibition and IR could be actively pursued as a potential novel adjuvant treatment for cancer in conjunction with radiotherapy [24, 34]. Another possible mechanism is the involvement of β-catenin could be suggested for enhanced antitumor and antimetastatic effects of radiotherapy by proteasome inhibition. According to recent reports, the cleavage of β-catenin by MG132 is associated with a decrease in the mRNA level of c-myc, whose downregulation has been proved to enhance the efficacy of radiotherapy [35, 36].
In this study, NSCLC-derived A549 and H1299 cells were used. A549 cells are P53 wild-type whereas H1299 cells are deficient in P53 expression. In our study, A549 cells were observed to have G1 arrest and H1299 cells had a slight increase in G2/M phase after irradiation. Although both kinds of cells were pretreated with MG132 and then irradiated with X-ray, MG132 enhanced the radiation-induced G1 arrest of A549 cells and G2/M arrest of H1299 cells. In A549 cells, increased P53 was observed to be treated with IR and is more pronounced by pretreatment of MG132, which is similar to a previous report by Jung et al. [35]. Comparatively, there was no impact of MG132 on G1 arrest in H1299 cells. However, pretreatment with MG132 significantly enhanced IR-induced G2/M arrest in H1299 cells. It has been reported that IR-induced DNA damage activates ATM kinase that phosphorylates P53, checkpoint kinase 1 (Chk1), checkpoint kinase 2 (Chk2), and H2AX, which plays important roles in IR-induced DNA damage response in irradiated cells [37–40]. Our results suggested that upregulation of P53 expression was related to the enhanced radiation-induced G1 arrest and the improved antitumor and antimetastatic effects of radiation by MG132 in P53 wild-type A549 cells. During this process, inhibited NF-κB activation might coordinately facilitate the increase of P53. Activation of P53 in ATM signaling pathways by IR can regulate multiple pathways such as TGF-β, which suppresses cell growth, apoptosis, and angiogenesis of cancer cells [41]. The P53-deficient H1299 cells showed G2/M arrest after MG132 together with irradiation, which is similar to our previous report of artesunate in P53 mutant Hela cells [42], suggesting a P53-independent manner.
In summary, we demonstrated that MG132 at a dose of 100 nM displayed sufficient enhancement of antitumor and antimetastatic effects of radiation in human NSCLC cells. However, animal models bearing NSCLC-derived xenograft tumor still need be established to further confirm the facilitation of antitumor and antimetastatic effects of radiation by MG132 in vivo. Our results suggest the feasibility of using reduced dose of MG132 for the treatment of human lung cancer.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
Langer CJ, Mok T, Postmus PE. Targeted agents in the third-/fourth-line treatment of patients with advanced (stage III/IV) non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2013;39(3):252–60.
Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–74.
de Boer RH, Arrieta Ó, Yang CH, Gottfried M, Chan V, Raats J, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2011;29(8):1067–74.
O'Rourke N, Roqué IFM, Farré Bernadó N, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010;6, CD002140.
Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25(26):4066–74.
Sandler A. State-of-the-art treatment for advanced non-small-cell lung cancer. Oncology (Williston Park). 2003;17(12 Suppl 13):15–22.
Choi EK, Terai K, Ji IM, Kook YH, Park KH, Oh ET, et al. Upregulation of NAD(P)H:quinone oxidoreductase by radiation potentiates the effect of bioreductive beta-lapachone on cancer cells. Neoplasia. 2007;9(8):634–42.
Jeong SY, Park SJ, Yoon SM, Jung J, Woo HN, Yi SL, et al. Systemic delivery and preclinical evaluation of Au nanoparticle containing beta-lapachone for radiosensitization. J Control Release. 2009;139(3):239–45.
Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5(5):417–21.
Sorokin AV, Kim ER, Ovchinnikov LP. Proteasome system of protein degradation and processing. Biochemistry (Mosc). 2009;74(13):1411–42.
Goktas S, Baran Y, Ural AU, Yazici S, Aydur E, Basal S, et al. Proteasome inhibitor bortezomib increases radiation sensitivity in androgen independent human prostate cancer cells. Urology. 2010;75(4):793–8.
Wu WK, Cho CH, Lee CW, Wu K, Fan D, Yu J, et al. Proteasome inhibition: a new therapeutic strategy to cancer treatment. Cancer Lett. 2010;293(1):15–22.
Yakovlev VA, Barani IJ, Rabender CS, Black SM, Leach JK, Graves PR, et al. Tyrosine nitration of IκBα: a novel mechanism for NF-κB activation. Biochemistry. 2007;46(42):11671–83.
Cao N, Li S, Wang Z, Ahmed KM, Degnan ME, Fan M, et al. NF-κB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res. 2009;171(1):9–21.
Veuger SJ, Hunter JE, Durkacz BW. Ionizing radiation-induced NF-κB activation requires PARP-1 function to confer radioresistance. Oncogene. 2008;28(6):832–42.
Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–8.
Fukuyama R, Ng KP, Cicek M, Kelleher C, Niculaita R, Casey G, et al. Role of IKK and oscillatory NFkappaB kinetics in MMP-9 gene expression and chemoresistance to 5-fluorouracil in RKO colorectal cancer cells. Mol Carcinog. 2007;46(5):402–13.
Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res. 1999;5(9):2638–45.
Han YH, Moon HJ, You BR, Park WH. The attenuation of MG132, a proteasome inhibitor, induced A549 lung cancer cell death by p38 inhibitor in ROS-independent manner. Oncol Res. 2010;18(7):315–22.
Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14(6):1649–57.
Escobar M, Velez M, Belalcazar A, Santos ES, Raez LE. The role of proteasome inhibition in nonsmall cell lung cancer. J Biomed Biotechnol. 2011;2011:806506.
Van Waes C, Chang AA, Lebowitz PF, Druzgal CH, Chen Z, Elsayed YA, et al. Inhibition of nuclear factor-kappaB and target genes during combined therapy with proteasome inhibitor bortezomib and reirradiation in patients with recurrent head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;63(5):1400–12.
Russo SM, Tepper JE, Baldwin Jr AS, Liu R, Adams J, Elliott P, et al. Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int J Radiat Oncol Biol Phys. 2001;50(1):183–93.
Pajonk F, van Ophoven A, Weissenberger C, McBride WH. The proteasome inhibitor MG-132 sensitizes PC-3 prostate cancer cells to ionizing radiation by a DNA-PK-independent mechanism. BMC Cancer. 2005;5:76.
Warren G, Grimes K, Xu Y, Kudrimoti M, St Clair W. Selectively enhanced radiation sensitivity in prostate cancer cells associated with proteasome inhibition. Oncol Rep. 2006;15(5):1287–91.
Grimes KR, Daosukho C, Zhao Y, Meigooni A, St Clair W. Proteasome inhibition improves fractionated radiation treatment against non-small cell lung cancer: an antioxidant connection. Int J Oncol. 2005;27(4):1047–52.
Bulk E, Yu J, Hascher A, Koschmieder S, Wiewrodt R, Krug U, et al. Mutations of the EPHB6 receptor tyrosine kinase induce a pro-metastatic phenotype in non-small cell lung cancer. PLoS One. 2012;7(12):e44591.
Zhang D, Chen C, Li Y, Fu X, Xie Y, Li Y, et al. Cx31. 1 acts as a tumour suppressor in non-small cell lung cancer (NSCLC) cell lines through inhibition of cell proliferation and metastasis. J Cell Mol Med. 2012;16(5):1047–59.
Dempke WC, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer. 2010;67(3):257–74.
Liu YC, Chiang IT, Hsu FT, Hwang JJ. Using NF-κB as a molecular target for theranostics in radiation oncology research. Expert Rev Mol Diagn. 2012;12(2):139–46.
Li F, Sethi G. Targeting transcription factor NF-κB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805(2):167–80.
Lin Y, Bai L, Chen W, Xu S. The NF-κB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14(1):45–55.
Pajonk F, McBride WH. Ionizing radiation affects 26 s proteasome function and associated molecular responses, even at low doses. Radiother Oncol. 2001;59(2):203–12.
Jung J, Kim EJ, Chung HK, Park HJ, Jeong SY, Choi EK. c-Myc downregulation is involved in proteasome inhibitor-mediated enhancement of radiotherapeutic efficacy in non-small cell lung cancer. Int J Oncol. 2012;40(2):385–90.
Cervello M, Giannitrapani L, La Rosa M, Notarbartolo M, Labbozzetta M, Poma P, et al. Induction of apoptosis by the proteasome inhibitor MG132 in human HCC cells: possible correlation with specific caspase-dependent cleavage of beta-catenin and inhibition of beta-catenin-mediated transactivation. Int J Mol Med. 2004;13(5):741–8.
Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60(21):5934–6.
Fernandez-Capetillo O, Chen HT, Celeste A, Ward I, Romanienko PJ, Morales JC, et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol. 2002;4(12):993–7.
Lavin MF, Birrell G, Chen P, Kozlov S, Scott S, Gueven N. ATM signaling and genomic stability in response to DNA damage. Mutat Res. 2005;569(1–2):123–32.
Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282(5395):1893–7.
Dancea HC, Shareef MM, Ahmed MM. Role of radiation-induced TGF-beta signaling in cancer therapy. Mol Cell Pharmacol. 2009;1(1):44–56.
Luo J, Zhu W, Tang Y, Cao H, Zhou Y, Ji R, et al. Artemisinin derivative artesunate induces radiosensitivity in cervical cancer cells in vitro and in vivo. Radiat Oncol. 2014;9:84. 25.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (81172597, 81372433, and 31300694), the Key Programs of Natural Science Foundation of Jiangsu Educational Committee (11KJA310001), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, J., Shen, W., Tang, Y. et al. Proteasome inhibitor MG132 enhances the antigrowth and antimetastasis effects of radiation in human nonsmall cell lung cancer cells. Tumor Biol. 35, 7531–7539 (2014). https://doi.org/10.1007/s13277-014-2012-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2012-z