Abstract
The aim of this study is to evaluate the diagnostic performance of human epididymis protein 4 (HE4), cancer antigen 125 (Ca125) and the risk of ovarian malignancy algorithm (ROMA) in discriminating ovarian cancer from other benign gynaecological diseases. Serum levels of HE4 and Ca125 were measured in 119 women with benign gynaecological diseases, 29 patients with primary ovarian cancer, 32 patients with ovarian cancer on chemotherapy treatment (18 of them with progressive disease), 6 patients treated and free of disease and 32 healthy women. Sensitivity, specificity, positive and negative predictive values and positive and negative likelihood ratios (LR±) were calculated. Receiver operator characteristic (ROC) curves were constructed, and the areas under the curve (AUC) were calculated. High serum levels for HE4, Ca125 and ROMA were observed in cancer patients. HE4 was elevated in 12.6 %, Ca125 in 21 % and ROMA in 9.2 % in the benign group, but HE4 was not elevated in endometriosis. The AUC values for HE4, Ca125 and ROMA were 0.92, 0.911 and 0.945 respectively. The sensitivity for discriminating ovarian cancer from benign gynaecological diseases was 86.2 % for HE4 and Ca125 and 93.1 % for ROMA. The specificity was 87.4, 78.9 and 90.7 % for HE4, Ca125 and ROMA. The overall positive likelihood ratio (LR+) was 6.84 for HE4, 4.1 for Ca125 and 10.01 for ROMA. In premenopausal women, LR + was 11.86 for HE4, 5.11 for ROMA and 2.02 for Ca125. HE4 might be significant in the differential diagnosis of ovarian cancer. HE4 seems to be superior to Ca125 in terms of diagnostic performance of all premenopausal women. ROMA could help to discriminate in cases with any doubt with a high diagnostic accuracy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial ovarian cancer (EOC) is the main cause of death from gynaecological malignancy in western world [1], and ovarian cancer has a poor prognosis because its diagnosis is usually detected in advanced stages. The early stages do not show any symptoms, or the symptoms are similar to the gynaecological benign diseases, and the clinical detection usually occurs in late stages. This delayed detection is common both in women with average risk of ovarian cancer and in women with high risk of ovarian cancer. If this tumour was diagnosed at early stage, it might be treated before spreading to surrounding tissues with a high possibility to have an optimal cytoreduction surgery [2, 3].
Biomarkers may be a powerful tool to improve diagnosis and prognosis for patients with ovarian cancer. So far, cancer antigen 125 (Ca125) is the tumour marker for ovarian cancer that the Scientific Societies Clinical Guidelines recommends for the detection of recurrence, monitoring of therapy and determination of prognosis in women with ovarian cancer but not for diagnosis in early stages. Ca125 is only recommended (with transvaginal ultrasound) for early detection of ovarian cancer in women at risk of this disease [4].
The Ca125 sensitivity is low for early stage, and the Ca125 specificity is low because the Ca125 level is high in other tumours that are not ovarian cancer as endometrial, cervix and lung cancers [5–8]. Elevated Ca125 serum level may be also found in benign gynaecological conditions as ovarian cysts, myomas and endometriosis [9]. It may be found elevated in other benign diseases as in patients with liver or renal failure or effusions [10]. For these reasons, Ca125 is not recommended for screening and diagnosis of ovarian cancer.
Nevertheless, some remarkable screening studies with Ca125 and transvaginal sonography (TVS) began at the end of 1980s and 1990s. The UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) had evaluated with a multimodal strategy using the age and the increased Ca125 serum levels measured annually, in order to select postmenopausal women for TVS [11]. Preliminary results suggest that multimodal strategy is effective at detecting early-stage cancer. This trial will report on the impact on ovarian cancer mortality in 2015. However, a randomized trial in postmenopausal women from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer trial in the USA found that the screening with Ca125 measurements with a fixed cutoff (>35 U/l) and annual transvaginal ultrasound leads to unnecessary surgery and did not decrease mortality from ovarian cancer and reported no mortality benefit with ovarian cancer (OC) screening [12].
In summary, there are controversies about the determination of Ca125 for the screening of ovarian cancer. But, Ca125 is related to tumour stage when the cancer is diagnosed. Abnormal Ca125 serum levels are found in approximately 50 % of stage I patients and 80–90 % in patients of stages III–IV, and Ca125 is a tumour marker for the follow-up because Ca125 serum levels decrease with the response to the treatment and increase with the recurrence [6, 13–15].
Other tumour markers for ovarian cancer have been proposed and investigated. Human epididymis protein 4 (HE4) has been identified as a potential serum marker in the diagnosis of a pelvic mass [16], and HE4 protein is frequently overexpressed in ovarian cancers, especially in serous and endometrioid histology [17, 18]. HE4 also increases sensitivity for detecting ovarian cancer, particularly in the stage I disease [18, 19]. Previous studies suggest that HE4 has a similar sensitivity to Ca125 but has an increased specificity in patients with malignant gynaecological disease as compared with those with benign gynaecological disease [20, 21]. However, HE4 is not specific for ovarian cancer, and abnormal levels also have been found in other malignancies as lung cancer [22] and endometrial adenocarcinomas [17, 21–23]. HE4 serum levels may be high in patients with renal failure or effusions. [21, 24].
It has been reported that HE4 was more specific than Ca125 in benign and malignant conditions so that HE4 had a better capacity to distinguish among healthy women and women with benign disease from those with malignant tumours [18, 20, 21, 23, 25–28]. On the other hand, Ca125 glycoprotein is not expressed in up to 20 % of ovarian cancer patients [14, 29].
Recent guidelines based on a meta-analysis approach have suggested HE4 to be used as an aid in ovarian cancer diagnosis [30], and some authors have proposed the use of a symptom index, Ca125 and HE4 as an annual first-line screen to select women for imaging if any two of three tests are positive warrants for further study [31].
In addition, different studies have proposed the use of a risk of ovarian malignancy algorithm (ROMA). ROMA relates Ca125, HE4 and the menopausal status, and it predicts the probability of risk of ovarian cancer. ROMA improves the sensitivity and specificity of the combined use of both tumour markers in patients with abdominal masses [24, 25, 27, 32].
Some authors showed that ROMA had better diagnostic performance than the widely used risk of malignacy index (RMI), where M is menopausal status, U is ultrasound findings and C is serum Ca125 level [28].
The aims of this study were the following:
-
1.
To evaluate HE4 and Ca125, alone or with the ROMA to be used for the diagnosis of ovarian cancer in patients with gynaecological symptoms
-
2.
To calculate our own cutoff for HE4 in premenopausal women.
Patients
Our hospital is a specialized centre on cancer treatment. We have analyzed and compared the Ca125, HE4 and ROMA of 218 women. The studied groups were the following:
-
1.
Twenty-nine patients who came to the hospital with gynaecological symptoms and were diagnosed for primary ovarian cancer.
-
2.
Thirty-eight patients with diagnosed ovarian cancer. The subgroups were as follows:
-
2.1
Eighteen patients with diagnosed ovarian cancer on chemotherapy treatment (T OC), they were patients diagnosed and treated with surgery before (stage I and II), on chemotherapy treatment at the moment of the inclusion in the study, and patients with advanced stages, on chemotherapy treatment at the moment of the analyses.
-
2.2
Fourteen patients with progressive ovarian cancer on chemotherapy treatment (PD), they were patients who had been diagnosed and treated before, and they were free of disease for some time that have relapsed and must be treated with chemotherapy.
-
2.3
Six ovarian cancer patients without evidence of disease after treatment (NED).
-
2.1
-
3.
One-hundred nineteen patients who came to the hospital with gynaecological symptoms and were diagnosed for gynaecological benign diseases (BDs) including ovarian simple cysts diagnosed by TVS (22 cases), serous cystadenoma (18 cases), endometriosis (18 cases), myoma (10 cases), teratoma (7 cases), mucinous cystadenoma (5 cases), atypical endometrial hyperplasia (5 cases) and other benign diseases in minor frequency.
-
4.
Thirty-two healthy women were taken as control group (CG).
The hormonal status was studied, and the menopause was defined as the absence of menstrual periods for 12 months.
The 29 patients with primary ovarian cancer were prospectively evaluated and staged according to conventional FIGO criterion. The patients with stages I and II were undergoing surgery and adjuvant chemotherapy. In patients with extensive stages III and IV, there were two options to treatment: a neoadjuvant chemotherapy, followed by interval debulking, or a debulking surgery, followed by chemotherapy.
Four patients were operated and classified as stage I (13.8 %). Two patients were operated and classified as stage II (6.9 %). Twenty-tree of the primary ovarian cancer patients were diagnosed in advanced stages (79.3 %): 15 patients were stage III (51.7 %), and 8 patients were stage IV (27.6 %).
Tissues were evaluated by experienced pathologists for diagnosis. The serous carcinoma histology was the most frequent diagnosis among the women with primary ovarian cancer patients (17 patients, 58.6 %); the histology of the other cases in these groups were 3 patients with endometrioid adenocarcinoma, 2 with mucinous adenocarcinoma, 2 patients with transitional cell carcinoma, 2 with clear cell carcinoma, 1 with müllerian, 1 with granulose cell, and 1 with fallopian tube.
The exclusion criteria were chronic liver disease or chronic renal failure.
Written informed consent was obtained from every patient.
Methods
All blood samples were collected on the same day prior to surgery and prior to chemotherapy. The blood samples were centrifuged, and the serum was processed within the 4 h of collection.
The marker serum levels were analyzed with automatic immunoassays ECLIA, Roche®, and Cobas e 601®. The cutoff of HE4 = 140 pmol/l (manufacturer’s protocol), and the cutoff of Ca125 = 35 U/ml.
Algorithm ROMA is a mathematical expression that calculates a predictive index (PI) for premenopausal/postmenopausal women:
For premenopausal women
For postmenopausal women
And the calculated PI is included in the equation:
For ROMA, in premenopausal women, the cutoff value was 11.4 %, and the cutoff value for postmenopausal women was 29.9 %.
The HE4 cutoff for premenopausal women was 77 pmol/l. It was calculated at 70 % of sensitivity and 94.1 % of specificity from the receiver operating characteristic (ROC) curves.
Surgical pathologic samples were tested by a gynaecological pathologist, and every diagnosis were reviewed and classified as either benign or malignant. Both tumour marker levels and ROMA were compared with the final pathologic diagnosis.
OC patient charts were reviewed to obtain all clinical and pathological features at the moment of diagnosis and during the follow-up.
EOC was defined as ovarian, fallopian tube and primary peritoneal cancer.
Statistical analyses
Statistical differences in marker levels among groups were evaluated using Kruskal-Wallis test and the Mann-Whitney U test. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for each marker and for ROMA as well.
The predictive probabilities for each model were used to construct ROC curves, and area under the curve (AUC) value was calculated. Efficacy value was calculated by the ratio among patients with cancer and positive results plus patients without cancer and negative results and the total number of patients studied.
Positive (LR+) and negative (LR−) likelihood ratios, corresponding to sensitivity/(1-specificity) and (1-sensitivity)/specificity were performed. The positive likelihood describes the discriminatory properties of a positive test result. A likelihood ratio greater than 1 indicates that the test result is associated with the disease. Positive likelihood ratios above 10 have been noted as providing convincing evidence, whereas those above 5 provide strong diagnostic evidence.
The negative likelihood describes the discriminatory properties of a negative test result. A likelihood ratio less than 1 indicates that the result is associated with absence of the disease. Negative likelihood ratios below 0.1 have been noted as providing convincing evidence, whereas those below 0.2 provide strong diagnostic evidence [33].
For all statistical comparisons, a p value of p ≤ 0.05 was considered significant. Statistical analyses were performed in SPSS ver. 15.0.
Results
We have compared tumour markers among women with primary ovarian cancer before any treatment, benign diseases and healthy women. Significantly higher serum concentrations of HE4, Ca125, and values of ROMA were found in patients with primary ovarian cancer than in those with benign diseases (p < 0.001) and healthy women (p value <0.001). HE4 and ROMA were higher in benign diseases than in control group (p = 0.008 and p = 0.001 respectively). Ca125 serum levels were higher in benign diseases than in healthy women, but the data were not statistically significant (p = 0.068). It is shown in Table 1.
We have compared among subgroups of patients with diagnosed ovarian cancer on treatment or after treatment (group 2). Significantly higher serum concentrations of HE4, Ca125, and values of ROMA were found in ovarian cancer patients on chemotherapy treatment (T OC) than in those patients without evidence of disease (NED) (p < 0.05, p < 0.001 and p < 0.05 respectively), and significantly higher serum concentrations of HE4 were found in patients with progressive disease (PD) than those ovarian cancer patients on chemotherapy treatment (p < 0.05), see Table 2. Serum mean values of HE4 and Ca125 in NED patients resemble those of the benign diseases and healthy women.
After these preliminary results, we have focused the study on the 29 patients with primary ovarian cancer and the 119 patients with gynaecological benign diseases that were prospectively evaluated.
Levels of both tumour markers and ROMA in patients with primary ovarian cancer were related to the stage. The 79.3 % of the patients were advanced stages (III and IV). The median serum levels and the sensitivity are shown in Table 3. The sensitivity of HE4 in stage I was 50 versus 100 % of Ca125, but in stage I, a patient has clear cell carcinoma, and another patient has transitional cell tumour, and in these histological types, HE4 is not expressed. In stage II, one of the two patients had peritoneal effusion.
Serous histology was the most common diagnosis in the primary ovarian cancer. The sensitivity for both HE4 and Ca125 were 94.1 % and for ROMA 100 %. The medians for these markers were 481.1 pmol/l (±540.6), 376.6 U/ml (±2083.9) and 89.9 (±16.8) respectively. The numbers of cases in the other group were insufficient to calculate statistics.
In patients with benign diseases, the most frequent pathologies were analyzed, and elevated serum levels of HE4 and Ca125 were found in 3.4 % (4 of 119) and 21 % (25 of 119) of patients respectively. These cases correspond to false-positive results. ROMA values were positives in 9.2 % of the cases. Ca125 was increased in these benign diseases especially in endometriosis (33.3 %), serous cyst (38.9 %), myoma (20 %) and teratoma (14.3 %). However, HE4 was not increased in endometriosis, and HE4 discriminated better than Ca125 and ROMA in this disease. In other benign pathologies, the percentage of false positives for HE4 was lower than those for Ca125. In benign pathology, false-positive results for HE4 were observed in postmenopausal women (Table 4).
The sensitivity for discriminating primary ovarian cancer from benign gynaecological diseases was 79.3 % for HE4, 86.2 % for Ca125 and 93.1 % for ROMA, and the specificity was 96.6 % for HE4, 78.9 % for Ca125 and 90.7 % for ROMA. The PPV was 85.2 % for HE4, 50 % for Ca125 and 71.1 % for ROMA. The NPV was 95 % for HE4, 95.9 % for Ca125 and 98.2 % for ROMA.
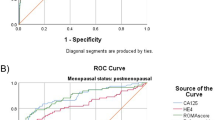
The diagnosis accuracy of HE4, Ca125 and ROMA was assessed by estimating ROC and AUC for patients with primary ovarian cancer versus benign diseases (Fig. 1). The AUC values for HE4, Ca125 and ROMA were 0.92 (confidence interval (CI) 95 %, 0.874–0.966), 0.911 (CI 95 %, 0.867–0.955) and 0.945 (CI 95 %, 0.94–0.987) respectively. The highest ROC-AUC was for ROMA, followed by HE4.
We have studied the hormonal status of the primary ovarian cancer patients, benign disease and control group, and we have divided them into subgroups of premenopausal and postmenopausal women. Most of these women were postmenopausal (66.1 %). Significantly higher HE4 serum levels were found in postmenopausal women than in premenopausal women (p < 0.001). For Ca125, higher concentrations were found in premenopausal women, but the values were not statistically significant (p = 0.061).
We have calculated the sensitivity, specificity, PPV and NPV and the ROC curves and the AUC in these subgroups based on hormonal status. In premenopausal women group, the sensitivity was 60 % for HE4, 90 % for Ca125 and 90 % for ROMA. The specificity was 100 % for HE4, 55.6 % for Ca125 and 82.4 % for ROMA. The PPV was 100 % for HE4, 37.5 % for Ca125 and 60 % for ROMA. The NPV was 89 % for HE4,95 % for Ca124 and 96.6 % for ROMA. The AUC was 0.915 (CI 95 %, 0.789–1.04) for HE4, 0.83 (CI 95 %, 0.699–0.961) for Ca125 and 0.917 (CI 95 %, 0.791–1.04) for ROMA. In postmenopausal women group, the sensitivity was 89.4 % for HE4, 84.2 % for Ca125 and 94.7 % for ROMA. The specificity was 95.3 % for HE4, 88.2 % for Ca125 and 94.1 % for ROMA. The PPV was 80.9 % for HE4, 61.5 % for Ca125 and 78.3 % for ROMA. The NPV was 97.5 % for HE4, 96.2 % for Ca125 and 98.8 % for ROMA. The AUC was 0.916 (CI 95 %, 0.864–0.967) for HE4, 0.94 (CI 95 %, 0.898–0.982) for Ca125 and 0.952 (CI 95 %, 0.91–0.994) for ROMA.
As in the premenopausal women, the AUC for HE4 was better than the AUC for Ca125; HE4 would have to be better tumour marker, but the HE4 sensitivity was 60 % versus 90 % for Ca125. Therefore, we have calculated another cutoff for HE4 for premenopausal women. The estimated cutoff for HE4 in premenopausal women was 77 pmol/l at 70 % of sensitivity and 94.1 % of specificity from the ROC. With this new cutoff for premenopausal women and the cutoff of 140 pmol/l for postmenopausal women, the sensitivity for HE4 in premenopausal women was 70 %, specificity was 94.1 %, PPV was 77.8 % and NPV was 91.4 %. The new overall sensitivity for HE4 was 86.2 % (79.3 % with cutoff = 140 pmol/l), specificity was 87.4 %, PPV was 62.5 % and NPV was 96.3 % (Table 5).
Therefore, with this new cutoff for HE4 in premenopausal women, the percentage of false positives in benign diseases increased from 3.4 to 12.6 %. Percentages of simple cyst and serous cyst increased as well, but no false positive was observed in endometriosis (Table 4).
We have calculated the LR ± for HE4, Ca125 and ROMA of patients with primary ovarian cancer and the subgroups of premenopausal and postmenopausal women (Table 5). The best LR ± for all cancer patients with primary ovarian cancer was for ROMA followed by HE4. However, if we evaluate LR ± in the subgroups of hormonal status, the best LR + in both premenopausal and postmenopausal groups was HE4 followed by ROMA with proved convincing evidence, and the best LR − was ROMA in all groups of patients.
The patients classified with ROMA as high risk for having ovarian cancer were 30.6 %, and all the cancer groups analyzed were 30.5 %. The patients classified with ROMA as low risk for having ovarian cancer were 69.3 %, and the benign groups together with healthy woman group were a 67.7 % of the studied cases. Therefore, the efficacy value for ROMA was 91.2 %.
Discussion
The great screening studies with Ca125 in combination with ultrasonography in asymptomatic women are not confluent. The positive predictive value of Ca125 is low to be used as an initial step in the screening of ovarian cancer [11, 12]. On the other hand, Ca125 is not an appropriate tool to be used in the diagnosis of a pelvic mass because of the lack of specificity of this marker, particularly in premenopausal women with benign gynaecological diseases, mainly related to endometriosis, and it would be of great help having other tumour markers that would help to assess the risk of ovarian cancer.
Previous published studies of HE4 have reported a higher specificity than Ca125 in different benign and malignant conditions, and algorithm ROMA improved the sensitivity and specificity, and both tumour markers were complementary [18–21, 25, 27].
Our hospital is a centre specialized in the treatment of cancer. Women who come to the hospital are patients that have gynaecological symptoms and suspicion of having tumour. So far, we used Ca125 as the tumour marker for ovarian cancer.
In this first evaluation, we wanted to assess whether HE4, Ca125 and ROMA could help to clarify the diagnosis of cancer in patients who came to the hospital with gynaecological symptoms and were diagnosed for primary ovarian cancer. This would help to optimize the rest of tests and the treatment for OC.
The results of our study show that serum levels of HE4 and Ca125 and ROMA values are significantly greater in patients with primary OC than in benign disease and in healthy women. These results are consistent with those published by other authors [16, 18, 20, 25, 27, 28, 34–37].
In benign diseases, only 12.6 % were positive for HE4 (cutoff = 77 pmol/l for premenopausal and cutoff = 140 pmol/l for postmenopausal women), while 21 % were positive for Ca125. These results were similar to those obtained by Moore et al. with 12 % for HE4 and 26 % for Ca125 in benign diseases using a cutoff for HE4 = 70 pM [18]. We have observed less false-positive cases in the benign diseases for HE4, particularly in the endometriosis in that none of the patients have abnormal results and Ca125 is abnormal in 33 % of these patients. These results were confirmed with other studies [18, 27, 31, 37]. Therefore, HE4 seems to be a valuable marker to distinguish patients with ovarian malignancies from those suffering from the benign ovarian endometriotic cysts.
Significantly higher HE4 serum levels were found in postmenopausal women than in premenopausal women (p < 0.001). These results were expected, given that HE4 increases with age in healthy people [21, 23, 31, 35]. Ca125 is higher in healthy premenopausal patients [34, 35], and higher concentrations of Ca125 in premenopausal women were found, but our values were not statistically significant (p = 0.061). The cutoff for HE4 and ROMA are vague yet. It would be essential to define a specific normal range and cutoff value for premenopausal and postmenopausal women. Differences in the results among several studies might have been caused by different numbers and characteristics of patients and controls, including age, types of benign diseases, cancer stages and histological subtypes.
HE4 is related with the stage in primary ovarian cancer, and HE4 increases the sensitivity for detecting ovarian cancer, particularly in the stage I [18, 19]. Our results are limited by the small number of cases in stages I–II. Besides, in stage I, a patient has clear cell carcinoma and another patient has transitional cell tumour, and in these histological types, HE4 is not expressed.
The diagnostic accuracy of HE4, Ca125 and ROMA in discriminating primary ovarian cancer from benign gynaecological conditions has been verified using ROC analysis. We have only used the group of primary ovarian cancer and benign disease patients for calculating the ROC curves. We did not include healthy women to calculate ROC-AUC values because the patients coming to our hospital have symptoms and suspicious of having tumours. The AUC values for HE4, Ca125 and ROMA were 0.92, 0.911 and 0.945 respectively. For other authors, the AUC ranges from 0.85 to 0.96 for HE4, from 0.81 to 0.95 for Ca125 and from 0.88 to 0.97 for ROMA [16, 19, 34, 38, 39]. We thought that the AUC among markers had similar values because of the 66.1 % of our patients were postmenopausal women, and in this group of women, Ca125 works well.
Therefore, we have separated the hormonal groups. The AUC in premenopausal and postmenopausal women indicated that in premenopausal women, HE4 discriminated better that Ca125, but in postmenopausal women, Ca125 was slightly better than HE4. As we have mentioned above, the 66.1 % of the patients were postmenopausal women where Ca125 have less false-positive results. The best results in both groups were for ROMA. Our results were similar to other studies [21, 33, 36] but are not agreed with those obtained by van Gorp et al. [36], whose AUC was slightly better for Ca125 than those for HE4 and ROMA, in overall patients and premenopausal and postmenopausal patients.
With the calculated cutoff value for HE4 in premenopausal of 77 and 140 pmol/l in postmenopausal women, the overall sensitivity was 86.2 % for both HE4 and Ca125, but the specificity was higher for HE4. The NPV were high and similar for both: 96.3 % for HE4 and 95.4 % for Ca125, and the PPV were low for both (62.2 and 50 %, respectively). This was one of the reasons because we calculated the LR±. The other one was that the group of patients with primary ovarian cancer was small, and the advantage of using the LR ± against PPV and NPV of the test lies in LR ± which does not depend on the prevalence or the proportion of analyzed patients but only of the sensitivity and specificity of the test.
The sensitivity was 86.2 % for both markers, but LR + for HE4 was 6.84, and LR + for Ca125 was 4.1, and this would mean that it would be more likely to be positive for HE4 in the presence of cancer. LR + gives the same information than sensitivity and specificity but expresses also how many times is more probable of having a positive result in women with ovarian cancer than in healthy women. This LR + value for HE4 provides strong diagnostic evidence.
In premenopausal patients, the best LR + was for HE4 with 11.86. This value provides convincing evidence, whereas LR + for ROMA was 5.1 (Table 5). This LR + of HE4 provides strong diagnostic evidence indicating more likely to have an ovarian cancer than a positive result of Ca125 or ROMA. In postmenopausal women, the best LR + was for HE4, but the LR + for ROMA was positive as well. As we have mentioned above, the 66.1 % of the patients were postmenopausal women, where Ca125 have less false-positive results.
The best LR − was for ROMA in every group. It means that a negative result for ROMA points at a smaller probability of risk of ovarian cancer than a negative result of HE4, or with a negative result of ROMA, there are less probability to have an ovarian cancer than those with a negative result of HE4.
NICE guidelines have reported results from studies comparing HE4 and Ca125 for the diagnosis of ovarian cancer in women with a pelvic mass and showed LR + of 7.75 (CI 95 %, 5.45–11.01) for HE4 versus LR + of 6.42 (CI 95 %, 4.02–10.26) for Ca125. LR − for HE4 was 0.26 (CI 95 %, 0.19–0.36) and LR − for Ca125 was 0.37 (CI 95 %, 0.31–0.45) [29].
Recent systematic reviews have reported LR + of 10.271 (CI 95 %, 6.982–15.109) and LR − of 0.228 (CI 95 %, 0.181–0.287) for HE4 [38]. Another study have published LR + and LR − of 13.0 (CI 95 %, 8.2–20.7) and 0.23 (CI 95 %, 0.19–0.28) for HE4 and 4.2 (CI 95 %, 3.1–5.6) and 0.27 (CI 95 %, 0.23–0.31) for Ca125 [39]. In the systematic review of Lin et al., they have also reported LR + of 8.04 (CI 95 %, 4.89–13.21) and LR − of 0.27 (CI 95 %, 0.22–0.34) for HE4 [40]. All these results of LR ± values for HE4 show strong diagnostic evidence.
Li et al. in a meta-analysis study with 11 published works have compared HE4, Ca125 and ROMA algorithm, and they have reported that LR + for HE4, Ca125 and ROMA were 12.21, 3.81 and 5.35 respectively and LR − for HE4, Ca125 and ROMA were 0.22, 0.23, 0.17 respectively. It means that the best probability of having ovarian cancer with a positive result is for HE4 followed by ROMA. So, a ROMA negative result indicates more probability of not having ovarian cancer that a negative result of HE4 or Ca125 [39]. Our results, considering the groups of premenopausal and postmenopausal, were agreed with by Li et al. [41].
Conclusions
-
1.
HE4 has similar sensitivity with Ca125 but has better specificity; HE4 discriminates ovarian cancer from benign diseases of all in endometriosis.
-
2.
HE4 measurement seems to be superior to Ca125 in terms of diagnostic performance of all in premenopausal women.
-
3.
HE4 and Ca125 serum concentrations and ROMA algorithm increase the accuracy of ovarian cancer diagnosis. ROMA can discriminate in cases with any doubt with a high diagnostic accuracy and provides strong diagnostic evidence (AUC 0.945 and LR − 0.08).
-
4.
It would be necessary for further studies with more patients to establish the definitive cutoff in premenpausal and postmenopausal groups for HE4 and ROMA algorithm.
References
Jemal A, Siegel RXJ, Sala E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300.
Paulsen T, Kjaerheim K, Kaern J, Tretli S, Trope C. Improved short-term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int J Gynecol Cancer. 2006;16 Suppl 1:11–7.
Engelen MJ, Kos HE, Willemse PH, Aalders JG, de Vries EG, Schaapveld M, et al. Surgery by consultant gynaecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006;106(3):589–98.
Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumour markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54(12):e11–79.
Jacobs I, Bast Jr RC. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
Molina R, Ojeda B, Filella X, Borras G, Jo J, Mas E, et al. A prospective study of tumour markers CA 125 and CA 19.9 in patients with epithelial ovarian carcinomas. Tumour Biol. 1992;13:278–86.
Molina R, Auge JM, Escudero JM, Marrades R, Vinolas N, Carcereny E, et al. Mucins CA 125, CA 19.9, CA 15.3 and TAG 72.3 as tumour markers in patients with lung cancer: comparison with CYFRA 21–1, CEA, SCC and NSE. Tumour Biol. 2008;29:371–80.
Molina R, Auge JM, Bosch X, Escudero JM, Vinolas N, Marrades R, et al. Usefulness of serum tumour markers, including progastrin-releasing peptide, in patients with lung cancer: correlation with histology. Tumour Biol. 2009;30:121–9.
Markman M. The role of CA-125 in the management of ovarian cancer. Oncologist. 1997;2:6–9.
Molina R, Filella X, Bruix J, Mengual P, Bosch J, Calvet X, et al. Cancer antigen 125 in serum and ascitic fluid of patients with liver diseases. Clin Chem. 1991;37:1379–83.
Menon U, Gentry-Maharaj A, Hallet R, Ryan A, Burnell M, Sharma A et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer , and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009; 10 (4):327-340. available http://www.ncbi.nlm.nih.gov/pubmed/21642681
Patridge E, Kreimer AR, Greenlee RT, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113 (4):775-782. available http://www.ncbi.nlm.nih.gov/pubmed/19305319
Bast Jr RC, Klug TL, Schaetzl E, Lavin P, Niloff JM, Greber TF, et al. Monitoring human ovarian carcinoma with a combination of CA 125, CA 19–9, and carcinoembryonic antigen. Am J Obstet Gynecol. 1984;149:553–9.
Bast Jr RC, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumour markers: CA 125 and beyond. Int J Gynecol Cancer. 2005;15 Suppl 3:274–81.
van Dalen A, Favier J, Hallensleben E, Burges A, Stieber P, de Bruijn HW, et al. Significance of serum CA 125 and TPS antigen levels for determination of overall survival after three chemotherapy courses in ovarian cancer patients during long-term follow-up. Eur J Gynaecol Oncol. 2009;30:609–15.
Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63(13):3695–700.
Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162–9.
Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumour biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–8.
Havrilesky L, Whitehead C, Rubatt J, Cheek R, Groelke J, He Q, et al. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008;110:374–82.
Nolen B, Velikokhatnaya L, Marrangoni A, De Geest K, Lomakin A, Bast Jr RC, et al. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol Oncol. 2010;117:440–5.
Escudero JM, Auge JM, Filella X, Torne A, Pahisa J, Molina R. The utility of serum human epididymis protein 4 (HE4) in patients with malignant and non malignant diseases: comparison with CA 125. Clin Chem. 2011;57(11):1534–44.
Galgano M, Hampton G, Frierson HJ. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006;19:847–53.
Moore RG, Brown AK, Craig Miller M, Badgwell D, Lu Z, Allard WJ, et al. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol. 2008;110:196–201.
Hertlein L, Stieber P, Kirschenhofer A, Krocker K, Nagel D, Lenhard M, et al. Human epididymis protein (HE4) in benign and malignant diseases. Clin Chem Lab Med. 2012;50(12):2181–8.
Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Craig Miller M, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and Ca125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40–6.
Park Y, Lee JH, Hong DJ, Lee EY, Kim HS. Diagnostic performances of HE4 and CA125 for the detection of ovarian cancer from patients with various gynecologic and non-gynecologic diseases. Clin Biochem. 2011;44(10–11):884–8. doi:10.1016/j.clinbiochem.2011.04.011.
Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009;100:1315–9.
Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, et al. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol. 2010;203:228–6.
Rosen DG, Wang L, Atkinson JN, Yu Y, Lu KH, Diamandis EP, et al. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005;99:267–77.
National Institute for Health and Clinical Excellence (NICE): Guidance. Ovarian cancer: the recognition and initial management of ovarian cancer. National Collaborative Centre for Cancer (UK). Cardiff, UK: National Collaborative Centre for Cancer, 2011.
Andersen MR, Goff BA, Lowe KA, Scholler N, Bergan L, Drescher CW, et al. Use of Symptom Idex, Ca125, and HE4 to predict ovarian cancer. Gynecol Oncol. 2010;116:378–83.
Lenhard M, Stieber P, Hertlein L, Kirschenhofer A, Fürst S, Mayr D, et al. The diagnostic accuracy of two human epididymis protein 4 (HE4) testing systems in combination with CA 125 in the differential diagnosis of ovarian masses. Clin Chem Lab Med. 2011;49(12):2081–8.
Deeks JJ, Almant DG. Diagnostics test 4: likelihood ratios. BMJ. 2004;329(7458):168–9.
Molina R, Escudero JM, Augé JM, Filella X, Foj L, Torne A, et al. HE4 a novel tumour marker for ovarian cancer: comparison with Ca125 and ROMA algoritm in patients with gynaecological diseases. Tumor Biol. 2011;32:1087–95.
Park Y, Kim Y, Lee EY, Lee JH, Kim HS. Reference ranges for HE4 and CA125 in a large Asian population by automated assays and diagnostic performances for ovarian cancer. Int J Cancer. 2012;130:1136–44.
Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, et al. HE4 and Ca125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer. 2011;104:863–70.
Bandiera E, Romani C, Specchia C, Zanotti L, Galli C, Ruggeri G, et al. Serum human epididymis protein 4 and risk for ovarian malignancy algorithm as new diagnositic and prognostic tools for epithelial ovarian cancer management. Cancer Epidemiol Biomarkers Prev. 2011;20:2496–506.
Yu S, Yang HJ, Xie SQ, Bao YX. Diagnostic value of HE4 for ovarian cancer: a meta-analysis. Clin Chem Lab Med. 2012;50(8):1439–46.
Ferraro S, Braga F, Lanzoni M, Boracchi P, Biganzolli EM, Panteghini M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review. J Clin Pathol. 2013;66:273–81.
Lin J, Qin J, Sangvatanakul V. Human epididymis protein 4 for differential diagnosis between benign gynaecologic disease and ovarian cancer: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2013;167(1):81–5.
Li F, Ruxiu T, Chang K, Wang F, Deng S, Lu W, et al. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and Ca125 in predicting epithelial ovarian cancer: A meta-analysis. BMC Cancer. 2012;12:258–76.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortiz-Muñoz, B., Aznar-Oroval, E., García, A.G. et al. HE4, Ca125 and ROMA algorithm for differential diagnosis between benign gynaecological diseases and ovarian cancer. Tumor Biol. 35, 7249–7258 (2014). https://doi.org/10.1007/s13277-014-1945-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1945-6