Abstract
The aim of this meta-analysis was to compare the effectiveness of combination of radiofrequency ablation (RFA) and transarterial chemoembolization (TACE) with that of RFA alone in patients with hepatocellular carcinoma (HCC). Randomized controlled trials comparing RFA plus TACE with RFA alone for HCC were included into this meta-analysis, and the search strategy followed the requirement of the Cochrane Library Handbook. Overall survival rate and recurrence-free survival rate were analyzed and compared by using Review Manager (version 5). We identified 7 randomized controlled trials comprising 571 patients who were treated by RFA plus TACE versus RFA alone for HCC. Meta-analyses showed that the combination of RFA and TACE was associated with a significantly higher overall survival rates (OR1 year = 2.39, 95 % CI, 1.35–4.21, P = 0.003; OR3 years = 1.85, 95 %CI 1.26–2.71, P = 0.002), and recurrence-free survival rate (OR1 year = 2.00, 95 % CI 1.26–3.18, P = 0.003; OR3 years = 2.13, 95 %CI 1.41–3.20, P < 0.001). Additionally, the quality of the evidence was high for the 1- and 3-year survival rate; no evidence of publication bias was observed. The combination of RFA with TACE can improve the overall survival rate and the recurrence-free survival rate for patients with HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third frequent cause of cancer death [1]. Besides, most patients with HCC are diagnosed at an intermediate stage, and only approximately 30 % of patients can benefit from hepatectomy [2, 3] Transcatheter arterial chemoembolization (TACE) can slow tumor progression, which has become one of the most widely performed treatments for intermediate-stage HCC [4]. Radiofrequency ablation (RFA), as a locoregional treatment, has been proved to be a safe and effective for local tumor control in patients with HCC, and is believed that it can be used as a first-line treatment for early HCC [5–7]. Either RFA or TACE has its own limitations; neither can achieve a complete control of medium or large HCC [4, 8]. Therefore, the combined use of RFA with TACE is an appealing approach and may offer opportunities for longer survival of HCCs. However, conflicting results has been reported by previous studies on assessing the effectiveness of the combination of RFA plus TACE and RFA alone [9–13]. A recently published meta-analysis which included seven randomized controlled trials (RCTs) show that RFA plus TACE significantly improved the survival rates compared with RFA alone in patients with HCC larger than 3 cm, and no advantage for HCC smaller than 3 cm [14]. However, the meta-analysis result was not precise for one included RCT study that has been retracted for a poor designation of randomized and controlled clinical trial [15].
The purpose of this paper is to present a meta-analysis of original research studies dealing with RFA plus TACE and RFA alone of HCC. We carried out an up-to-date meta-analysis of all RCTs to obtain a more precise estimate on effectiveness of combination of RFA and TACE with that of RFA alone in patients with HCC.
Materials and methods
Search strategy
We searched for relevant studies according to the search strategy of the Cochrane Collaboration. Three of the authors independently completed an online search of PubMed, Embase, Web of Science, and CBM databases for studies for RCTs that compared RFA plus TACE with RFA alone for HCC. We excluded registry data and case series which investigated either RFA or TACE for HCC. The literature search used terms (“radiofrequency ablation” or “RFA”) and (“TACE” or “Transarterial chemoembolization”) and (“hepatocellular carcinoma” or “liver cancer” or “HCC”). Language restriction was not imposed in this search. All the results were limited by “randomized controlled trials”.
Inclusion and exclusion criteria of trials
In the meta-analysis, the following inclusive selection criteria were set: (a) study design, prospective randomized controlled clinical trial; (b) the patients were scheduled to undergo TACE plus RFA vs RFA in the treatment of HCC; and (c) the age of the patient population should be over 18 years. The following exclusive selection criteria were set: (a) nonrandomized or lacking control group studies, (b) no clinical data were collected for primary or secondary outcomes (e.g., overall and recurrence-free survival rate), and (c) liver metastases or recurrence of HCC after hepatectomy.
Qualitative analysis
The risk of bias in RCTs was assessed following Cochrane recommendations, considering adequate sequence generation, allocation concealment, blinding, incomplete outcome data addressed, free of selective reporting, and free of other bias [16]. Each category was assessed as yes (low risk of bias), nuclear, or no (high risk of bias), and summarized in a table with plus, question mark, or minus signs, respectively. Publication bias was evaluated by funnel plots and Egger’s regression.
Statistical analysis
For each trial, odds ratio (OR) with the 95 % confidence interval (95 % CI) of the survival rate was derived and calculated using either the fixed-effects model or the random-effects model [17, 18]. For each meta-analysis, the Cochrane’s Q statistic was first calculated to assess the heterogeneity of the included trials. For P values less than 0.1, the assumption of homogeneity was deemed invalid, and the random effects model was used; otherwise, data were assessed using the fixed-effects model. In addition, a funnel plot was used to test a potential publication bias. Statistical analysis was performed using the software programs Review manager (version 5).
Results
Study identification and quality assessment
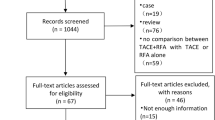
There were 605 potentially relevant studies. Most of these studies were not suitable for the present analysis because they included nonrandomized cohort studies, retrospective studies, and other study subjects irrelevant to our question; one study has been retracted [15]. By limiting the search to only RCTs, 7 studies involving a total of 571 HCC patients were included into this meta-analysis [19–25] (Table 1). The quality assessment of included RCTs was performed using Cochrane Collaboration’s tool, and the outcome is shown in Fig. 1. The most obvious risk of bias in the RCTs was the blinding procedure. Meanwhile, all RCTs had no adequate sequence generation and allocation concealment, thus the risk of bias is apparent. The risk of incomplete outcome data addressing, selective reporting, and other bias were not apparent across studies (Fig. 1).
Meta-analysis results
Overall survival rate
Data for 1-year survival rate were reported in seven trials, and there was no heterogeneity among those trials (P = 0.67), thus the fixed-effects model was used to pool the results. Meta-analysis showed the combination of RFA and TACE was associated with a higher 1-year survival rate compared with the RFA-alone group (OR = 2.39, 95 % CI 1.35–4.21, P = 0.003) (Fig. 2a). Data for 3-year survival rate were reported in six studies, and there was no heterogeneity among those trials (P = 0.61), thus the fixed-effects model was used to pool the results. Meta-analysis showed that the combination of RFA and TACE was associated with a higher 3-year survival rate compared with the RFA-alone group (OR = 1.85, 95 % CI 1.26–2.71, P = 0.002) (Fig. 2b) (Table 2).
Recurrence-free survival rate
Data for 1-year recurrence-free survival rate were reported in three trials, and there was no heterogeneity among those trials (P = 0.91), thus the fixed-effects model was used to pool the results. Meta-analysis showed the combination of RFA and TACE was associated with a higher 1-year recurrence-free survival rate compared with the RFA-alone group (OR = 2.00, 95 % CI 1.26–3.18, P = 0.003) (Fig. 3a). Data for 3-year recurrence-free survival rate were reported in three trials, and there was no heterogeneity among those trials (P = 0.20), thus the fixed-effects model was used to pool the results. Meta-analysis showed the combination of RFA and TACE was associated with a higher 3-year recurrence-free survival rate compared with the RFA-alone group (OR = 2.13, 95 % CI 1.41–3.20, P < 0.001) (Fig. 3b).
Major complications
Three trials reported relevant data on major complications [19, 22, 24], Which included segmental hepatic infarction (n = 1), bile duct stenosis (n = 1), gastric hemorrhage (n = 1), moderate ascites (n = 1), and liver failure (n = 1) in the TACE-RFA group, subcapsular hemorrhage (n = 1), abdominal infection (n = 1), small intestinal obstruction (n = 1), severe ascites (n = 1), and persistent jaundice (n = 1) in the RFA group. Two trials reported that there were no major complications observed in the patients of the two groups [20, 23]. There was no heterogeneity among those trials (I 2 = 0 %); thus, the fixed-effects model was used to pool data. Meta-analysis showed that there was no difference in terms of major complications between those two groups (fixed-effects OR = 1.00, 95 % CI 0.28–3.50, P = 1.00; Fig. 3c).
Assessment of publication bias
The publication bias in this meta-analysis was assessed using the funnel plot of the meta-analysis result. In the analysis of the effect of 1- and 3-year overall survival rates, and 1- and 3-year recurrence-free survival rates, the symmetry of the funnel plot shape suggested that there was no obvious publication bias in this meta-analysis. The results of Egger’s test did not show any evidence of publication bias in all comparisons.
Discussion
HCC is a serious fatal disease worldwide and causes serious damage to human health, and was the third most common cause of cancer mortality. HCC is a tumor with a highly variable biology that often occurs in the setting of chronic liver disease and cirrhosis. Most HCC patients are diagnosed at an intermediate or late stage, with poor baseline liver function, intrahepatic metastases, or overtumor burden, and not suitable for surgical resection. The established locoregional treatment options for HCC mainly include TACE, RFA, ethanol injection, and microwave coagulation; however, the optimal treatment choice continues to be debated [19, 26–28]. TACE is recommended as the first-line palliative treatment for inoperable HCC in the 2005 practice guideline issued by the American Association for the Study of Liver Disease, which can induce tumor ischemic necrosis by arterial injection of chemotherapeutic drugs and embolizing agents. RFA is typically performed percutaneously by advancing an electrode or multiple electrodes into the tumor and applying radiofrequency energy to generate a zone of thermal coagulation necrosis. Previous studies have reported that the combination of TACE and RFA was more effective than RFA alone for HCC [12, 19, 22, 29, 30]. However, some other studies have reported conflicting results [23, 24], and the number of patients in those studies were relatively small and failed to confirm a strong and consistent association.
Previous meta-analysis has shown that the efficacy of TACE combined with RFA was significantly better than that of TACE alone in patients with HCC [14]; however, the meta-analysis result was not precise for one included RCT study that has been retracted for a poor designation of randomized and controlled clinical trial. Hence, to provide good-quality evidence on its efficacy and safety, we carried out this up-to-date meta-analysis in which eight randomized controlled trials were finally included. Meanwhile, the overall survival rates together with recurrence-free survival rates were assessed in our meta-analysis. The present study showed that the combination of RFA and TACE was associated with higher survival rates (fixed-effects OR1 year = 2.39, 95 % CI 1.35–4.21, P = 0.003; fixed-effects OR3 years = 1.85, 95 %CI 1.26–2.71, P = 0.002) and recurrence-free survival rate (fixed-effects OR1 year = 2.00, 95 % CI 1.26–3.18, P = 0.003; fixed-effects OR3 years = 2.13, 95 %CI 1.41–3.20, P < 0.001) compared with RFA alone. The results of our study suggested that the combination of TACE and RFA was better than RFA alone for HCC.
In the previous meta-analysis, it showed that there was no significant difference between the combination of TACE plus RFA and RFA monotherapy on 1-year recurrence-free survival rate [12]. In contrast, in our data analysis, we found that the combination of TACE plus RFA was associated with significantly better 1-year recurrence-free survival rate than RFA monotherapy was in the treatment of HCC patients. The results could be explained as follows: first, the blood supply to a HCC is primarily provided by the hepatic artery, occlusion of hepatic arterial flow by means of TACE before RFA can reduce the cooling effect of hepatic blood flow on thermal coagulation. Thus, the necrotic area induced by RFA would be increased. Second, previous study showed that micrometastasis of HCC was occurred even at an early T stage when the tumor was small and solitary [31]. Lipiodol and anticancer agents used in TACE can improve the chance of clearance of micrometastasis. Therefore, an enlarged ablation zone and the effect of anticancer agents on hepatic cancer cells during the treatment might reduce the chance of tumor recurrence [22]. However, there were only three RCTs included, thus, they still need to be further confirmed by large sample randomized controlled trials.
Several limitations in this meta-analysis should be acknowledged. First, the inclusion criteria of HCC patients were heterogeneity (tumor size and location, number of tumors, and stage of liver function), which might influence the consistency of results and cause the between-study heterogeneity, which could affect the overall quality of our study. Second, the Cochrane Library’s tool was used to assess the risk of bias of all RCTs; it showed that the risk of bias was rather obvious for blinding procedure. However, it is hardly to precede blinding techniques due to differences between the interventions and the associated adverse effects. Third, the safety, complication, and adverse effects of the combination of TACE plus RFA were not fully assessed in these studies owing to the lack of data from original researches. Thus, future researches can further assess the safety, complication, and adverse effects of the combination of TACE and RFA.
In conclusion, our study indicated that the combination of RFA with TACE can improve the overall survival rate and the recurrence-free survival rate for patients with HCC. In addition, large-scale RCTs with long-term follow-up are needed to validate this outcome.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17.
Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–24.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42.
N'Kontchou G, Mahamoudi A, Aout M, Ganne-Carrie N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 western patients with cirrhosis. Hepatology. 2009;50:1475–83.
Rossi S, Ravetta V, Rosa L, Ghittoni G, Viera FT, Garbagnati F, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53:136–47.
Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–9.
Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9.
Kim JW, Kim JH, Won HJ, Shin YM, Yoon HK, Sung KB, et al. Hepatocellular carcinomas 2–3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. Radiofrequency ablation alone. Eur J Radiol. 2012;81:e189–93.
Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, et al. Medium-sized (3.1–5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18:1624–9.
Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210–24.
Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Transarterial chemoembolization combined with percutaneous radiofrequency ablation versus TACE and PRFA monotherapy in the treatment for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:653–9.
Wang W, Shi J, Xie WF. Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int. 2010;30:741–9.
Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872–82.
Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL, Zhang ZL, et al. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669–77.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
MANTEL N, HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–32.
Aikata H, Shirakawa H, Takaki S. Radiofrequency ablation combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. Hepatology 2006;44:A487nt 2013;33:375-383.
Kang CB, Xu HB, Wang SL, Rui B. Treatment of large hepatoma by TACE in combination with RFA. Zhonghua Gandan Waike Zazhi. 2007;13:828–30.
Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus rf ablation alone: a prospective randomized trial. Radiology. 2012;262:689–700.
Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–60.
Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–13.
Yang P, Liang M, Zhang Y, Shen B. Clinical application of a combination therapy of lentinan, multi-electrode RFA and TACE in HCC. Adv Ther. 2008;25:787–94.
Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgro G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol. 2010;52:380–8.
Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–58.
Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–9.
Kong QF, Jiao JB, Chen QQ, Li L, Wang DG, Lv B. Comparative effectiveness of radiofrequency ablation with or without transarterial chemoembolization for hepatocellular carcinoma. Tumour Biol. 2014;35:2655–9.
Jiang G, Xu X, Ren S, Wang L. Combining transarterial chemoembolization with radiofrequency ablation for hepatocellular carcinoma. Tumour Biol. 2014;35:3405–8.
Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–81.
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grant No. 81371654) and the Major Project of Science and Technology of Guangzhou (Grant number 132400027).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhenyin Liu and Fei Gao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Z., Gao, F., Yang, G. et al. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: an up-to-date meta-analysis. Tumor Biol. 35, 7407–7413 (2014). https://doi.org/10.1007/s13277-014-1976-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1976-z