Abstract
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related deaths among female population worldwide. Metastases are the common cause of morbidity and mortality in breast cancer and can remain latent for several years after surgical removal of the primary tumour. Thus, the identification and functional characterisation of molecular factors that promote oncogenic signalling in mammary tumour development and progression could provide new entry points for designing targeted therapeutic strategies for metastatic breast cancer. In the present study, we investigated the expression of proteins involved in cell signalling (growth hormone receptor (GHR) and NEDD9) and cell-cell adhesion (plakoglobin) in epithelial and stromal compartments of primary ductal invasive breast carcinomas and their axillary lymph node metastases versus non-metastatic tumours. Obtained data revealed remarkable increase in the expression levels of GHR and NEDD9 proteins in both epithelial and stromal components of axillary lymph node metastases in comparison with those of non-metastatic tumours, suggesting that the expression of these two proteins may provide biomarkers for tumour aggressiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related deaths among female population worldwide. From the cellular, chromosomal and molecular genetic point of view, breast cancer is a heterogeneous disease whose management is hampered by variable response to treatment and differences in prognosis [1]. Traditionally, breast cancer can be classified based on distinctive histological features and molecular markers including histological grade, oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (HER2)/ERBB2 status as well as p53 mutational status. Metastases are the common cause of morbidity and mortality in breast cancer and can remain latent for several years after surgical removal of the primary tumour [2]. In some cases, it is possible to forecast the risk of metastatic recurrence in breast cancer based on distinctive features of the primary lesion including tumour size, histological grade and gene expression signatures [3]. However, there are still many gaps in current understanding of genetic alterations underlying metastatic process in breast cancer, either locoregionally into lymph nodes or systemically into distant organs. For example, it is not clear whether the same genes regulate metastasis in different histological types of breast cancer such as infiltrating ductal in comparison with lobular carcinoma. The advent of high-throughput technologies such as genome-wide expression analysis using cDNA microarray technology has offered a new perspective on breast cancer biology and showed that intrinsic gene expression signatures in combination with histopathological characteristics might prove efficient for more subtle tumour classification [4]. Still, these studies fail to completely delineate metastasis genetic programme in breast cancer resulting in the proposal of negligible number of protein products with potential therapeutic implications in blocking metastatic progression.
For metastasis to take place, tumour cells should detach from the primary site and penetrate through the vessel walls. Breakdown of the extracellular matrix (ECM) allows the cells to invade the surrounding tissue, enter the circulation and ultimately adhere to distant organs. In this process, cell-cell adhesion molecules occupy a central position. Plakoglobin (also known as γ-catenin) is an adhesion molecule found in adherens junctions and desmosomes, where it regulates cell-cell adhesion. Its expression has already been correlated with metastatic process in breast cancer, where plakoglobin seems to be involved in tumour cell shedding from the primary site into the bloodstream [5]. Down-regulation of plakoglobin expression detected in metastatic tissues in comparison with that in primary tumours from breast cancer patients indicates that plakoglobin might play a different role in metastatic lesions than in primary tumours [6]. Plakoglobin has a recognised role in controlling the motility of epithelial cells, where its high levels are associated with lower motility and vice versa [7, 8]. More insights into how plakoglobin contributes to the aggressiveness of breast cancer cells were provided by Bailey et al. [9], who showed that metastasis regulator zinc finger protein SNAI2 (SLUG) represses plakoglobin gene expression in the triple-negative (negative in the expression of oestrogen, progesterone, and ERBB2 receptors) breast cancer cells resulting in their increased motility. Expectedly, SLUG-negative non-aggressive breast cancer cells expressed high levels of plakoglobin.
Several well-known signalling pathways including the Myc, TGF-β, and Src pathways mediate some components of breast cancer metastasis progression [4]. In addition, there is evidence that growth hormone receptor (GHR)-mediated signalling pathways can be linked with the development of human breast cancer, possibly via autocrine or paracrine mechanisms [10, 11]. Besides growth hormone (GH), other hormones could be associated with breast cancer progression including oestrogen [10, 11], whose biological effects are mediated by its binding to one of the structurally and functionally distinct oestrogen receptors. Recent study has demonstrated that oestrogen negatively regulates cell spreading mediated by NEDD9 in human breast cancer cells giving rise to the importance of this protein in the breast cancer metastasis [12]. NEDD9 is involved in integrin-dependent signalling cascades, which trigger the focal adhesion kinase (FAK) and Src kinase responsible for cell migration, and also plays a role in Ras signalling cascades [13]. Growing body of evidence supports the role of NEDD9 as a tumour-promoting factor whose high levels in tumours correlate with poor prognosis and treatment resistance.
The aim of this study was to evaluate the expression of proteins involved in cell signalling (GHR and NEDD9) and cell-cell adhesion (plakoglobin) in epithelial and stromal compartments of primary ductal invasive breast carcinomas and their axillary lymph node metastases in relation to their expression in non-metastatic tumours.
Materials and methods
Patients
Forty tissue samples of non-metastatic (group I) and metastatic invasive ductal carcinoma (group IIA) along with their corresponding axillary lymph node metastases (group IIB) obtained upon surgical resection and confirmed by standard immunohistochemical staining methods were analysed in the present study. Tissue samples were obtained during regular surgery at the Sestre Milosrdnice Clinical Hospital Center in Zagreb, Croatia. All tumour tissue samples were of grade 2. Clinicopathological features of patients enrolled in this study are presented in Table 1. This study was approved by the hospital’s ethics committee.
Immunohistochemistry
Immunohistochemical analyses were performed using formalin-fixed, paraffin-embedded tissue sections (thickness 5 μm). After deparaffinisation, the tissue sections were stained by standard haematoxylin-eosin procedure. Deparaffinisation and immunohistochemical staining were carried out following microwave streptavidin immunoperoxidase (MSIP) protocol and by use of labelled streptavidin-biotin (LSAB) method on DAKO TechMateTM Horizon automated immunostainer. Monoclonal antibodies raised against oestrogen receptor, progesterone receptor and HER2 (Dako, Denmark), GHR (DAKO, Denmark), and plakoglobin (Abcam, UK) and NEDD9 (Abcam, UK) were used. Immunoreactive reactions were determined in stromal and epithelial tumour components, as well as in stromal and epithelial components of metastatic tumours in lymph nodes. Epithelial and stromal reactions were determined at the site of strongest activity («hot spot») under magnification of × 400 for a total of 1,000 tumour cells. The «hot spot» was established upon the whole section inspection (magnification of × 40). The results were presented semi-quantitatively by determination of immunohistochemical staining index by taking into account the intensity of reaction and upon calculation of cells with positive reaction. The intensity of the reaction was scored in the following way: 0, for no reaction; 1, for weak reaction; 2, for moderate to strong reaction; and 3, for very strong reaction. The percentage of immunoreactive (positive) tumour cells was scored as follows: 0, for no reaction; 1, for weak reaction (up to 10 % of the positive cells); 2, for moderate reaction (more than 10–25 % of the positive cells); and 3, for strong reaction (over 25 % of the positive cells).
Cell culture, cell substrate attachment and spreading assays
Cell culture
Freshly collected tissue from the surgical resection of mammary gland (invasive ductal carcinomas with distant metastases growth-hormone-positive cells (HGH+) and growth-hormone-negative cells (HGH−) of the enrolled patients were minced and dispersed by discontinuous incubation with neutral protease (Boehringer Meinheim, Germany) and collagenase (Flow Laboratories LA, CA, USA). For single cell experiments, freshly isolated cells were cultured at a density of 3 × 104 cells/cm2 in tissue culture flasks (Falcon Becton Dickinson, Oxnard, CA, USA).
The method for cultivation of primary human tumour cells and of their characterisation was performed as described previously [14, 15]. Briefly, primary breast cancer cells cultured in 24-well plates were maintained in RPMI supplemented with 10 % fetal bovine serum (FBS, Gibco), 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere with 5 % CO2 at 37 °C followed by serum deprivation for 12 h. Two primary mammary carcinoma cell cultures were studied: HGH + and HGH − as described previously [16]. The cells were washed in phosphate-buffered saline (PBS), detached in Ca2+- and Mg2+-free PBS-based cell dissociation buffer and collected by centrifugation at 300 g for 10 min (Eppendorf, Germany). The cells were re-suspended in the serum-free medium and plated in a crosswise mixing movement on the ECM to a final concentration of 1 × 104 cells in a total volume of 2 ml and incubated for 30, 60, 120 and 240 min. The medium was gently aspirated upon each treatment, and the cells were rinsed twice in the fresh serum-free medium to remove unattached cells. The experiment has been repeated in three biological replicates. When suspensions of single breast cancer cells were allowed to adhere to collagen-coated dishes, they initially attached as phase-bright rounded cells and then spread to form phase-dark epitheloid cells. The number of attached cells was counted under a light microscope. Data are expressed as the mean number of cells per grid field, and ten grid fields were counted for each sample. For statistical analysis, the ANOVA test was performed, and differences were considered statistically significant if p < 0.05.
Cell spreading was also assessed as the percentage of total number of attached cells by counting the number of cells that possessed a ‘spread morphology’ (epitheloid with lamelloid extensions) in each microscope field. Spreading cells (with lamelloid extensions during the spreading) came into contact with each other and remained in association as groups of epithelioid cells. Cell aggregates incubated in control medium with HGH showed a characteristic sequence of morphological changes as they were spreading. Cell spreading from aggregates included and orderly sequence of morphological changes: (1) appearance of cells at the margins of aggregates, (2) formation of monolayer patches and (3) eventual formation of confluent patches (monolayer patch). Spread cells were expressed as the proportion of total cells attached in each respective microscope field.
Statistical analysis
Statistical analyses were performed using specialised software for statistical analysis MedCalc (MedCalc Inc., Mariakerke, Belgium) and Statistica (version 7.0). For differential expression analysis, chi-square test and ANOVA (p < 0.05) were used. Post hoc analysis was conducted by t test between proportions. Differences were considered statistically significant if p < 0.05.
Results
Immunohistochemical analyses of the expression of epithelial and stromal growth hormone receptors in non-metastatic breast cancer (I), metastatic breast cancer (IIA) and corresponding axillary lymph node metastasis (IIB)
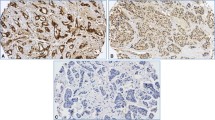
GHR expression in tissues derived from group I breast cancer, IIA and IIB are shown in Fig. 1a and Supplementary Tables 1, 2 and 3. Weak cytoplasmic immunostaining of the epithelial component and moderate stromal staining for GHR in group I tumours and strong epithelial and stromal staining in group IIA were detected (Fig. 1a, left and middle). GHR expression was markedly increased in group IIB in comparison with that in group I in both epithelial and stromal compartments (chi-square test; p = 0,001) (Fig. 3). In stromal compartment, we detected strong and moderate staining reactions in 47.5 % of cases of group I tumours vs 75 % of group IIB (Fig. 1a right). Interestingly, in epithelial compartment, we observed neither strong nor moderate reaction for GHR in group I as opposed to 12.5 % (group IIB, Fig. 1a right) and 10 % (group IIA). Furthermore, there was statistically significant difference in GHR expression in tumours without metastases between epithelial and stromal compartments (chi-square test; p <0.001). Its expression was increased in stroma in comparison with that in epithelium (strong reaction 25 vs 0 %, respectively; p = 0.001; moderate reaction 22.5 vs 0 %, respectively; p = 0.032) (Supplementary Table 1). In metastatic tumours, statistically significant reduction in epithelial GHR expression in comparison with its expression in stroma was observed (strong reaction 7.5 vs 30 %, respectively; p = 0.012) (Supplementary Table 2). Similarly, there was statistically relevant decline in GHR expression in epithelium in relation to stroma in axillary lymph node metastases (strong reaction 7.5 vs 47.5 %, respectively; p < 0.001) (Supplementary Table 3). Finally, no correlation was found between epithelial and stromal GHR expressions and clinicopathological parameters including tumour size and grade, oestrogen and progesterone receptor status, HER2 status and the TNM classification (data not shown).
Immunohistochemical analyses of the expression of epithelial and stromal NEDD9 protein in non-metastatic breast cancer (I), metastatic breast cancer (IIA) and corresponding axillary lymph node metastasis (IIB)
Immunohistochemical staining of breast cancer tissues for NEDD9 is shown in Fig. 1b. Strong immunostaining of the epithelial component of group I tumours and group IIA breast cancers was observed for NEDD9. There was no statistically significant difference in epithelial and stromal NEDD9 expressions between groups I and IIA. However, the expression of NEDD9 was markedly increased in group IIB in comparison with that in group I in epithelial compartment (chi-square Test; p = X). There were strong and moderate epithelial staining reactions in 77.4 % of the cases (group I) vs 100 % in group IIB and 80.0 % in group IIA) (Fig. 1b). Interestingly, strong staining reaction for NEDD9 was also observed in 5 % of the group IIB cases in stromal component, whereas groups I and IIA showed either absence or weak staining reaction for stromal NEDD9 (Fig. 1b). NEDD9 expression was predominantly detected in epithelial compartment of group I and group IIA in comparison with their stroma as evident from strong reaction in 67.4 vs 0 % of the cases (p < 0.001) and 80 vs 0 % of the cases (p < 0.001), respectively (Supplementary Tables 1 and 2). Similarly, statistically significant difference in NEDD9 expression between epithelial and stromal compartments in group IIB was noticed as inferred from strong reaction in 90 vs 5 % of all the cases, respectively (p < 0.001) (Supplementary Table 3).
Immunohistochemical analyses of the expression of epithelial and stromal plakoglobin in non-metastatic (I), metastatic breast cancer (IIA) and corresponding axillary lymph node metastasis (IIB)
Immunohistochemical staining of breast cancer tissues for plakoglobin is shown in Fig. 1c. In general, strong immunostaining of the epithelial component and negative or weak stromal staining were observed in groups I and IIA. Similarly, immunohistochemical detection revealed strong cytoplasmic immunostaining of the epithelial component in group IIB (Fig. 1c). Plakoglobin expression was significantly increased in epithelial compartment of group IIA and IIB tumours in comparison with that of group I tumours as evident from strong and moderate staining reactions observed in 92.5 % (group IIA) and 87.5 % (group IIB) of the cases, respectively, vs 65 % in group I tumours (Figs. 1c and 3). Interestingly, either complete absence or only weak staining of plakoglobin was detected in stromal components of all tissue samples (Fig. 1c). A tendency towards a decrease in plakoglobin expression in epithelial component of nodal metastases in comparison with that of the corresponding primary tumour could be noticed. In addition, there was statistically significant increase in plakoglobin expression in epithelium in comparison with that in stroma in non-metastatic (strong reaction 65 vs. 0 %, respectively; p < 0.001) and metastatic tumours (strong reaction 85 vs 0 %, respectively; p < 0.001) as well as in axillary lymph node metastases (strong reaction 87.5 vs 0 %, respectively; p < 0.001) (Supplementary Tables 1, 2 and 3). Interestingly, expression of plakoglobin was significantly higher in group I tumours in comparison with that in group IIB (p < 0.02).
Cell substrate attachment and spreading assay
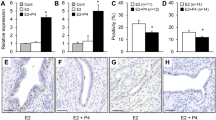
The relation between HGH expression and the spreading ability of cells in culture were assessed. The ability to spread upon adhesive substrate is a characteristic feature of many cultured cells. Cell spreading reflects the adhesive interaction between cells and their substrate. Changes in cell substrate adhesion may contribute to cell aggregation, in a complex sequence of changes from adsorption of cells to substrate, attachment (where they become resistant to shear forces) and eventually spreading. These changes normally form a continuous process in culture but can be experimentally separated and are likely to be mediated by different adhesive mechanisms [14]. Whereas the spreading of single cells primarily reflects cell-substrate adhesion, spreading of cells from aggregates is likely to be influenced by the balance between cell-cell and cell substrate adhesions as well. Thus, stimulation of cell-cell adhesion by HGH is predicted to shift the balance of adhesive forces acting upon the cells to favour aggregation [16]. Conversely, a decrease in cell-cell adhesion may alter that adhesive balance sufficiently to cause cell spreading without any concomitant change in cell adhesiveness for substrate. For that purpose, single cells were incubated in ECM alone or in serum-free medium supplemented with 100-nM HGH. After the incubation, wells were washed to remove non-attached cells. Cell attachment (cells per field) and cell spreading (% of the total number of attached cells) were measured and presented as a mean + SD. The cell substrate attachment and spreading assay results confirmed observed immunohistochemical data (Table 2; Fig. 2). The HGH-positive cells showed higher attachment rates in comparison with HGH-negative cells ranging between 14 and 32 %. The lowest attachment rate was observed upon 30 min of treatment with HGH and diminished with increased incubation time. However, the treatment of HGH-negative cells with HGH reversed the attachment rates to normal.
Cell attachment assay (upper panel) and cell spreading assay (lower panel). Isolated primary single breast cancer cells were cultured in collagen-coated dishes. After varying periods of time, dishes were washed to remove non-adherent cells. Cell attachment (cells per field) and cell spreading (% of total number of attached cells) were measured, and data are presented as mean ± SD
Similar results were obtained for cell spreading assay, where spreading rates for HGH-positive cells in comparison with those for HGH-negative cells ranged between 19 and 48 % (Fig. 2). Again, the treatment of HGH-negative cells with HGH reversed the spreading rates to normal. When suspensions of single breast cancer cells were allowed to adhere to collagen-coated dishes, they initially attached as phase-bright rounded cells and then spread to form phase-dark epitheloid cells (with lamelloid extensions during the spreading). This change in morphology was accompanied by increased spreading. A similar effect was observed by Gabriele Handschuh et al. [17] where mammary carcinoma cell line bearing mutant E-cadherin showed an increased cell motility which was accompanied with morphological changes including epitheloid morphology.
Discussion
Taking into account high mortality rates associated with metastatic breast cancer, the identification and functional characterisations of cellular and molecular factors driving breast cancer invasion and metastasis are of major health significance. Several experimental studies and clinical observations have brought into focus the possible role of pituitary hormones including GH and its receptor (GHR) in human breast carcinogenesis. It has been found that expression of GHR in tumour cells might be increased due to autocrine stimulation [11] and that more than 50 % of all breast cancer cases stain positively for GHR [18]. Kovari et al indeed have shown that positive staining for the GHR was more frequent in grade 2 tumours (86 %) in comparison with that in grade 1 (18 %) or grade 3 (47 %) cancers. Moreover, Gebre-Medhin et al. [10, 11] analysed 48 human breast carcinomas and 17 adjacent normal breast tissues to establish the expression status of GHR and found that GHR expression was up-regulated in breast cancer compared with adjacent normal breast tissue. GHR was predominantly expressed within the epithelial compartment of tumours, and its expression was detected in some stromal cells of the breast carcinoma as well. Our study showed remarkable increase in GHR expression in both epithelial and stromal components of axillary lymph node metastases in comparison with that of non-metastatic tumours and normal breast tissue (data not shown), indicating that deregulation of GHR expression might be associated with the development of metastases in breast cancer (Figs. 1, 2 and 3). However, several similar studies do not completely support the correlation between the GHR expression status and breast cancer progression. For example, Mertani et al. [19] detected GHR expression in epithelial cells of normal, benign proliferative breast disease (fibroadenoma, papilloma, adenosis, epitheliosis) and neoplastic lesions of the breast (intraductal carcinoma or lobular carcinoma in situ, and invasive ductal, lobular or medullary carcinoma) and concluded that the putative role of GH in the progression of proliferative mammary disorders cannot be ascribed to major alterations in the GHR expression levels. Similarly, Gebre-Medhin et al. [10, 11] postulated that increased GHR expression does not correspond to aggressive biological behaviour per se based on an inverse correlation found between GHR expression and proliferative activity and tumour grade, and GHR expression detected in both stromal and epithelial cells of a benign phyllode tumour [10, 11]. However, if epithelial expression of GHR was compared with plakoglobin and NEDD9 in specific histological types, GHR expression was significantly lower (p < 0.0001) in comparison with plakoglobin and NEDD9 in the luminal A and luminal B histological types and HER2+ subtype (Supplementary Fig. 1). Moreover, the patients’ survival rates were significantly higher for the group of non-metastatic tumours in comparison with those of metastatic tumours and HER2+ subtype (Supplementary Fig. 2). Due to inconsistent data published in the literature so far, we might postulate that the expression of GHR cannot be used as a sole marker for tumour aggressiveness and invasiveness but might be used in addition to standard immunohistochemical data and other markers, i.e. plakoglobin and NEDD9 as substantiated by the presented results.
Plakoglobin is a member of the Armadillo protein family and is a structural and functional homologues of β-catenin involved in the regulation of cell-cell adhesion and cell signalling [20]. Several studies are reported on the association between decreased plakoglobin expression and increased invasive behaviour of breast cancer cells proposing its possible clinical utility as potential prognostic factor of increased risk of breast cancer progression and as a therapeutic target [5, 21–23]. For example, Mukhina et al. [24] showed that autocrine production of GH in human mammary carcinoma cells specifically reduced the expression of plakoglobin associated with relocalisation of E-cadherin to the cytoplasm, disruption of cell-cell contacts and altered cellular morphology and motility. This decreased plakoglobin expression triggered by autocrine GH was indispensable for acquisition of an invasive phenotype in human mammary carcinoma cells, as forced expression of plakoglobin in human mammary carcinoma cell line MCF-7 stably transfected with the GH gene greatly reduced invasive capacity of these cells [24]. Presented in vitro results support the role of human GH in increased motility of primary breast cancer cells as well where human GH-negative cells treated with human GH increasingly spread in culture (Table 2; Fig. 2). Moreover, Holen et al. [5] showed that reducing expression of plakoglobin in human breast cancer cell lines increases cell motility and invasion and reduces cell-cell adhesion. In addition, these authors found very low levels of plakoglobin in the highly metastatic breast cancer cell lines, higher levels of plakoglobin in weakly metastatic cells and very high levels in non-metastatic cells, providing evidence that reduced plakoglobin expression increases the metastatic phenotype of breast cancer cells.
These in vitro results support a tumour suppressor role for plakoglobin, previously described in the literature, i.e. through inhibition of the c-Myc gene expression [25] or indirectly, through regulation of tumour suppressor gene expression, including PML in renal cells [26], and modulation of the cytoskeleton and Rho GTPase signalisation [8]. Indeed, several independent clinical examinations of plakoglobin expression patterns in primary breast carcinoma detected reduced expression of plakoglobin [22, 23, 27, 28]. Importantly, invasive ductal carcinomas showed statistically significant higher expression of plakoglobin in the metastases in comparison with that in the corresponding primary tumours, as supported by our results as well, whereas in invasive lobular carcinoma, there was no statistically different expression in the metastatic lesions compared with that in the corresponding primary tumours [22]. On the contrary, Bukholm et al. [6] reported on down-regulation of plakoglobin in metastatic lesions from invasive ductal carcinoma in comparison with that in primary tumour. Results presented in the current study revealed statistically significant increase in plakoglobin expression in the epithelial compartment of primary metastatic breast cancer tissues in comparison with that in non-metastatic tumours, indicating that plakoglobin in metastatic breast carcinomas might bear a biological function different from that in benign breast tumours. Indeed, literature evidence supports both the tumour suppressive and oncogenic roles of plakoglobin. The oncogenic activity of plakoglobin, as observed in the presented study as well, may be a result of modulation of the protein levels and signalling ability of β-catenin [29, 30].
A growing line of evidence supports the role of NEDD9/HEF1/Cas-L, a member of the Cas family of adhesion docking molecules, in breast cancer progression. In vitro studies showed that overproduction of HEF1 in MCF-7 breast carcinoma cells promotes cell spreading and migration and increases levels of mRNA transcripts encoding proteins that are associated with motility, cell transformation and invasiveness [31]. The first in vivo evidence linking NEDD9 with breast cancer aggressiveness came from the study demonstrating that genetic deletion of NEDD9 significantly limits early tumour development and diminishes cell spreading and migration in a mouse model of mammary tumour development [32]. Lack of NEDD9 caused marked down-regulation of pro-oncogenic signalling pathways including AKT, Src, FAK, and ERK in the late-appearing Nedd9 (−/−) tumours, pointing to the role of Nedd9 as a scaffolding protein for these proteins. Further elucidation of the role of NEDD9 in breast cancer progression was provided by Kong et al. [33] who showed that NEDD9 activates epithelial-mesenchymal transition, as overexpression of NEDD9 in human mammary epithelial cells led to the reduction of the epithelial markers with concomitant increase in the mesenchymal markers and promoted cell migration and invasion. Additionally, inhibition of endogenous NEDD9 expression in highly aggressive triple-negative breast cancer (TNBC) cell lines reduced the migration, invasion and proliferation of these cells and partially reversed the EMT process. Immunohistochemical analysis of mammary tissue sections from breast cancer patients showed increased expression of NEDD9 in breast carcinoma cells in comparison with that in normal mammary epithelial cells. Importantly, high levels of NEDD9 expression were associated with the aggressive breast cancers, including TNBC and ERBB2-positive subtypes [33]. Our results also support the role of NEDD9 as metastasis marker in breast cancer, as we observed significant rise in NEDD9 expression in both epithelial and stromal components of axillary lymph node metastases in comparison with that of their corresponding primary tumours. In light of the finding that the absence of NEDD9 greatly sensitises cells to the Src-family targeting agent dasatinib, clinically used for the treatment of breast and of other cancers [13] , pharmacological inhibition of NEDD9-mediated signalling pathways might provide an entry point for the development of novel therapeutic strategies for treating metastatic breast cancer.
In conclusion, our study revealed remarkable increase in the expression levels of GHR and NEDD9 proteins in both epithelial and stromal components of axillary lymph node metastases in comparison with those of non-metastatic tumours, suggesting that the expression of these two proteins may provide biomarkers for breast cancer aggressiveness. In addition, reduced expression of epithelial plakoglobin in nodal metastases in comparison with primary ductal invasive breast carcinomas indicates that deregulation of plakoglobin expression might play a critical role in the acquisition of an invasive phenotype in human mammary carcinoma cells.
References
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–52.
Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602.
Blanco MA, Kang Y. Signaling pathways in breast cancer metastasis—novel insights from functional genomics. Breast Cancer Res. 2011;13:206.
Holen I, Whitworth J, Nutter F, Evans A, Brown HK, Lefley DV, et al. Loss of plakoglobin promotes decreased cell-cell contact, increased invasion and breast cancer cell dissemination in vivo. Breast Cancer Res. 2012;14:R86.
Bukholm IK, Nesland JM, Borresen-Dale AL. Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients [seecomments]. J Pathol. 2000;190:15–9.
Yin T, Getsios S, Caldelari R, Kowalczyk AP, Muller EJ, Jones JC, et al. Plakoglobin suppresses keratinocyte motility through both cell-cell adhesion-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2005;102:5420–5.
Todorovic V, Desai BV, Patterson MJ, Amargo EV, Dubash AD, Yin T, et al. Plakoglobin regulates cell motility through Rho- and fibronectin-dependent Src signaling. J Cell Sci. 2010;123:3576–86.
Bailey CK, Mittal MK, Misra S, Chaudhuri G. High motility of triple-negative breast cancer cells is due to repression of plakoglobin gene by metastasis modulator protein SLUG. J Biol Chem. 2012;287:19472–86.
Gebre-Medhin M, Kindblom LG, Wennbo H, Tornell J, Meis-Kindblom JM. Growth hormone receptor is expressed in human breast cancer. Am J Pathol. 2001;158:1217–22.
Chiesa J, Ferrer C, Arnould C, Vouyovitch CM, Diaz JJ, Gonzalez S, et al. Autocrine proliferative effects of hGH are maintained in primary cultures of human mammary carcinoma cells. J Clin Endocrinol Metab. 2011;96(9):E1418–26.
Bradshaw LN, Zhong J, Bradbury P, Mahmassani M, Smith JL, Ammit AJ, et al. Estradiol stabilizes the 105-kDa phospho-form of the adhesion docking protein NEDD9 and suppresses NEDD9-dependent cell spreading in breast cancer cells. Biochim Biophys Acta. 1813;2011:340–5.
Singh MK, Izumchenko E, Klein-Szanto AJ, Egleston BL, Wolfson M, Golemis EA. Enhanced genetic instability and dasatinib sensitivity in mammary tumor cells lacking NEDD9. Cancer Res. 2010;70:8907–16.
Yap AS. Manley SW Contact inhibition of cell spreading: a mechanism for the maintenance of thyroid cell aggregation in vitro. Exp Cell Res. 1993;208:121–7.
Pavelic K, Bulbul MA, Slocum HK, Pavelic ZP, Rustum YM, Niedbala MJ, et al. Growth of human urological tumors on extracellular matrix as a model for the in vitro cultivation of primary human tumor explants. Cancer Res. 1986;46:3653–62.
Kaulsay KK, Mertani HC, Lee K-O, Lobie PE. Autocrine human growth hormone enhancement of human mammary carcinoma cell spreading is Jak2 dependent. Endocrinology. 2000;141:1571–84.
Handschuh G, Candidus S, Luber B, Reich U, Schott C, Oswald S, et al. Tumour-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene. 1999;18:4301–12.
Kővári B, Rusz O, Schally AV, Kahán Z, Cserni G. Differential immunostaining of various types of breast carcinomas for growth hormone-releasing hormone receptor—apocrine epithelium and carcinomas emerging as uniformly positive. APMIS. 2014. doi:10.1111/apm.12224.
Mertani HC, Garcia-Caballero T, Lambert A, Gerard F, Palayer C, Boutin JM, et al. Cellular expression of growth hormone and prolactin receptors in human breast disorders. Int J Cancer. 1998;79:202–11.
Aktary Z, Pasdar M. Plakoglobin: role in tumorigenesis and metastasis. Int J Cell Biol. 2012;2012:189521.
Sommers CL, Gelmann EP, Kemler R, Cowin P, Byers SW. Alterations in beta-catenin phosphorylation and plakoglobin expression in human breast cancer cells. Cancer Res. 1994;54:3544–52.
Park D, Karesen R, Axcrona U, Noren T, Sauer T. Expression pattern of adhesion molecules (E-cadherin, alpha-, beta-, gamma-catenin and claudin-7), their influence on survival in primary breast carcinoma, and their corresponding axillary lymph node metastasis. APMIS. 2007;115:52–65.
Bukholm IK, Nesland JM, Karesen R, Jacobsen U, Borresen-Dale AL. E-cadherin and alpha-, beta-, and gamma-catenin protein expression in relation to metastasis in human breast carcinoma. J Pathol. 1998;185:262–6.
Mukhina S, Mertani HC, Guo K, Lee KO, Gluckman PD, Lobie PE. Phenotypic conversion of human mammary carcinoma cells by autocrine human growth hormone. Proc Natl Acad Sci U S A. 2004;101:15166–71.
Williamson L, Raess NA, Caldelari R, Zakher A, de Bruin A, Posthaus H, et al. Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. EMBO J. 2006;25:3298–309.
Shtutman M, Zhurinsky J, Oren M, Levina E, Ben-Ze'ev A. PML is a target gene of β-catenin and plakoglobin, and coactivates β-catenin-mediated transcription. Cancer Res. 2002;62:947–54.
Goyal A, Martin TA, Mansel RE, Jiang WG. Real time PCR analyses of expression of E-cadherin, alpha-, beta- and gamma-catenin in human breast cancer for predicting clinical outcome. World J Surg Oncol. 2008;6:56.
Schonborn I, Zschiesche W, Behrens J, Herrenknecht K, Birchmeier W. Expression of E-cadherin/catenin complexes in breast cancer. Int J Oncol. 1997;11:1327–34.
Salomon D, Sacco PA, Roy SG, Simcha I, Johnson KR, Wheelock MJ, et al. Regulation of β-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system. J Cell Biol. 1997;139:1325–35.
Klymkowsky MW, Williams BO, Barish GD, Varmus HE, Vourgourakis YE. Membrane-anchored plakoglobins have multiple mechanisms of action in Wnt signaling. Mol Biol Cell. 1999;10:3151–69.
Fashena SJ, Einarson MB, O'Neill GM, Patriotis C, Golemis EA. Dissection of HEF1-dependent functions in motility and transcriptional regulation. J Cell Sci. 2002;115:99–111.
Izumchenko E, Singh MK, Plotnikova OV, Tikhmyanova N, Little JL, Serebriiskii IG, et al. NEDD9 promotes oncogenic signaling in mammary tumor development. Cancer Res. 2009;69:7198–206.
Kong C, Wang C, Wang L, Ma M, Niu C, Sun X, et al. NEDD9 is a positive regulator of epithelial-mesenchymal transition and promotes invasion in aggressive breast cancer. PLoS One. 2011;6:e22666.
Acknowledgments
The work was supported by the Ministry of Science Education and Sports projects 335-0982464-239, 335-0000000-3532 and 108-1081870-1884.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Štajduhar, E., Sedić, M., Leniček, T. et al. Expression of growth hormone receptor, plakoglobin and NEDD9 protein in association with tumour progression and metastasis in human breast cancer. Tumor Biol. 35, 6425–6434 (2014). https://doi.org/10.1007/s13277-014-1827-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1827-y